Abstract
The aim of this study was to investigate the physiological effects of compression tights on blood flow following exercise and to assess if the placebo effect is responsible for any acute performance or psychological benefits. Twenty-two resistance-trained participants completed a lower-body resistance exercise session followed by a 4 h recovery period. Participants were assigned a post-exercise recovery intervention of either compression tights applied for 4 h (COMP), placebo tablet consumed every hour for 4 h (PLA) or control (CON). Physiological (markers of venous return, muscle blood flow, blood metabolites, thigh girth), performance (countermovement jump, isometric mid-thigh pull), and psychological measures (perceived muscle soreness, total quality of recovery) were collected pre-exercise, immediately post-exercise, at 30 (markers of venous return and muscle blood flow) and 60 min (blood metabolites, thigh girth and psychological measures) intervals during 4 h of recovery, and at 4 h, 24 h and 48 h post-exercise. No significant (P > 0.05) differences were observed between interventions. However, effect size analysis revealed COMP enhanced markers of venous return, muscle blood flow, recovery of performance measures, psychological measures and reduced thigh girth compared to PLA and CON. There were no group differences in blood metabolites. These findings suggest compression tights worn after resistance exercise enhance blood flow and indices of exercise recovery, and that these benefits were not due to a placebo effect.
Similar content being viewed by others
Introduction
Exercise-induced muscle damage (EIMD) frequently occurs following unaccustomed exercise1, particularly if it is comprised of a large eccentric component. The etiology of EIMD is characterised by structural damage to the myofibrils during the initial exercise insult, followed by secondary inflammation from leukocyte infiltration into the damaged tissue2,3. The signs and symptoms associated with EIMD include muscle soreness, muscle swelling, reduced muscle function, and elevated concentration of myofibrillar proteins in the blood (e.g., creatine kinase (CK), lactate dehydrogenase (LDH))2,3,4,5. These can become evident within a few hours after exercise3 and can persist for several days2. Although EIMD is an important process for the adaptive response to exercise training4, reducing the symptoms associated with EIMD is beneficial for individuals aiming to maintain short-term exercise performance and training quality6. Post-exercise recovery strategies are commonly utilised to alleviate the symptoms of EIMD7, with one of the most prevalent techniques being sports compression garments (SCG)8.
The mechanisms by which SCG enhance recovery following exercise remain unclear but may be closely associated with venous and muscle blood flow alterations9. Compression-induced increases in venous blood flow9 is linked with accelerating the removal of myofibrillar proteins from the muscle10,11. For example, lower concentrations of plasma CK have been reported with compression garment use during the post-exercise recovery12,13. Compression may also reduce exercise-induced oedema by limiting the space available for swelling to form and by promoting lymphatic outflow, thus attenuating the inflammatory response and preventing further muscle damage8,14. In addition, compression may improve blood flow to the muscle following exercise and aid recovery by increasing nutrient delivery post-exercise. Nonetheless, a limitation with compression research to date is that most studies have focused on blood flow responses during exercise15,16,17,18,19, and within a short period (< 1 h) post-exercise17,18. Considering the inflammatory response begins in the early hours (1–4 h) during exercise recovery5, the use of compression garments beyond the first hour post-exercise may serve to reduce the symptoms of EIMD. The assessment of compression-induced changes in blood flow during this time will provide valuable insight into the proposed mechanism (i.e., increased blood flow) attributed to the effectiveness of SCG as a post-exercise recovery strategy.
A further limitation of the compression research to date is that the contribution of psychological factors to the ergogenic effects of compression on exercise performance and recovery outcomes is currently unknown. Considering many studies report favourable psychological outcomes (e.g., perceived muscle soreness, quality of recovery) with a concomitant lack of effect on physiological and/or performance measures20,21,22, it is possible that psychological factors may contribute, at least in part, to the benefits associated with compression23. In further support of this, participants’ positive perception and belief in SCG are suggested to improve exercise performance and recovery21,24,25. Research in other recovery techniques, including massage26 and cold water immersion27, suggest enhanced exercise recovery might occur via psychophysiological mechanisms (i.e., placebo effect)27; however, this has not been appropriately investigated with sports compression garments.
Due to the high level of pressure exerted on the limb with compression garments, a challenge with compression research is the inability to blind participants. Previous research has attempted to blind participants using similar-looking garments with a low level of pressure28,29. Alternatively, compression research has used 'sham' placebo interventions (e.g., drink30 and ultrasound20,31,32), in which participants were deceived into thinking these interventions are beneficial for exercise performance and/or recovery. However, a limitation of these studies is that belief in the placebo intervention (as compared with compression) was not assessed, meaning the presence of a placebo effect is still unable to be discounted20,30,31,32. In addition, no study has incorporated a placebo intervention promoting the beneficial effect on blood flow, despite the general consensus that the ergogenic effects associated with SCG are closely related to improvements in blood flow8,11,33. In order to determine the effectiveness of SCG on exercise recovery, it is crucial to assess this placebo effect.
This study aimed to investigate the influence of sports compression tights on post-exercise recovery and markers of blood flow compared to those of a placebo condition that participants were informed was as effective as compression for the recovery from exercise. We hypothesised that sports compression tights would enhance post-exercise blood flow and subsequently improve indices of muscle recovery, and that these benefits would not be related to the placebo effect—i.e., compression would elicit superior benefits when compared with the placebo and control conditions.
Results
A detailed summary of statistical data for all between-group effects for blood flow measures (markers of venous return and muscle blood flow), performance measures (CMJ and IMTP), and perceptual/swelling measures (MS, TQR and thigh girth) are presented in Tables 1, 4 and 6, respectively.
Garment details
The compression tights applied 14.0 ± 2.2 mmHg, 17.4 ± 2.6 mmHg, 20.1 ± 2.5 mmHg, 13.9 ± 2.5 mmHg, 13.9 ± 2.5 mmHg and 12.6 ± 2.4 mmHg of pressure to the lower-limb of COMP group at landmarks A (upper ankle) to F (upper thigh), respectively.
Belief questionnaires
The average belief in the interventions for all participants was 3.3 ± 0.7 for COMP, and 3.3 ± 0.9 for PLA. Participants assigned to COMP had a baseline belief in their intervention of 3.0 ± 0.6, which was significantly lower (P = 0.037) than participants in the PLA condition (3.9 ± 0.8). Belief in the COMP intervention (3.9 ± 0.8) was significantly higher (P = 0.040) than that of the PLA intervention (3.0 ± 0.5) at the end of the study.
Popliteal markers of venous return
There were no interaction effects for popliteal CSA (F22, 228 = 0.30, P = 1.000; Fig. 1a), Vmean (F22, 228 = 0.94, P = 0.543; Fig. 1b), Vpeak (F22, 228 = 0.78, P = 0.766; Fig. 1c), and venous blood flow (F22, 228 = 0.14, P = 1.000; Fig. 1d), or main effects of time for popliteal CSA (F11, 228 = 0.13, P = 1.000) and venous blood flow (F11, 228 = 1.54, P = 0.110). There were main effects of time for elevated popliteal Vmean (F11, 228 = 8.01, P < 0.001; Fig. 1b) and Vpeak (F11, 228 = 7.08, P < 0.001; Fig. 1c) post-exercise. Effect size analysis revealed medium to large effects for increased popliteal Vmean during the 4-h post-exercise recovery in the COMP group compared to the CON and PLA groups, respectively (Table 1).
Popliteal vein markers of venous return for CON, PLA and COMP conditions. Markers measured include (a) cross-sectional area (CSA), (b) mean flow velocity (Vmean), (c) peak flow velocity (Vpeak) and (d) venous blood flow. Time points are before exercise (PRE), immediately post-exercise (POST), 4 h recovery (30–240 min), and 24 and 48 h post-exercise. All data presented as mean ± SD. †Significant time effect as compared with all other time points. *Significant time effect as compared with 30 min post-exercise. aSmall effect as compared with CON. bMedium effect as compared with CON. eMedium effect as compared with PLA. fLarge effect as compared with PLA.
Femoral markers of venous return
There were no interaction effects for femoral CSA (F22, 228 = 0.09, P = 1.000; Fig. 2a), Vmean (F22, 228 = 0.27, P = 1.000; Fig. 2b), Vpeak (F22, 228 = 0.13, P = 1.000; Fig. 2c), and venous blood flow (F22, 228 = 0.39, P = 0.966; Fig. 2d), or main effects of time for femoral CSA (F11, 228 = 0.33, P = 0.984). There were main effects of time for elevated femoral Vmean (F11, 228 = 11.39, P < 0.001; Fig. 2b), Vpeak (F11, 228 = 7.05, P < 0.001; Fig. 2c,) and venous blood flow (F11, 228 = 6.91, P < 0.001; Fig. 2d) post-exercise. Effect size analysis revealed medium to large effects for increased Vmean and venous blood flow during the 4-h post-exercise recovery in the COMP group compared to the CON and PLA groups (Table 1).
Common femoral vein markers of venous return for CON, PLA and COMP conditions. Markers measured include (a) cross-sectional area (CSA), (b) mean flow velocity (Vmean), (c) peak flow velocity (Vpeak) and (d) venous blood flow. Time points are before exercise (PRE), immediately post-exercise (POST), 4 h recovery (30–240 min), and 24 and 48 h post-exercise. All data presented as mean ± SD. †Significant time effect as compared with all other time points. *Significant time effect as compared with 30 min post-exercise. #Significant time effect as compared with 60 min post-exercise. aSmall effect as compared with CON. bMedium effect as compared with CON. cLarge effect as compared with CON. eMedium effect as compared with PLA. fLarge effect as compared with PLA.
Muscle blood flow
There was no interaction effect for muscle blood flow (F22, 228 = 0.28, P = 1.000). There were main effects of time (F11, 228 = 11.94, P < 0.001) for muscle blood flow (Fig. 3). Specifically, muscle blood flow was elevated post-exercise compared to all other time points (P < 0.001) and elevated at 30 min as compared with baseline and 120 min, 150 min, 180 min, 210 min, 240 min, 24 h, and 48 h post-exercise (P < 0.001). Effect size analysis revealed small to large effects for increases in muscle blood flow during the 4-h post-exercise recovery in the COMP group compared to the CON and PLA groups (Table 1).
Muscle blood flow (millilitres of blood per min per 100 g of tissue) for CON, PLA and COMP conditions. Time points are before exercise (PRE), immediately post-exercise (POST), 4 h recovery (30–240 min), and 24 and 48 h post-exercise. All data presented as mean ± SD. †Significant time effect as compared with all other time points. *Significant time effect as compared with 30 min post-exercise. bMedium effect as compared with CON. cLarge effect as compared with CON. dSmall effect as compared with PLA. eMedium effect as compared with PLA.
Performance
There were no interaction (F8, 95 < 1.00, P > 0.05) or main effects of time (F4, 95 < 1.00, P > 0.05) for all CMJ (Table 2) and IMTP (Table 3) variables measured. For CMJ variables, there were small to large effects for improved performance post-exercise in the COMP group compared to the CON and PLA groups (Table 4). There were also medium effects for improved IMTP performance at 4 h post-exercise in the COMP group compared to the CON and PLA groups (Table 4).
Perceptual measures
There were no interaction effects for muscle soreness (F14, 152 = 0.75, P = 0.720) or TQR (F14, 152 = 0.53, P = 0.914). There were main effects of time for muscle soreness (F7, 152 = 15.70, P < 0.001) and TQR (F7, 152 = 14.37, p < 0.001). Specifically, muscle soreness was higher (P < 0.05), and TQR lower (P < 0.05), at every time point as compared with baseline (Table 5). For muscle soreness, there were medium to large effects for lower ratings post-exercise in the COMP group compared to CON and PLA groups (Table 6). There were also large effects for increased TQR at all time-points post-exercise in the COMP group compared to the CON and PLA groups (Table 6).
Blood analysis
There were no interaction effects for plasma LDH (F14, 152 = 0.19, P = 0.999) or CK (F14, 152 = 0.43, P = 0.965), and no main effect of time for CK (F7, 152 = 1.30, P = 0.254). There were main effects of time (F7, 152 = 2.85, P = 0.009) for LDH. Specifically, LDH was increased at 60 min, 120 min, 180 min and 240 min as compared with baseline, 24 h, and 48 h (P < 0.05; Table 5).
Thigh girth
There were no interaction effects (F14, 152 = 0.04, P = 1.000) or main effects of time (F7, 152 = 0.20, P = 0.985) for thigh girth circumference (Table 5). Effect size analysis revealed small effects for reduced thigh girth during the 4-h post-exercise recovery in the COMP group compared to the CON and PLA groups (Table 6).
Discussion
The aim of this study was to assess the effects of sports compression tights on post-exercise blood flow, and to determine if the placebo effect was responsible for any acute performance or psychological benefits during the recovery from an eccentric lower-body resistance exercise session. Despite no significant group differences, the main findings from ES analysis were that compression tights increased blood flow during the 4-h post-exercise recovery period, and that this increase coincided with enhanced recovery of exercise performance and improved subjective ratings of soreness and recovery. Additionally, our findings suggest that compression was more effective in improving markers of venous return (ES range: 0.49 to 2.21), muscle blood flow (ES range: 0.44 to 1.15), exercise performance (ES range: 0.27 to 0.99), and subjective ratings of soreness and recovery (ES range: 0.64 to 4.05) as compared with both the placebo and control conditions. These results highlight that the ergogenic benefits associated with compression garments are paralleled with physiological alterations (e.g., increased blood flow), and that compression-induced improvements in exercise recovery are not explained by the placebo effect.
A novel component of this study was to monitor the effects of wearing sports compression tights for 4 h post-exercise on markers of venous return and muscle blood flow. The external pressure applied by sports compression is suggested to assist muscle pump action and enhance venous return by decreasing vein diameter, increasing venous flow velocity, and reducing venous pooling in the lower limbs34,35,36. Although no changes in venous diameter were present in the current study, there was medium to large effects for compression tights to enhance venous flow velocity during the 4-h recovery period. The increase in venous flow velocity is likely due to the shunting of blood from superficial veins to the deep venous system (i.e., popliteal and common femoral veins)34,35,36. Previous findings have observed compression-induced increases in similar markers of venous return at rest37,38. However, this is the first study to investigate the effect of compression tights on these markers of venous return post-exercise, and our findings suggest that these effects are maintained for up to 4 h post-exercise.
Our findings of enhanced markers of venous return coincided with large effects of attenuated EIMD symptoms including improved CMJ and IMTP performance recovery, TQR ratings and reduced muscle soreness and thigh girth swelling, which supports compression use for 4 h post-exercise as being beneficial for recovery. Furthermore, compression-induced increases in venous return may also serve as a protective mechanism against post-exercise hypotension, which can persist for several hours if individuals remain in a supine position during recovery after intense exercise39. Post-exercise hypotension, observed in trained40 and untrained41 individuals, is characterised by a reduction in blood pressure, and occurs due to a combination of an inactive muscle pump42, pooling of blood in previously active muscles40, decreased end-diastolic filling43, and reduced stroke volume40. The increase in venous velocity observed in this study, combined with compression garments resulting in a pronounced increase in stroke volume33, highlights the beneficial effects compression may have in preventing post-exercise hypotension. Furthermore, the enhanced venous return observed with compression may also serve to increase muscle blood flow via increases in arteriovenous pressure gradient and/or endothelial shear stress37,44,45.
Similar to markers of venous return, the effects of compression to enhance muscle blood flow were evident throughout the 4-h recovery period. Previous research has highlighted compression to enhance muscle blood flow during exercise15,16, and immediately post-exercise18,19. From ES analysis, the current study is the first to show that increased muscle blood flow is still present for 4 h post-exercise while wearing sports compression tights. Although the underlying mechanisms associated with compression-induced increases in muscle blood flow are less clear, and may be attributed to enhanced venous return37,44,46, it is frequently suggested that a myogenic response may provide an explanation46,47. The garment’s compressive effect is proposed to increase extravascular tissue pressure, subsequently reducing arteriolar transmural pressure and resulting in a reflex increase in arteriole vessel size (i.e., vasodilation)46,48. In turn, this leads to a decrease in arterial flow resistance, thus improving muscle blood flow46,47. Considering muscle blood flow is positively correlated with glucose uptake49 and rates of MPS50, compression may enhance the delivery of nutrients to the muscle, consequently enhancing the recovery and restoration process10,15,31. The only study to investigate the effect of compression garments on post-exercise nutrient delivery reported no effect of compression on glucose uptake17, likely due to the high level of pressure (37 mmHg) exerted to the limb (i.e., mechanically reduced muscle blood flow). Other post-exercise strategies promoting increases in limb (femoral artery) blood flow, such as hot water immersion51, have been reported to improve glucose metabolism52 and key markers of MPS and muscle hypertrophy53. However, these findings are not consistent54,55, and future research is required to investigate the effect of SCG on post-exercise nutrient uptake and rates of MPS due to their correlations with muscle blood flow.
A crucial component of this study, and the first in compression research, was the effective deception of participants administered the placebo intervention. To achieve this, participants in the PLA were given information sheets that highlighted similar benefits and mechanisms that are associated with compression (i.e., improved blood flow and reduced muscle damage/inflammation). After reading the information sheets, the PLA group had a higher belief rating for this intervention (3.9 ± 0.8) than the COMP group (3.0 ± 0.6). Thus, in support of our hypothesis, this study highlights that compression's performance and perceptual benefits are likely due to compression-induced physiological alterations and not a placebo effect. This is also supported by the increase in blood flow (i.e., venous flow velocity and muscle blood flow) coinciding with medium to large effects of improved performance and perceptual indices of recovery in the COMP group. These benefits were not present in the PLA or CON groups.
In the present study, the use of sport compression tights during recovery did not affect CK or LDH at any time point, in line with previous studies32,56,57. However, apart from a small increase in LDH concentrations during the 4-h recovery period, there were no significant increases in the measured blood parameters following exercise in all three groups. It is not uncommon for blood markers to remain unchanged following resistance exercise58,59, with the reliability of blood markers as an indicator of muscle damage questioned60. Several factors may explain the high variability in blood markers of EIMD and lack of effect reported in this study. A potential explanation for the lack of change in blood markers is that participants muscle were already in an exercise-induced damaged state, as evident in the high LDH and CK values pre-exercise. Although participants were asked to refrain from strenuous exercise 48 h prior to testing Session 2, an earlier exercise session (i.e., 72 h prior to testing Session 2) could be responsible for the elevated LDH and CK values pre-exercise. These blood markers can reach a peak level from 24 to 96 h following an exercise bout and may remain elevated for up to 7 days post-exercise1. Also, considering the magnitude of change in blood biomarkers of EIMD are typically greater in untrained than trained individuals61, as well as trained individuals possessing a more efficient mechanism of myofibrillar protein clearance following exercise62, the exercise intervention in the current study may not have been sufficient to elicit a significant response58 in this resistance-trained cohort. In addition, the inclusion of both male and female participants may have masked any impact of the exercise protocol on blood biomarkers, as females are reported to exhibit lower muscle damage marker activity following damaging exercise63. Despite the limited changes in blood markers, the exercise protocol resulted in elevations in muscle soreness and fatigue, and performance decrements.
The applied pressure from compression showed small effects in reducing thigh girth circumference during the 4-h recovery period, and potentially limiting the space available for fluid accumulation and swelling to occur12. Attenuating muscle swelling post-exercise may reduce the secondary inflammatory response and soreness64. The changes in muscle soreness and TQR reported in this study support this mechanism and are consistent with previous research20,22,59. In comparison, this is the first study to highlight that these benefits of compression garments on perceptual measures are not due to a placebo effect or prior belief in the efficacy of compression. The reduction in muscle soreness with COMP in this study, further highlights that compression tights used post-exercise may help limit muscle damage and decrease inflammation, thus improving exercise performance recovery.
Compression tights used for 4 h after an eccentric lower-body resistance exercise session appear to enhance (small to large effects) the recovery of CMJ and IMTP performance. Damage to the contractile elements of muscle following resistance exercise leads to oedema formation, resulting in muscle soreness and decrements in exercise performance4,5. The improved recovery of CMJ variables with COMP, observed in this study and consistent with previous research59,64, has been attributed to the enhanced repair of the muscle contractile elements32. In support of this, improved ratings of muscle soreness and exercise performance recovery were evident with COMP. Regarding IMTP, COMP was beneficial at 4 h post-exercise only (Force at 100 ms, Force at 200 ms, RFD 0 to 100 ms, RFD 0 to 200 ms). The application of compression is suggested to positively influence muscle fibre recruitment65 and muscle contraction efficiency66 due to reduced muscle movements67. Although speculative, the enhanced motor unit activation, important for maximising force development (i.e., RFD measures)68, may explain the enhanced RFD at 4 h post-exercise for COMP in the current study. However, this proposed mechanism requires further investigation.
Sports compression tights used for 4 h post a lower-body resistance exercise session appear to increase blood flow and improve perceptual and performance indices of exercise recovery. Furthermore, the addition of a successful placebo by deception in this study highlights that the benefits observed in the current study with compression were likely not due to a placebo effect. Therefore, sports compression tights might be a beneficial strategy to improve recovery when used for 4 h following an eccentric lower-body resistance exercise session. This finding might be valuable to athletes/individuals that may be in a supine position following exercise, training or competition for several hours (e.g., watching a movie, travelling via bus or plane) or when there is a short recovery period between training sessions (e.g., morning and evening sessions) or competitive events.
Methods
Participants
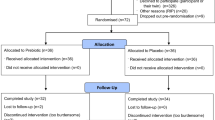
Thirteen males (mean ± SD: age, 24.9 ± 5.9 years; height, 179.5 ± 7.8 cm; body mass, 85.6 ± 10.7 kg) and nine females (mean ± SD: age, 27.3 ± 2.9 years; height, 167.4 ± 7.0 cm; body mass, 62.5 ± 11.8 kg) completed the study. The sample size was powered to detect a moderate difference (d = 0.54)69 in muscle blood flow with and without compression with an α value of 0.05 and 80% statistical power (G*Power Version 3.1.9.2; Universität Düsseldorf, Düsseldorf, Germany). Participants were required to be performing a minimum of two lower-body resistance exercise sessions a week, for a minimum of 6 months, to be eligible to participate. Written informed consent was obtained before participation. All participants were screened to ensure no contraindications were present for study participation, including cardiovascular risk factors (i.e., personal or family history of cardiovascular disease) and exercise capacity (e.g., musculoskeletal injury or joint pain). All procedures and methods were approved by the Victoria University Human Research Ethics Committee (HRE18-227) and performed in accordance with the relevant guidelines and regulations. The experimental approach was a between-subject, parallel-group design. Participants were assigned one of three recovery conditions in a randomised fashion and matched on belief in the interventions (as assessed during the familiarisation session) to control for the placebo effect. These conditions were sport compression tights [COMP, 4 males and 3 females (n = 7)], placebo by deception [PLA, 5 males and 3 females (n = 8)] or a passive control [CON, 4 males and 3 females (n = 7)]. A parallel-group design was chosen to avoid a repeated bout effect70 that would be present in a cross-over design. Participant characteristics are described in Table 7.
Experimental overview
Participants reported to the laboratory on four separate occasions (Fig. 4). Session one involved leg press one repetition max (1RM) testing, and familiarisation of the performance tests, blood flow measurements, and perceptual questionnaires. Anthropometric measurements of height, body mass, and quadriceps’ skinfold of the right leg were also taken. Following the reading of individual information sheets that highlighted the benefits of the COMP and PLA conditions for exercise recovery, participants completed belief questionnaires for both recovery interventions. Session one was conducted 10–14 days before session two.
Following a period of refraining from strenuous (< 48 h) or unaccustomed (< 7 days) exercise, participants reported to the laboratory in the morning for three experimental sessions. Participants were also asked to refrain from other exercise or recovery interventions (e.g., massage, water immersion) until the final testing session (session four) was complete. Session two started with pressure measurements of the sports compression tights (COMP only), followed by a 20-min supine rest period during which the sites for blood flow and thigh girth measurements were identified and prepared. Immediately after this, baseline measures of blood flow, perceptual questionnaires, thigh girth, and performance tests, as well as a venous blood sample, were collected. Participants then completed the leg press exercise protocol, and all baseline measures were repeated immediately after exercise. Following the post-exercise measures, participants performed their assigned recovery intervention (i.e., CON, PLA, or COMP) while supine for 4 h, and measurements were repeated at 30 (blood flow) or 60 (blood samples, perceptual questionnaires, thigh girth) min intervals. These measurements were collected in approximately 8 min at each time point. Performance tests were repeated at the end of 4 h supine rest only. Sessions three and four involved re-testing of baseline measures at 24 and 48 h post-exercise, respectively. At the end of the 48-h testing session, participants repeated the belief questionnaires in their completed recovery intervention. Testing sessions two, three, and four, started at the same time of day to minimise diurnal variations.
Recovery information and belief questionnaires
During the familiarisation session, participants were given an information sheet on the efficacy of two recovery interventions, SCG and l-Arginine supplementation. These information sheets illustrated peer-reviewed data on the effectiveness of either SCG71 or l-Arginine72 in enhancing recovery post-exercise, with a particular focus on their capacity to improve blood flow and reduce the symptoms of EIMD. The benefits of l-Arginine72 were used to create a placebo by deception, with participants assigned to the PLA group falsely led to believe they were receiving L-Arginine tablets (detailed below) during 4 h of recovery post-exercise. A ‘belief’ questionnaire27 was used to assess the participants anticipated effectiveness of SCG and L-Arginine for exercise recovery. Participants were instructed to mark an ‘X’ on a 5-point likert scale, with 0 representing 'not effective at all' and 5 representing ‘extremely effective’. From this questionnaire, participants were assigned their recovery intervention (CON, PLA, or COMP). A participant was randomly assigned to either COMP or CON if they answered a higher belief in COMP than L-Arginine (PLA) after reading the information sheets, and vice versa for a higher belief in l-Arginine (PLA) (i.e., participant was randomly assigned to either PLA or CON). If a participant rated both interventions equally, they were then randomly assigned to one of the three recovery interventions. A similar ‘belief’ questionnaire was used at the end of testing to measure the participants’ perceived effectiveness of their completed recovery intervention (i.e., COMP or PLA).
One repetition max testing
Prior to testing of 1RM, participants performed a standardised warm-up consisting of 5 min of cycling at 1 W per kg body mass, 10 repetitions of bodyweight squats, 10 repetitions on each leg of bodyweight walking lunges, high knee run over 20 m, heel kick run over 20 m, 3 submaximal counter-movement jumps (CMJ), and 1 maximal CMJ. Following the warm-up, participants performed two warm-up sets of the leg press protocol, with each set consisting of 10 reps with no weight and 5 reps at 50% of a participant’s self-estimated 1RM (Supplementary File). A participants 1RM was determined as previously described73. This 1RM was used to prescribe the workload for the lower-body resistance exercise session.
Dietary control
Participants completed a 24-h diet diary before session two, and were asked to replicate this diet for the 24 h before sessions three and four. Participants were asked to refrain from caffeine and alcohol consumption (< 12 h) before all testing sessions. A snack (Aussie Bodies, Protein FX Super Bar, New Zealand) containing 25.6 g protein and 18.4 g carbohydrate was provided to each participant at 2 h and 30 min into recovery. The same snack was provided after session 3 to help maintain nutrition adherence post-testing.
Venous return
Markers of venous return were measured at the popliteal and common femoral veins via Doppler ultrasound. The ultrasound examinations were performed using a CX50 Ultrasound System (Philips, USA), L12-3 MHz linear transducer and venous presets. Flow studies were performed by a single experience sonographer in a temperature-controlled (22 °C) environment. All measurements were obtained in a supine position and conducted as previously described9. Briefly, the common femoral veins were examined 2 cm above the saphenofemoral junction, with the compression garments turned down slightly to gain access. The popliteal veins were examined at the level of the knee crease. Prior to participants’ wearing compression tights, a small incision was made in the garment at the knee crease to create a window for the transducer to access the popliteal vein. Pilot data confirmed that the pressure of the compression tights was not altered by the small incision. The inner vessel transverse cross-sectional area (CSA; cm2), time-averaged mean blood flow velocity (Vmean; cm/s) and time-averaged peak blood flow velocity (Vpeak; cm/s) measurements for popliteal and common femoral veins were obtained for at each time point (Fig. 4).
Muscle blood flow
Muscle blood flow was assessed in the vastus lateralis muscle using near-infrared spectroscopy (NIRS) and multiple venous occlusion, as previously described9,15. Muscle blood flow was assessed immediately following markers of venous return measures at each time point (Fig. 4), with the average of the three occlusions (coefficient of variation (CV); 14.9 ± 10.9%) used for data analysis.
Perceptual measures
Participants assessed their level of perceived muscle soreness (MS) via self-manual palpation of the gluteal and thigh muscles followed by rating their level of soreness using a visual analogue scale (0 = no soreness, 10 = extremely high soreness)74. Each participant’s level of perceived recovery and fatigue was assessed using the Total Quality of Recovery (TQR) scale, which is a scale from 6 (no recovery) to 20 (maximal recovery)75.
Thigh girth
Girth measurements were taken at the midpoint of the right thigh to evaluate potential swelling of muscles resulting from exercise. In a standing position, the thigh midpoint was determined and marked as 4 cm distal to halfway between the greater trochanter and lateral epicondyle27. For the COMP group, the thigh midpoint was also determined with participants wearing the garments prior to 4 h supine rest, and the site marked with tape. Thigh circumference was measured around the thigh while the participant lay supine on the table, with the foot flat on the table surface and the knee at 90°27. Measurements were repeated twice at each time point (Fig. 4), with the average value used for analysis.
Blood analyses
A 22-gauge indwelling venous catheter (Optiva IV Catheter 22G X 1′′, Smiths Medical, USA) was inserted into the antecubital vein. The catheter was kept patent with 0.9% saline (~ 3 mL; Pfizer, Australia) after each blood draw in session two. Blood samples at 24 and 48 h post-exercise were drawn via venepuncture (Winged Infusion Set 21G X 0.75′′, Smiths Medical, USA). Blood samples (~ 10 mL each) were collected into an EDTA tube (K2EDTA, Smiths Medical, USA) at each time point (Fig. 4). A portion (100 µL) of blood from baseline, 24 and 48 h blood samples were analysed immediately for total haemoglobin concentration (KX-21 N; Sysmex, Japan). The remaining whole blood was centrifuged at 1000×g and 4 °C for 10 min. The acquired plasma was stored at − 80 °C for subsequent analysis. All samples were analysed using commercially available kits for CK (Creatine Kinase Activity Assay Kit, Abcam, Melbourne, Australia) and LDH (Lactate Dehydrogenase Assay Kit, Abcam, Melbourne, Australia). All samples were analysed in duplicate. The intra-assay coefficients of variation were 2.6% for CK and 3.8% for LDH.
Exercise performance testing
The CMJ and isometric mid-thigh pull (IMTP) performance tests were performed on a force platform (400S, Fitness Technology, Adelaide, Australia). Prior to all performance testing, participants performed the same standardised warm-up as completed before 1RM testing during session one. The CMJ test was chosen as it is a valid and reliable test for assessing the fatigue levels of lower body power76. Each participant completed five maximal jumps as previously described76, separated by 10 s of rest. The average of the five maximal jumps was used to derive jump height (m), relative peak force (N/kg), relative peak power (W/kg), and total duration (s).
The IMTP test is commonly used to assess fatigue and changes in maximum strength and rate of force development capabilities77. The IMTP protocol required participants to pull upward on an immovable bar for 3 s while standing on the force platform. The mid-thigh position and hip and knee angle positions were determined from previous protocols78. Two repetitions were performed, separated by a 2-min rest. A third repetition was performed if a > 250 N difference was observed between peak forces of the first two efforts. Force–time variables calculated from IMTP included absolute peak force (N), relative peak force (N/kg), force at 100 ms (N), force at 200 ms (N), rate of force development (RFD; ∆Force/∆Time) from 0 to 100 ms (N/s), and RFD from 0 to 200 ms (N/s). The average value reported across the two trials was used for analysis.
The raw force–time data for both CMJ and IMTP, sampled at 600 Hz, was collected using Ballistic Measurement System software (Fitness Technology, Adelaide, Australia). Raw force–time data was exported to and analysed in Microsoft Excel using spreadsheets specifically formulated for analysing CMJ79 and IMTP80.
Exercise intervention
An eccentric focused leg press exercise protocol, consisting of 8 sets of 6 repetitions at 85% of 1RM, was used to induce lower-limb muscle damage. Participants assumed a seated position on the leg press machine (Hammer Strength Linear, Schiller Park, IL, USA) and placed feet shoulder-width apart and flat on the platform. Prior to the beginning of 8 sets, participants performed a total of two warm-up sets, 10 repetitions with no weight on the leg press machine and 8 repetitions at 50% of 1RM. Participants lowered the resistance platform slowly for a duration of 4 s (time recorded by the investigator), and then pushed the platform back to its starting position as quickly as possible by extending the legs for each repetition. A 3-min rest period was provided between each set.
Recovery intervention
For the COMP group, lower-body sports compression tights (Refresh Recovery Tights, 2XU, Melbourne, Australia) were assigned to participants based on height and weight (manufacturer guidelines), with garments pressure measured at the beginning of session two via the Kikuhime device (mediGroup, Australia) at six different landmarks along the lower limb. The landmarks were 5 cm proximal to the distal border of the medial malleolus (A), 5 cm proximal to A (B), medial aspect of maximal calf girth (C), anterior aspect of the thigh 10 cm below landmark E (F), midpoint between the inguinal crease and the superior-posterior border of the patella (E) and 5 cm proximal to landmark E (F)9. The PLA group were given a sugar-free tablet (Hermesetas, Stevia Sweet 220 Tablets, Woolworths) from a de-identified container at 0, 1, 2, and 3 h following post-exercise performance testing. Participants assigned to CON did not participate in any recovery intervention but remained supine for 4 h.
Statistical analysis
Data are presented as mean ± SD and were analysed using IBM SPSS Statistics (Version 19, IBM Corp., Chicago, IL, USA). Normality was confirmed using the Shapiro–Wilk test. Belief effect (COMP vs. PLA) was assessed using independent sample t-tests. Comparisons between recovery interventions were analysed using a two-way linear mixed model (ANOVA) with repeated measures for time, where the between-subject factor is the recovery intervention (CON vs. COMP vs. PLA), and the within-subject factor is time. Significance was set at P < 0.05. Where significant time or interaction (time x condition) effects were found, a Fisher LSD post-hoc analysis was used. Due to the small sample sizes, effect sizes (ES) analysis were incorporated as these are independent of sample size81, and were used to compliment null hypothesis statistical testing. The measures analysed included markers of venous return, muscle blood flow, perceptual measures (PMS, TQR), thigh girth, blood plasma measures (CK, LDH) and performance tests (CMJ, IMTP). Cohen’s conventions for ES (with 95% confidence intervals) were used for interpretation and were defined as small (0.20 to 0.49), medium (0.50 to 0.79), and large (≥ 0.80)82.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author upon reasonable request.
References
Clarkson, P. M. & Hubal, M. J. Exercise-induced muscle damage in humans. Am. J. Phys. Med. Rehabil. 81, 52–69 (2002).
Harty, P. S., Cottet, M. L., Malloy, J. K. & Kerksick, C. M. Nutritional and supplementation strategies to prevent and attenuate exercise-induced muscle damage: A brief review. Sports Med. Open 5, 1–17 (2019).
Bongiovanni, T. et al. Nutritional interventions for reducing the signs and symptoms of exercise-induced muscle damage and accelerate recovery in athletes: Current knowledge, practical application and future perspectives. Eur. J. Appl. Physiol. 120, 1965–1996 (2020).
Owens, D. J., Twist, C., Cobley, J. N., Howatson, G. & Close, G. L. Exercise-induced muscle damage: What is it, what causes it and what are the nutritional solutions? Eur. J. Sport Sci. 19, 71–85 (2019).
Peake, J. M., Neubauer, O., Della Gatta, P. A. & Nosaka, K. Muscle damage and inflammation during recovery from exercise. J. Appl. Physiol. 122, 559–570 (2017).
Kellmann, M. et al. Recovery and performance in sport: Consensus statement. Int. J. Sports Physiol. Perform. 13, 240–245 (2018).
Minett, G. M. & Costello, J. T. Specificity and context in post-exercise recovery: It is not a one-size-fits-all approach. Front. Physiol. 6, 130 (2015).
Brown, F. et al. Compression garments and recovery from exercise: A meta-analysis. Sport. Med. 47, 1–23 (2017).
O’Riordan, S. F., McGregor, R., Halson, S. L., Bishop, D. J. & Broatch, J. R. Sports compression garments improve resting markers of venous return and muscle blood flow in male basketball players. J. Sport Heal. Sci. https://doi.org/10.1016/J.JSHS.2021.07.010 (2021).
Kraemer, W. J., French, D. N., Barry, M. & Spiering, A. Compression in the treatment of acute muscle injuries in sport. Int. Sport. J. 5, 200–208 (2004).
Hill, J., Howatson, G., van Someren, K., Leeder, J. & Pedlar, C. Compression garments and recovery from exercise-induced muscle damage: A meta-analysis. Br. J. Sports Med. 48, 1340–1346 (2014).
Davies, V., Thompson, K. G. & Cooper, S.-M. The effects of compression garments on recovery. J. Strength Cond. Res. 23, 1786–1794 (2009).
Kraemer, W. J. et al. Effects of a whole body compression garment on markers of recovery after a heavy resistance workout in men and women. J. Strength Cond. Res. 24, 804–814 (2010).
Marqués-Jiménez, D., Calleja-González, J., Arratibel, I., Delextrat, A. & Terrados, N. Are compression garments effective for the recovery of exercise-induced muscle damage? A systematic review with meta-analysis. Physiol. Behav. 153, 133–148 (2016).
Broatch, J. R., Bishop, D. J. & Halson, S. Lower-limb sports compression garments improve muscle blood flow during repeated-sprint cycling. Int. J. Sports Physiol. Perform. 13, 882–890 (2018).
Kerhervé, H. A. et al. Calf compression sleeves change biomechanics but not performance and physiological responses in trail running. Front. Physiol. 8, 247 (2017).
Sperlich, B., Born, D. P., Kaskinoro, K., Kalliokoski, K. K. & Laaksonen, M. S. Squeezing the muscle: Compression clothing and muscle metabolism during recovery from high intensity exercise. PLoS ONE 8, e60923 (2013).
Ménétrier, A. et al. Effects of three post-exercice recovery treatments on femoral artery blood flow kinetics. J. Sport. Med. Phys. Fit. 55, 258–266 (2015).
Vaile, J., Stefanovic, B. & Askew, C. D. Effect of lower limb compression on blood flow and performance in elite wheelchair rugby athletes. J. Spinal Cord Med. 39, 206–211 (2016).
Hill, J. A., Howatson, G., van Someren, K. A., Walshe, I. & Pedlar, C. R. Influence of compression garments on recovery after marathon running. J. Strength Cond. Res. 28, 2228–2235 (2014).
Duffield, R., Cannon, J. & King, M. The effects of compression garments on recovery of muscle performance following high-intensity sprint and plyometric exercise. J. Sci. Med. Sport 13, 136–140 (2010).
Pruscino, C. L., Halson, S. & Hargreaves, M. Effects of compression garments on recovery following intermittent exercise. Eur. J. Appl. Physiol. 113, 1585–1596 (2013).
Weakley, J. et al. Putting the squeeze on compression garments: Current evidence and recommendations for future research: A systematic scoping review. Sport. Med. https://doi.org/10.1007/s40279-021-01604-9 (2021).
Brophy-Williams, N. et al. Wearing compression socks during exercise aids subsequent performance. J. Sci. Med. Sport 22, 123–127 (2019).
Engel, F. A., Holmberg, H. C. & Sperlich, B. Is there evidence that runners can benefit from wearing compression clothing? Sport. Med. 46, 1939–1952 (2016).
Weerapong, P., Hume, P. A. & Kolt, G. S. The mechanisms of massage and effects on performance, muscle recovery and injury prevention. Sports Med. 35, 235–256 (2005).
Broatch, J. R., Petersen, A. & Bishop, D. J. Postexercise cold water immersion benefits are not greater than the placebo effect. Med. Sci. Sports Exerc. 46, 2139–2147 (2014).
Mizuno, S., Arai, M., Todoko, F., Yamada, E. & Goto, K. Wearing compression tights on the thigh during prolonged running attenuated exercise-induced increase in muscle damage marker in blood. Front. Physiol. 8, 834 (2017).
Mizuno, S., Arai, M., Todoko, F., Yamada, E. & Goto, K. Wearing lower-body compression garment with medium pressure impaired exercise-induced performance decrement during prolonged running. PLoS ONE 12, e0178620 (2017).
Upton, C. M., Brown, F. C. & Hill, J. A. The efficacy of compression garments on recovery from a simulated rugby protocol. J. Strength Cond. Res. 31, 2977–2982 (2017).
Brown, F. et al. Custom-fitted compression garments enhance recovery from muscle damage in rugby players. J. Strength Cond. Res. https://doi.org/10.1519/jsc.0000000000003408 (2020).
Hill, J. et al. The effects of compression garment pressure on recovery from strenuous exercise. Int. J. Sports Physiol. Perform. 12, 1–22 (2017).
Lee, D. C. W., Ali, A., Sheridan, S., Chan, D. K. C. & Wong, S. H. S. Wearing compression garment enhances central hemodynamics ? A systematic review and meta-analysis. J. Strength Cond. Res. 36, 2349 (2020).
Agu, O., Baker, D. & Seifalian, A. M. Effect of graduated compression stockings on limb oxygenation and venous function during exercise in patients with venous insufficiency. Vascular 12, 69–76 (2004).
Lawrence, D. & Kakkar, V. V. Graduated, static, external compression of the lower limb: A physiological assessment. Br. J. Surg. 67, 119–121 (1980).
Mosti, G. & Partsch, H. High compression pressure over the calf is more effective than graduated compression in enhancing venous pump function. Eur. J. Vasc. Endovasc. Surg. 44, 332–336 (2012).
Liu, R., Lao, T. T., Kwok, Y. L., Li, Y. & Ying, M. T. C. Effects of graduated compression stockings with different pressure profiles on lower-limb venous structures and haemodynamics. Adv. Ther. 25, 465–478 (2008).
Benko, T., Kalik, I. & Chetty, M. N. The physiological effect of graded compression stockings on blood flow in the lower limb: An assessment with colour Doppler ultrasound. Phlebology 14, 17–20 (1999).
MacDonald, J. R. Potential causes, mechanisms, and implications of post exercise hypotension. J. Hum. Hypertens. 16, 225–236 (2002).
Dujic, Z. et al. Postexercise hypotension in moderately trained athletes after maximal exercise. Med. Sci. Sport. Exerc. 38, 318–322 (2006).
Halliwill, J. R. Mechanisms and clinical implications of post-exercise hypotension in humans. Exerc. Sport Sci. Rev. 29, 65–70 (2001).
Halliwill, J. R., Buck, T. M., Lacewell, A. N. & Romero, S. A. Postexercise hypotension and sustained postexercise vasodilatation: What happens after we exercise? Exp. Physiol. 98, 7–18 (2013).
Jones, H., George, K., Edwards, B. & Atkinson, G. Is the magnitude of acute post-exercise hypotension mediated by exercise intensity or total work done? Eur. J. Appl. Physiol. 102, 33–40 (2007).
Charles, T. et al. Efficacy of micromobile foot compression device in increasing lower limb venous blood flow. Int. J. Vasc. Med. https://doi.org/10.1155/2013/948769 (2013).
Valic, Z., Buckwalter, J. B. & Clifford, P. S. Muscle blood flow response to contraction: Influence of venous pressure. J. Appl. Physiol. 98, 72–76 (2005).
Bochmann, R. P. et al. External compression increases forearm perfusion. J. Appl. Physiol. 99, 2337–2344 (2005).
Mayrovitz, H. N. & Larsen, P. B. Effects of compression bandaging on leg pulsatile blood flow. Clin. Physiol. 17, 105–117 (1997).
Broatch, J. R. et al. Reduced post-exercise muscle microvascular perfusion with compression is offset by increase muscle oxygen extraction: Assessment by contrast-enhanced ultrasound. Fed. Am. Soc. Exp. Biol. 35, e21499 (2021).
Ebeling, P. et al. Mechanism of enhanced insulin sensitivity in athletes increased blood flow, muscle glucose transport protein (GLUT-4) concentration, and glycogen synthase activity. J. Clin. Investig. 92, 1623–1631 (1993).
Tipton, K. D. et al. Timing of amino acid-carbohydrate ingestion alters anabolic response of muscle to resistance exercise. Am. J. Physiol. Metab. 281, 197–206 (2001).
Amin, S. B. et al. Whole body passive heating versus dynamic lower body exercise: A comparison of peripheral hemodynamic profiles. J. Appl. Physiol. 130, 160–171 (2021).
Hoekstra, S. P., Bishop, N. C., Faulkner, S. H., Bailey, S. J. & Leicht, C. A. Acute and chronic effects of hot water immersion on inflammation and metabolism in sedentary, overweight adults. J. Appl. Physiol. 125, 2008 (2008).
Yoshihara, T. et al. Heat stress activates the Akt/mTOR signalling pathway in rat skeletal muscle. Acta Physiol. 207, 416–426 (2013).
Fuchs, C. J. et al. Hot-water immersion does not increase postprandial muscle protein synthesis rates during recovery from resistance-type exercise in healthy, young males. J. Appl. Physiol. 128, 1012–1022 (2020).
Leicht, C. A., James, L. J., Briscoe, J. H. B. & Hoekstra, S. P. Hot water immersion acutely increases postprandial glucose concentrations. Physiol. Rep 7, 14223 (2019).
Martínez-Navarro, I. et al. Effects of wearing a full body compression garment during recovery from an ultra-trail race. Eur. J. Sport Sci. 21, 811–818 (2021).
Mizuno, S., Morii, I., Tsuchiya, Y. & Goto, K. Wearing compression garment after endurance exercise promotes recovery of exercise performance. Int. J. Sports Med. 37, 870–877 (2016).
Magal, M. et al. Relationship between serum creatine kinase activity following exercise-induced muscle damage and muscle fibre composition. J. Sports Sci. 28, 257–266 (2010).
Goto, K. & Morishima, T. Compression garment promotes muscular strength recovery after resistance exercise. Med. Sci. Sports Exerc. 46, 2265–2270 (2014).
Fridén, J. & Lieber, R. L. Serum creatine kinase level is a poor predictor of muscle function after injury. Scand. J. Med. Sci. Sport. 11, 126–127 (2001).
Newton, M., Morgan, G., Sacco, P. & Chapman, D. Comparison of responses to strenuous eccentric exercise of the elbow flexors between resistance-trained and untrained men. J. Strength Cond. Res. 22, 597–607 (2008).
Vincent, H. K. & Vincent, K. R. The effect of training status on the serum creatine kinase response, soreness and muscle function following resistance exercise. Int. J. Sport. Med 28, 431–437 (1997).
Amorim, M. Z., Machado, M. & Hackney, A. C. Sex differences in serum ck activity but not in glomerular filtration rate after resistance exercise: Is there a sex dependent renal adaptative response? J. Physiol. Sci. 64, 31–36 (2014).
Goto, K., Mizuno, S. & Mori, A. Efficacy of wearing compression garments during post-exercise period after two repeated bouts of strenuous exercise: A randomized crossover design in healthy, active males. Sport. Med. 3, 25 (2017).
Ehrström, S. et al. Acute and delayed neuromuscular alterations induced by downhill running in trained trail runners: Beneficial effects of high-pressure compression garments. Front. Physiol. 9, 1627 (2018).
Fu, W., Liu, Y., Zhang, S., Xiong, X. & Wei, S. Effects of local elastic compression on muscle strength, electromyographic, and mechanomyographic responses in the lower extremity. J. Electromyogr. Kinesiol. 22, 44–50 (2012).
Broatch, J. R. et al. Compression garments reduce muscle movement and activation during submaximal running. Med. Sci. Sports Exerc. 52, 685–695 (2020).
Vecchio, A. D. et al. You are as fast as your motor neurons: Speed of recruitment and maximal discharge of motor neurons determine the maximal rate of force development in humans. J. Physiol. 597, 2445–2456 (2019).
Bringard, A., Denis, R., Belluye, N. & Perrey, S. Effects of compression tights on calf muscle oxygenation and venous pooling during quiet resting in supine and standing positions. J. Sports Med. Phys. Fitness 46, 548 (2006).
Nosaka, K., Sakamoto, K. E. I., Newton, M. & Sacco, P. How long does the protective effect on eccentric exercise-induced muscle damage last? Med. Sci. Sport. Exerc. 33, 1490–1495 (2001).
Jakeman, J. R., Byrne, C. & Eston, R. G. Lower limb compression garment improves recovery from exercise-induced muscle damage in young, active females. Eur. J. Appl. Physiol. 109, 1137–1144 (2010).
Campbell, B. et al. Pharmacokinetics, safety, and effects on exercise performance of L-arginine a-ketoglutarate in trained adult men. Nutrition 22, 872–881 (2006).
Baechle, T. R. & Earle, R. W. Essentials of Strength Training and Conditioning (Human Kinetics, 2008).
Vaile, J., Halson, S., Gill, N. & Dawson, B. Effect of hydrotherapy on the signs and symptoms of delayed onset muscle soreness. Eur. J. Appl. Physiol. 102, 447–455 (2008).
Kenttä, G. & Hassmén, P. Overtraining and recovery: A conceptual model. Sport Med. 26, 1–6 (1998).
Slinde, F., Suber, C., Suber, L., Elam Edwé, N. C. & Svantesson, U. Test-retest reliability of three different countermovement jumping tests. J. Strength Cond. Res. 22, 640–644 (2008).
Brady, C. J., Harrison, A. J. & Comyns, T. M. A review of the reliability of biomechanical variables produced during the isometric mid-thigh pull and isometric squat and the reporting of normative data. Sport. Biomech. 19, 1–25 (2020).
Comfort, P., Jones, P. A., McMahon, J. J. & Newton, R. Effect of knee and trunk angle on kinetic variables during the isometric midthigh pull: Test-retest reliability. Int. J. Sports Physiol. Perform. 10, 58–63 (2015).
Chavda, S. et al. Force-time characteristics of the countermovement jump: Analyzing the curve in excel. Strength Cond. J. 40, 67–77 (2018).
Chavda, S. et al. A practical guide to analyzing the force-time curve of isometric tasks in excel. Strength Cond. J. 42, 1 (2019).
Sullivan, G. M. & Feinn, R. Using effect size—Or why the p value is not enough. J. Grad. Med. Educ. 4, 279–282 (2012).
Cohen, J. Statistical Power Analysis for the Behavioral Sciences (L. Erlbaum Associates, 1988).
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. S.F.O., J.R.B., S.L.H., and D.J.B. received a research grant from compression manufacturer 2XU Pty Ltd (Melbourne, Australia), which also supported S.F.O. and J.R.B. 2XU had no input into the study design, the collection and analysis of data, the writing of the manuscript, or the decision to submit the article for publication.
Author information
Authors and Affiliations
Contributions
The study design was performed by S.F.O., J.R.B., D.J.B. and S.L.H. Data collection was performed by S.F.O. Analysis and interpretation of data were performed by S.F.O., J.R.B., D.J.B. and S.L.H. The manuscript was written by S.F.O. and J.R.B., while D.J.B. and S.L.H. critically revised the manuscript. All authors approved the final version of the manuscript. All data collection and data analysis for the study was performed at the exercise physiology and biochemistry laboratories at IHES.
Corresponding author
Ethics declarations
Competing interests
This study was supported using research funds from the compression garment manufacturer 2XU (Australia). The results of this study do not constitute an endorsement of the product by the authors.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
O’Riordan, S.F., Bishop, D.J., Halson, S.L. et al. Compression-induced improvements in post-exercise recovery are associated with enhanced blood flow, and are not due to the placebo effect. Sci Rep 12, 16762 (2022). https://doi.org/10.1038/s41598-022-21029-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-21029-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.