Abstract
A novel type of agarose gel microcapsule (AGM), consisting of an alginate picolitre sol core and an agarose gel shell, was developed to obtain high-quality, single-cell, amplified genomic DNA of bacteria. The AGM is easy to prepare in a stable emulsion with oil of water-equivalent density, which prevents AGM aggregation, with only standard laboratory equipment. Single cells from a pure culture of Escherichia coli, a mock community comprising 15 strains of human gut bacteria, and a termite gut bacterial community were encapsulated within AGMs, and their genomic DNA samples were prepared with massively parallel amplifications in a tube. The genome sequencing did not need second-round amplification and showed an average genome completeness that was much higher than that obtained using a conventional amplification method on the microlitre scale, regardless of the genomic guanine–cytosine content. Our novel method using AGM will allow many researchers to perform single-cell genomics easily and effectively, and can accelerate genomic analysis of yet-uncultured microorganisms.
Similar content being viewed by others
Introduction
Microorganisms are ubiquitously distributed in diverse environments. They are often associated with other organisms and play important roles in ecosystems. However, the majority of microbial species are difficult to culture with conventional methods and are called yet-uncultured microorganisms1. In the past two decades, they have been extensively studied using culture-independent methods such as amplicon sequencing analysis of small subunit ribosomal RNA (SSU rRNA) genes and shotgun sequencing analysis of metagenomes. Metagenomics is a powerful tool for investigating the ecological and physiological functions of microbiota based on their encoded genetic repertoires. The recent development of the computational binning of metagenomic fragments into respective microbial taxonomic assemblies has reinforced the utility of metagenomics2,3. However, computational binning based on the sequence composition, homology to database sequences, and sequence read coverage of each fragment can often fail to discriminate genomes of closely related (sub)species and to correctly sort genomic regions exhibiting distinct features from others, such as rRNA genes, prophages and plasmids4,5. Furthermore, a massive sequencing effort is required to obtain the genome sequences of minor species in the microbial community3.
Single-cell genomics, in which whole genomic DNA is amplified from physically isolated single cells6, has been used as an alternative or complementary method to reveal the metabolic capacity of yet-uncultured microorganisms1,7. Single-cell isolation can be achieved in many cases by using technologies such as fluorescence-activated cell sorting (FACS)1, micromanipulators8, microfluidic devices9,10 and encapsulation in water-in-oil droplets11. Although various methods for whole genome amplification have been developed12, multiple displacement amplification (MDA) using phi29 DNA polymerase with random primers is most widely used due to its relative simplicity, reliability and applicability6,13. However, MDA inherently demonstrates extreme amplification bias among genome regions1, which has been the major technical limitation in single-cell genomics. Additionally, the high guanine–cytosine (GC) content of genomic DNA tends to increase the amplification bias1,14. Amplification bias can be suppressed using small reaction vessels; for example, microchannel chambers (60 nL)10, nanolitre microwells (12 nL)15, virtual microfluidics (MDA in a gel sheet, 60 nL)16, digital droplets generated by using a microchannel (9.5–240 pL)17,18,19 and gel beads20. However, these microfabrication techniques are not available for many microbiologists, and the amount of the amplification product is often too little for direct genome sequencing; therefore, a second round of MDA is often required, which ultimately enhances the amplification bias21.
A hollow-core hydrogel microcapsule22, which consists of a hydrogel shell and a sol core, has potential as an efficient vessel for single-cell MDA. Many hollow-core hydrogel microcapsules have been reported to be useful for cell cultivation and cell transplantation23,24,25. However, the MDA product from single-cell genomic DNA amplified in a picolitre-scale hollow core in a microcapsule has not been fully evaluated for single-cell genomics.
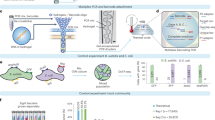
Here, we introduce a novel single-cell genomics method using an AGM consisting of an agarose gel shell and an alginate sol hollow core, which enables single-cell isolation and picolitre-scale MDA (Fig. 1a). The AGM fabrication was optimised here for easier preparation using inexpensive equipment and reagents, such as a vortex mixer, centrifuges, and commercially available reagents (Fig. 1b), although the size uniformity of AGM is sacrificed compared to the methods using microfabrication techniques26,27. Thus, this AGM can be easily prepared and is scalable for many microbiologists. By using AGM, a single bacterial cell can be encapsulated in its picolitre-scale sol core and then subjected to MDA just by exchanging solutions (designated here ‘MDA-in-AGM’). We demonstrate, using E. coli, a mock mixture of human gut bacteria, and the termite gut microbiota, that MDA-in-AGM enables massively parallel single-cell genomics with much improved genome completeness compared to a conventional method using FACS and microlitre-scale MDA (FACS-MDA).
Schematic view of an agarose gel microcapsule (AGM) and its preparation process. (a) Schematic diagram of the AGM developed in this study for single-cell isolation and multiple displacement amplification (MDA). The AGM consists of an agarose hydrogel shell and an alginate sol core. The alginate core provides a picolitre-scale reaction chamber for MDA, while the agarose shell acts both as a permeable envelope for enzymes and small molecules and as a protective wall against larger particles and molecules such as bacterial cells and genomic/amplified DNA. (b) Preparation scheme for AGMs containing Escherichia coli cell(s). The alginate cores and agarose shells are gelated with Ca2+ from CaCO3 and cooled in an emulsion, respectively. Polyglyceryl-6 octacaprylate (PGO) can suppress the aggregation of AGMs during agarose gelation by cooling. Finally, the alginate gel cores are solated with EDTA by chelating Ca2+. ISA isostearyl alcohol.
Results
Preparation of AGMs containing single bacterial cells
For AGM cores, alginate gel beads containing bacterial cells were prepared using the emulsification/internal gelation method27 (Fig. 1b). Briefly, a mixture of alginate solution, bacterial cells and CaCO3 nanoparticles was emulsified with isostearyl alcohol (ISA). The resulting microdroplets were gelated with calcium ions that were released from CaCO3 nanoparticles by the addition of acetic acid to the water-in-oil emulsion. The concentration of bacterial cells was optimised using serial dilutions so that one alginate microdroplet contained one or no bacterial cells. The resulting alginate gel cores were washed sequentially with diethyl ether, 1-butanol and Tris–HCl (pH 7.5) buffer.
Agarose solution (SeaPlaque, Lonza; final concentration 2%) was then added to the alginate gel cores suspended in Tris–HCl (pH 7.5) at 35 °C, and the mixture was emulsified with 0.25% (v/v) Span 85 in polyglyceryl-6 octacaprylate (PGO) at 35 °C (Fig. 1b). PGO was used here because its density is water-equivalent (0.997 g/mL), and hence PGO stably emulsified water (Supplementary Fig. S1a), prevented agarose gel aggregation (S1b), and increased the diameters (S1c and S1d) and yields (S1e) of agarose gel droplets. The agarose droplets were gradually gelated in the stable emulsion by cooling at 4 °C. After PGO was removed by mixing with diethyl ether and further with 1-butanol, the alginate gel cores were solated with a one-tenth volume of 0.5 M ethylenediaminetetraacetic acid (EDTA). The solation of alginate cores was verified by labelling the alginate cores with rhodamine 123 and observing the diffusion of the dye into buffer when melting the agarose gel shells under the presence of EDTA whereas the alginate cores remained in a gel form with 50 mM CaCl2 (Supplementary Fig. S2). The alginate sol core provides an appropriate space for the MDA reaction, which was shown by the much greater yields of amplified DNA than in reactions with an alginate gel core or agarose gel bead as described below. The AGMs were washed with Tris–EDTA (TE) buffer (pH 7.4) to remove excess EDTA, suspended in a one-half volume of TE buffer, and stored at 4 °C. The entire process of the AGM preparation can be done in 7 h.
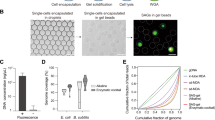
Escherichia coli DH5α was used as a model microorganism. E. coli cells were added at a concentration of 3 × 106 cells mL−1 to alginate-CaCO3 solution and were encapsulated in 5.64 ± 1.72 × 105 AGMs (mean ± SD) in three independent experiments, which corresponded to 12.5% ± 5.4% of the total AGMs. Of the AGMs containing E. coli cells, approximately 94% harboured a single cell in the core (Supplementary Table S1). In the three independent experiments, the diameters of the prepared AGMs were 39.6 ± 22.2 µm, 51.4 ± 13.3 µm and 49.3 ± 19.6 µm (Supplementary Fig. S3a). AGM diameters and core volumes showed normal distributions and log-normal distributions, respectively (Supplementary Figs. S3b, S4). The average median AGM core volume in the three experiments was 15.1 ± 6.3 pL (n = 3).
MDA of single-cell genomes within AGMs
AGMs (in 50 µL suspension) were washed with sterile water and collected by centrifugation. The lysis of bacterial cells and denaturation of double-stranded DNA within the AGMs were carried out at room temperature for 30 min with 50 µL of an alkaline solution, which was Buffer D2 of the REPLI-g UltraFast Mini Kit (Qiagen). Denaturation was stopped by neutralisation with the addition of an equal volume of Stop solution from the kit, and the supernatant was then removed by centrifugation. Reaction buffer (93.5 µL) containing 11 µL of REPLI-g UltraFast DNA Polymerase from the kit was added, and the resulting mixture was incubated at 30 °C for 3 h. The reaction was stopped with one-tenth volume of 0.5 M EDTA, and the AGMs were washed three times with 200 µL of TE buffer. All the reagents used in MDA and the AGM preparation were irradiated with ultraviolet light to degrade any contaminating DNA prior to use.
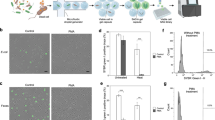
Among the 4.6 ± 2.9 × 105 AGMs prepared with E. coli cells, 8.9% ± 0.8% exhibited DNA amplification, which corresponded to 77.0% ± 27.2% of the AGMs containing E. coli cells (n = 3) (Supplementary Table S1). As shown in Fig. 2, amplified DNA filled the alginate sol core, whereas AGMs with alginate gel cores that were prepared without the solation step with EDTA or agarose gel beads containing no alginate core exhibited only limited or no DNA amplification by MDA.
Yield enhancement of multiple displacement amplification (MDA) by alginate core solation. The effect of alginate core solation on the productivity of MDA within agarose gel microcapsules (AGMs) was evaluated. The genomic DNA of Escherichia coli encapsulated within AGMs was amplified by MDA with or without alginate solation using EDTA. The amplified DNA was detected with SYBR Green I. A phase contrast image (red) and epifluorescent image (green, SYBR Green I) are overlaid. MDA in agarose gel beads (without an alginate core), which was prepared using gelation of agarose droplets containing E. coli in oil emulsion, was also conducted as a control experiment. Arrows indicate E. coli cells stained with SYBR Green I. Bar = 100 µm.
Quality of single-cell genome sequences compared between MDA-in-AGM and a conventional method using FACS-MDA
The quality of single-cell amplified genomes (SAGs) using MDA-in-AGM was evaluated and compared with that obtained by FACS-MDA. AGMs containing amplified DNA that was stained with SYBR Green I were individually transferred to 0.2 mL PCR tubes with 29 µL of sterile water under an epifluorescence microscope equipped with a TransferMan NK2 micromanipulator (Eppendorf), which required approximately 5 min per AGM. The micromanipulator was used in this study due to its low initial cost. Several tens of the picked AGMs were sequenced as follows. Amplified DNA in the AGM was released into water by heating the tubes at 60 °C for 5 min and directly used for the preparation of sequencing libraries using the QIAseq FX DNA Library Kit (Qiagen). No second round of MDA was necessary to obtain libraries suitable for genome sequencing. Paired-end sequence reads were generated on an Illumina MiSeq platform with the Reagent Kit V3 (600 cycles), and a fixed number of read pairs were randomly chosen and assembled de novo into contigs using SPAdes 3.13.028 after standard quality filtering. Contigs > 1 kb were used for the subsequent analyses. Several factors describing the genome sequence quality, including completeness and contamination rate, were evaluated using CheckM29 and QUAST30 and compared between the SAGs obtained using MDA-in-AGM and FACS-MDA.
The genome completeness and the total sequence length of E. coli SAGs obtained by MDA-in-AGM rapidly increased during the sequencing effort, and the average genome completeness reached 93.3% ± 4.1% (n = 10) for assemblies using 60-fold coverage, while it was 33.7% ± 17.3% (n = 10) in FACS-MDA (Student’s t test, P < 0.01) (Fig. 3a, Supplementary Table S2). The covered genome calculated by mapping reads to the reference E. coli DH5α genome sequence (BOCF01000000) was 97.4 ± 2.1% in MDA-in-AGM (n = 10), which was much higher than 47.8% ± 13.5% in FACS-MDA (n = 10) for 60-fold coverage reads (P < 0.01) (Supplementary Table S2). The steep decrease in the number of contigs, i.e. smooth assembly of contigs, in MDA-in-AGM along the sequencing effort’s depth was probably due to the lower amplification bias (Fig. 3a). In addition, the amplification bias among genome regions, which is inherent to MDA, was much improved in MDA-in-AGM compared to FACS-MDA (Fig. 3b). The amplification bias of SAGs was further evaluated using Gini coefficients and Lorenz curves, which indicate the population disparity as indices and plots. The Gini coefficients of the SAGs obtained by MDA-in-AGM were much lower than those of FACS-MDA (Welch’s t test, P < 0.01), indicating higher uniformity of the MDA-in-AGMs than the FACS-MDAs (Supplementary Fig. S5a). In the Lorenz curve, the SAGs obtained by MDA-in-AGM showed higher amplification uniformity than those by FACS-MDA (Supplementary Fig. S5b). Other quality metrics of SAGs using all sequence reads are shown in Supplementary Table S3.
Comparison of genome completeness and amplification bias of single-cell genomes obtained by MDA-in-AGM and FACS-MDA. (a) Completeness and number of contigs of SAGs during the sequencing process. Box-and-violin plots (blue: MDA-in-AGM; red: FACS-MDA) are shown for Escherichia coli and two bacterial species as examples from the mock community of human gut bacteria (Supplementary Tables S2, S5 and S6). Datapoints (purple circles) and their arithmetic means (rhombuses) are shown on the plots. In the E. coli graphs, the results for DNA prepared from cultured E. coli cells without MDA are also shown (green circles). In the left panels, significant differences are indicated with asterisks (P < 0.05) or double asterisks (P < 0.01). (b) The number of sequence reads mapped against genome regions is shown as an indicator of amplification bias caused during MDA. The numbers of reads corresponding to different coverages used for de novo assembly (shown as ‘Cov.’) and genome completeness (%) (‘Compl.’) estimated using CheckM are shown.
Single-cell genome analyses of a mock community and a natural sample
To evaluate the feasibility of MDA-in-AGM in a more realistic sample, we constructed a mock community comprising 15 cultured strains of human gut bacteria. The taxonomy and compositions of each bacterial strain estimated using 16S rRNA amplicon analysis or metagenome analysis are shown in Supplementary Table S4. SAGs from the mock community were obtained using either MDA-in-AGM or FACS-MDA in the same way as above. Taxonomic compositions of SAGs were similar between MDA-in-AGM and FACS-MDA, both of which successfully recovered SAGs of most strains accounting for > 5% of the mock community with the exception of Faecalibacterium prausnitzii (Supplementary Table S4). For example, SAGs of Bacteroides thetaiotaomicron and Parabacteroides distasonis exhibited clear differences between MDA-in-AGM and FACS-MDA in genome completeness, number of contigs, and amplification bias, as seen in the E. coli SAGs (Fig. 3, Supplementary Fig. S5). Since many SAGs obtained from environmental samples were difficult to compare at the same coverage due to lack of their reference genomes, the mock SAGs were assembled using 0.3 M read pairs in the following experiments. The overall genome completeness of SAGs with < 5% contamination was significantly higher in MDA-in-AGM (68.0% ± 23.3%, n = 39) than in FACS-MDA (42.4% ± 20.5%, n = 36) (P < 0.01) (Supplementary Tables S5, S6). The SAGs of most other strains also showed similar results to the E. coli SAGs (Supplementary Fig. S6).
Most sequence reads were mapped to their respective reference genomes: the median mapping rates were 95.1% and 97.6% for SAGs from MDA-in-AGM and FACS-MDA, respectively (Supplementary Table S7). Although the rates of cross-contamination were slightly higher in MDA-in-AGM than in FACS-MDA, both were lower than 5% (Supplementary Table S7). In Ba. thetaiotaomicron SAGs, sequence reads from a Ba. thetaiotaomicron plasmid were also obtained (Supplementary Table S7). Our results indicated that MDA-in-AGM is applicable to various bacterial species and improves genome completeness in both gram-positive and gram-negative bacteria, regardless of their GC content (Fig. 4). For Fa. prausnitzii, it is possible that the cell lysis process using KOH was not effective, and hence no SAGs were obtained in either MDA-in-AGM or FACS-MDA.
Relationships between genome completeness and GC contents of single-cell genomes in the mock community of human gut bacteria. Single-cell amplified genomes (SAGs) from the mock community comprising 15 strains of human gut bacteria are plotted with their GC contents against their genome completeness. The SAGs were assembled from 0.3 M randomly chosen read pairs, while MS485-1-51 of MDA-in-AGM (0.26 M read pairs) and CS1–94 of FACS-MDA (0.29 M read pairs) used all read pairs since they were fewer than 0.3 M. The bacterial species comprising the mock community and detailed results are shown in Supplementary Tables S4–S6. Blue and red indicate MDA-in-AGM and FACS-MDA, respectively. Squares, circles and rhombuses indicate gram-positive, gram-negative and gram-variable bacteria, respectively.
SAGs from MDA-in-AGM with > 5% contamination, which would be caused either by contaminating extracellular DNA or by multiple cells being encapsulated in a single AGM, were binned into each bacterial species using metaWRAP31, although discrimination between contaminating DNA and DNA from captured multiple cells was difficult. As a result, 30 additional SAGs with < 5% contamination were obtained, and their genome completeness and contamination rates were 73.5% (median) and 0.9% (median), respectively (Supplementary Table S8).
DNA rearrangement during MDA, i.e. the formation of chimeras, can complicate the de novo assembly of single-cell genomes32. In E. coli SAGs, the rates of chimeric reads were slightly higher in MDA-in-AGMs (6.28% ± 1.09%) than in FACS-MDAs (4.49% ± 1.32%) (Welch’s t test, P < 0.01; Supplementary Table S3). In SAGs from the mock community, the rates of chimeric reads were higher in MDA-in-AGM (8.22% ± 2.28%) than in FACS-MDA (3.55% ± 1.07%) (Welch’s t test, P < 0.01; Supplementary Tables S5, S6). These rates of chimeric reads in MDA-in-AGM were similar to or slightly higher than the rate of 6.19% in a previous study using a conventional MDA method for sequencing human haploid genomes33.
Finally, we applied MDA-in-AGM to a natural environmental sample. We used the microbiota in the gut of the termite Reticulitermes speratus, which comprises several hundred bacterial species from diverse phyla34. The termite gut microbiota has attracted researchers especially for the complex symbiotic system enabling the highly efficient plant biomass degradation35,36,37. The termite gut microbiota is also superior for a benchmarking study using a natural environmental sample because its bacterial community structure is highly consistent within a termite species and has been well characterised34,36,38,39 and, in addition, the complete genome sequences of dominant bacterial species in R. speratus guts have been reported40,41,42.
The entire guts of five worker termites were removed, and the gut contents were suspended. Then, bacterial cells were collected at 18,000×g for 5 min by centrifugation after digesting the extracellular DNA with DNase I, and the cells were washed with sterile water as described previously43. The subsequent procedures were the same as those described above. To evaluate MDA-in-AGM using environmental specimens from the termite guts, we analysed 48 SAGs obtained by MDA-in-AGM. The SAGs showed a high genome completeness (78.6% ± 18.2%) and a low contamination rate (1.0% ± 1.0%) (Table 1). The SAGs were taxonomically classified using the Genome Taxonomy Database Tool Kit (GTDB-Tk)44 and were affiliated with 13 bacterial classes, including known dominant groups in the termite gut, Spirochaetia, Bacteroidia, Alphaproteobacteria, Betaproteobacteria, Deltaproteobacteria, Epsilonproteobacteria and Clostridia34,38. In contrast, the genome completeness of SAGs obtained by FACS-MDA was only 37.7% ± 19.0% (n = 161). Although a different sequencing method, i.e. a combination of the Nextera XT DNA Library Prep Kit (Illumina) and the Illumina HiSeq 2500 platform (500 cycles), was used for the latter case, the number of reads (bases) used for assembly was much larger on average (551 Mb ± 64 Mb, n = 161) than in the analysis of SAGs obtained by MDA-in-AGM (256 Mb ± 49 Mb, n = 48) (Supplementary Table S9).
In addition to the 48 SAGs, we analysed 18 SAGs of the class Endomicrobia with small genome sizes (~ 1 Mb)40 obtained by MDA-in-AGM, and 91.2% ± 9.8% genome completeness was obtained. Furthermore, among them, eight SAGs of phylotype Rs-D17 were compared to the complete genome sequences obtained previously40,41. The genome coverages calculated by mapping reads onto the two genomovars of Rs-D17 (Ri200840 and Ti200541) reached 95.7% ± 1.3% and 96.7% ± 0.9%, respectively (Supplementary Table S10). The composition of the termite gut community was estimated from SAGs obtained by MDA-in-AGM or 16S rRNA amplicon analysis (Supplementary Table S11). The taxonomic composition of the SAGs showed a high positive correlation (R = 0.834, P < 0.01; Pearson’s correlation coefficient) with that shown by the 16S rRNA amplicon analysis, whereas the frequencies of gram-positive bacteria were lower in SAGs.
Discussion
MDA-in-AGM, which consisted of massively parallel MDAs in the picolitre-scale AGM cores, drastically improved the genome completeness of SAGs of E. coli, a mock community of human gut bacteria, and bacteria in the termite gut, compared to conventional microlitre scale FACS-MDA. Among them, the high-coverage SAGs obtained from the termite gut bacteria using MDA-in-AGM (Table 1, Supplementary Table S9) will prompt the understanding of the complex gut symbiotic system and the mechanisms of the highly efficient lignocellulose-decomposing system34,36,40,41,43. Thus, this method can reveal the metabolic capacity of yet-uncultured prokaryotes in environments such as animal intestinal tracts, soil and the hydrosphere.
The improvement of genome completeness in single-cell genomics by reducing the reaction volume of MDA using microfabrication techniques has previously been reported10,15,17,20,45, and the comparisons of MDA-in-AGM with those other MDA methods are shown in Supplementary Table S12. Although high genome completeness is also achieved using other miniaturized MDA methods, MDA-in-AGM does not require specialised equipment and provides scalable sample preparation. In addition, the use of alginate sol core successfully enhanced the yield of MDA, leading to the elimination of a second-round MDA step20,45 and hence leading to less amplification bias and simpler workflow. This high yield is probably attributable to the enhanced diffusivity of amplified DNA in the sol core46. Our MDA-in-AGM is applicable to both gram-positive and gram-negative bacteria and also to genomes with high GC content (Fig. 4). Additional advantages of the AGM are its physical stability and permeability to small molecules, which make buffer exchange and physical handling mush easier.
The formation of chimeric sequences during MDA can affect the efficiency and preciseness of the de novo assembly of SAGs47. In the mock community, the rates of chimeric reads were comparable with those in droplet MDA19 and slightly higher than in conventional MDA methods (Supplementary Table S12)33. Since the formation of chimeras is likely attributable to the branch migration of amplified DNA32, a higher MDA reaction temperature by using thermostable phi29 DNA polymerase14 may decrease erroneous hybridisation between replicons.
The uniformity of the alginate core sizes, which correspond to MDA reaction volumes (Supplementary Table S1), may have affected the yields of DNA-amplified AGM, the uniformity of the quantities of MDA products, and the quality of the AGM libraires. Although alginate cores with < 100-µm diameter were used for AGM preparations, a narrower size selection of the alginate cores, for example, 20–40 µm in diameter, would improve the uniformity of the AGM cores. Extrusion48 and microchannels17,18,19 would also improve the AGM core uniformity without size selection. However, introduction of these processes will complicate the workflow and increase the cost.
The manual picking procedure using a micromanipulator is the rate-limiting step in the single-cell genomics using MDA-in-AGM. To increase the throughput, AGM sorting using FACS or molecular barcoding49 of SAGs may be adapted, although these approaches also reduce the simplicity and inexpensiveness of the present protocol.
The taxonomic composition of SAGs obtained by MDA-in-AGM was largely consistent with those shown by 16S rRNA amplicon and metagenomic analyses; however, a SAG of the dominant Fa. prausnitzii was not recovered in either MDA-in-AGM or FACS-MDA (Supplementary Table S4). Thus, this failure was not specific to MDA-in-AGM but was attributable to the MDA procedure. Similar discrepancies in the taxonomic composition were found in gram-positive bacterial classes in experiments using termite gut microbiota (Supplementary Table S11), so modification of the cell lysis procedure, including the addition of lysozyme, may be required for certain gram-positive bacteria. Although we have not yet attempted, lysozyme with the size of 4 nm50 will diffuse through an agarose gel shell consisting of 2% low melting agarose (200-nm pore size)51 and lyse the bacterial cell wall in the core. To optimise such conditions, AGM is also superior because the exchange of the solution is easier compared to other methods.
The lower average genome completeness of the mock community (68%) and the termite gut microbiome (79%) compared to that of E. coli (93%) (Supplementary Tables S2, S5, Table 1) may be due to the differences in the ease of cell lysis. Low genome completeness of SAGs in certain bacterial lineages and environmental samples, compared to SAGs of cultured E. coli, has been reported in previous studies, irrespective of the MDA methods1,52. For example, in droplet single-cell MDA, the genome completeness of E. coli and soil microbiota has been found as 96.5% ± 2.2% (n = 16) and 52.8% ± 24.3% (n = 17), respectively19. Cell lysis optimisation for different prokaryotic strains and environmental samples is also important for increasing genome completeness.
Recently, as our experiments were on going, a report was published that shows MDA using a hollow-core hydrogel microcapsule consisting of a crosslinked PEG gel shell and a dextran sol core, a capsule structure similar to AGM46. Although amplification bias has not been evaluated, single-cell MDA in the core increases the amount of MDA product when compared with MDA in the gel bead. In addition, the shell of a hollow-core hydrogel microcapsule prevents genomic DNA from leaking from the core after alkaline denaturation prior to MDA. However, near-ultraviolet light (365 nm) used for photochemical crosslinking of the PEG shell damages cells and DNA due to photooxidation53. We selected agarose as the AGM shell because it allows thermally mild reversible gelation, is commercially available, and is stable in the presence of MDA reagents, especially alkaline solution, neutralisation buffer, and EDTA.
AGMs or other reaction chambers containing single bacterial cells inevitably produce a proportion of chambers containing multiple bacterial cells21, and it is difficult to completely eliminate contaminating DNA in our experimental procedures, as in other previously developed methods1. Nevertheless, the application of a computer program for binning metagenomic fragments, such as metaWRAP31, enabled us to recover a considerable number of at least median-quality SAGs from mini-metagenome bins that resulted from multiple cells by eliminating contaminating sequences (Supplementary Table S8).
In recent years, numerous studies analysing metagenome-assembled genomes from environmental samples have been reported54. Although single-cell genomics has different advantages and potentially increases the quality of research in combination with metagenomics, for example, by revealing strain-level heterogeneity55 and by providing information on horizontally transferred genetic components45, it is difficult for many microbiologists to perform. MDA-in-AGM is a much easier and less expensive method of single-cell genomics, and in addition, only a small amount of sample is needed. Thus, this method is also suitable for tiny specimens, such as the intestinal tracts of small insects and protist cells with endo- and ecto-symbiotic bacterial communities.
Methods
Reagents
Ultrapure DNase/RNase-Free Distilled Water (Thermo Fisher Scientific, Waltham, MA) was used for all solutions prepared in-house. The solutions were autoclaved at 121 °C for 15 min and further decontaminated by UV radiation at 7.2 mW/cm2 overnight (12 h) in a laminar flow cabinet.
A suspension of uniform CaCO3 nanoparticles (9.4% w/v, 100-nm diameter, pH 10) was provided by Shiraishi Central Laboratories (Hyogo, Japan). The conductivity and pH of the CaCO3 suspension were reduced by washing the particles using three volumes of water. In addition to Shiraishi's CaCO3, fine calcium carbonate such as precipitated calcium carbonate BioUltra (approximately 3-µm diameter; Sigma, St. Louis, MO) was also used for alginate core gelation. Alginate solution (5%, w/v) was prepared by dissolving sodium alginate (80–120 cP at 1%, w/v; Wako, Osaka, Japan) in water and stored at 4 °C. Agarose solution was prepared by dissolving SeaPlaque (final 2%, w/v; Lonza, Basel, Switzerland) in water and stored at 4 °C. Polyglyceryl-6 octacaprylate (PGO; Nisshin Oillio, Tokyo, Japan), Tris–EDTA buffer (TE; BioUltra for molecular biology, pH 7.4; Sigma), and REPLI-g UltraFast Mini Kit (Qiagen, Hilden, Germany) were used as described below.
Evaluation of PGO for agarose microdroplet gelation
After an agarose solution (2 mL) was mixed with PGO or ISA, each containing 0.25% sorbitan monooleate (Span 85; Wako) (v/v) (20 mL, 35 °C), by vortexing and gelated on ice, agarose beads were recovered from PGO or ISA as described below. Large agarose aggregates were removed from the agarose beads using 300-µm cell strainers (pluriSelect, Leipzig, Germany). The agarose beads were observed under an inverted fluorescence microscope (IX71; Olympus, Tokyo, Japan) equipped with a CCD camera (BU-51LN; Bitran, Saitama, Japan). Diameters of the agarose beads over one pixel on the images (1.28 µm) were measured with ImageJ (https://imagej.nih.gov/ij/). The yields and diameters of the agarose beads were statistically analysed with R (https://www.r-project.org/).
Preparation of Escherichia coli stock culture
Escherichia coli DH5α (TaKaRa Bio Inc., Shiga, Japan) from a single colony on an LB plate was cultured in two flasks with LB media (200 mL). E. coli cells were then harvested and washed twice in PBS (20 mL) by centrifugation. The cells were resuspended in PBS (10 mL), and their density was determined with a cell-counting chamber. The E. coli cells (3.05 × 1010 cell/mL)were divided into aliquots in microtubes (0.5 mL each) and stored at − 80 °C. As mentioned below, single cells of E. coli, the mock sample, and the termite gut microbiome were analysed using cells stored at − 80 °C, a mixture of glycerol stocks stored at − 80 °C, and live specimens from termite guts, respectively.
Preparation of alginate cores containing E. coli cells
Escherichia coli cells were encapsulated in alginate cores by the emulsification/internal gelation method27 (Fig. 1b). E. coli cells from the stock culture (10 µL) were mixed with 990 µL of 2% sodium alginate solution containing 1% CaCO3 and 50 mM acetate buffer (pH 7.0, Sigma). The mixture was emulsified with 9 mL of isostearyl alcohol (ISA; Kokyu Alcohol Kogyo Co., Ltd., Chiba, Japan) containing 3% lecithin (Wako) in a 50-mL tube by vortexing for 1 min. The emulsion was mixed with 2% acetic acid in ISA (0.1 mL each) by vortexing ten times for 1 min each. During this procedure, the pH of the mixture gradually decreased to 4.0 and the CaCO3 nanoparticles were dissolved. The released calcium ions from CaCO3 gelated the alginate microdroplets in the emulsion. ISA was removed by mixing with 9 mL of diethyl ether and centrifuging at 4 °C for 3 min using a swing rotor (3000×g, 5702R, Eppendorf, Hamburg, Germany). The residual ISA was further removed from the alginate cores by mixing with 50 mM Tris–HCl buffer (pH 7.5) containing 0.2% (v/v) Tween 20 (Tris-Tween 20) (5 mL), followed by centrifugation, and then by repeating the procedure twice with 1-butanol (5 mL). The resulting alginate cores were suspended in one volume of 50 mM Tris–HCl (pH 7.5) and filtered through a 100-µm cell strainer (pluriSelect) to remove large alginate aggregates. The filtrated alginate cores were further washed with Tris–HCl (pH 7.5) and collected in 2-mL microtubes. The volume of the alginate cores was calculated from its weight and stored at 4 °C after mixing with a one-half volume of Tris–HCl (pH 7.5). To adjust the number of E. coli cells in a single alginate core to less than two, 10 µL of 3.05 × 106–108 E. coli cells from the E. coli stock were mixed with 990 µL of the alginate containing CaCO3 suspension. After alginate cores containing E. coli were prepared from the mixture, E. coli in the alginate cores were observed using SYBR Green I (TaKaRa Bio Inc.) (Supplementary Fig. S7). At 3.05 × 106 cells/mL of the alginate mixture, 17.6% of the cores contained E. coli, and 78.8% of those carried single cells. Thus, 3.05 × 106 cells/mL of E. coli was used for alginate core preparation in subsequent experiments.
Preparation of AGMs containing E. coli cells
The supernatant of the alginate core suspension (600 µL) was removed after centrifugation in a 50-mL tube. The residual alginate cores (400 µL) were mixed with the agarose solution as prepared above (2 mL). The mixture was emulsified with 0.25% (v/v) Span 85 in PGO (20 mL) by vortexing for 1 min (Fig. 1b). The alginate cores, the agarose solution, and the PGO were preincubated at 35 °C for 10 min to prevent the formation of gelated large agarose aggregates. Then, the agarose was gelated by cooling on ice for 1 h. PGO was removed from the emulsion by washing as described above. The alginate gel core was solated by chelating calcium ions with the addition of a 1/10 volume of 0.5 M EDTA (Thermo Fisher Scientific). The AGMs were then suspended in one volume of TE buffer. Large debris in the suspension was removed through a 300-µm cell strainer, and the AGMs were further washed with TE. The AGMs were diluted in 50 mM Tris–HCl (pH 7.5) containing 0.5% SeaPlaque agarose, 0.1% p-phenylene diamine (Wako), and 10,000-fold diluted SYBR Green I (TaKaRa Bio Inc.), and the morphology, density, diameter, and number of encapsulated E. coli cells were observed as mentioned above. The AGMs were stored at 4 °C. For long-term storage (more than a week), the AGMs were stored in 40% ethanol at − 80 °C and washed three times in ten volumes of water before use. The quality of genomic DNA of AGMs stored in 40% EtOH was confirmed via their amplification using MDA.
Evaluation of alginate core solation in AGM
Alginate gel cores without E. coli were labelled with rhodamine 123 (Wako) using 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (Tokyo Chemical Industry Co., Ltd., TCI, Tokyo, Japan) and N-hydroxysuccinimide (Wako)56. AGMs containing rhodamine 123-labelled cores were heated at 65 °C for 5 min in the presence of 50 mM EDTA or 50 mM CaCl2. Residual cores were observed as mentioned above.
Effect of alginate core gelation and solation on MDA
Aliquots (50 µL) of the AGMs containing E. coli cells before solation of the alginate cores were mixed with one volume of cell lysis solution containing 400 mM KOH with or without 10 mM EDTA and they were neutralised with 100 µL of Stop buffer from the REPLI-g UltraFast Mini Kit. The AGMs containing E. coli, AGMs without E. coli (negative control), and agarose beads containing E. coli (control) were subjected to MDA as described below, and their amplified DNAs were observed.
MDA-in-AGM
A 50-µL aliquot of AGMs was centrifuged, and the collected AGMs were washed with 200 µL water. Cell lysis and DNA denaturation were carried out with Buffer D2 (50 µL) of the REPLI-g UltraFast Mini Kit at room temperature for 30 min. After denaturation was stopped with the Stop buffer (50 µL), the supernatant was removed by centrifugation. The Reaction buffer (93.5 µL) containing 11 µL phi29 DNA polymerase from the kit was added and incubated at 30 °C for 3 h (MDA-in-AGM). The reaction was stopped with 1/10 volume of 0.5 M EDTA, and the AGMs were washed with 200 µL of TE buffer three times. The washed AGMs were stored at 4 °C.
FACS-MDA
Single-cell MDA using a MoFlo XDP fluorescence-activated cell sorter (Beckman Coulter Inc., Brea, CA) was performed as a control43. After E. coli, the mock bacterial community and termite gut bacteria were stained with SYBR Green I or CellTracker Green (Thermo Fisher Scientific), and the stained bacteria were sorted into a single cell per well of 96-well PCR plates. Cell lysis was carried out with Buffer D2 (1.5 µL) of the REPLI-g UltraFast Mini Kit at room temperature for 30 min. After denaturation was stopped with the Stop buffer (1.5 µL), Reaction buffer (7.5 µL) containing 0.5 µL phi29 DNA polymerase from the kit was added and incubated at 30 °C for 3 h and 65 °C for 15 min. To check genome amplification and the degree of contamination, the MDA products were evaluated by direct Sanger sequencing of the 16S rRNA. PCR was performed with Bacteria-universal 16S rRNA gene primers: 27F (5′-AGAGTTTGATCMTGGCTCAG) and 1390R (5′-ACGGGCGGTGTGTACAA).
Mock community of human gut bacteria
A mock community of human gut bacteria was prepared using 15 isolates (Supplementary Table S4) obtained from the Japan Collection of Microorganisms. A mixture of all glycerol stocks in equal amounts was used as the mock bacterial community. After the mock bacterial community was diluted 100-fold using water, the diluent (10 µL) was treated with DNase I (20 µL) at 30 °C for 30 min. The cells were washed three times by suspending in water (1 mL), collected by centrifuging at 18,000×g for 5 min, and suspended in water (10 µL). After the MDA-in-AGM as described above, the AGMs were observed with SYBR Green I using a microscope. Among the large numbers of AGMs in the microscopic field of view, only a few AGMs contained amplified DNAs, which were isolated using a micromanipulator as described below.
To determine the composition of the mock bacterial community, the total DNA was extracted using the DNeasy Ultra Clean Microbial Kit (Qiagen). Metagenome sequencing was performed by preparing a library with the QIAseq FX DNA Library Kit (Qiagen). Amplicon sequencing of 16S rRNA genes in the mock community was also performed by preparing a library with the Nextera XT Index Kit 96 indices (Illumina, Inc., San Diego, CA) and the MiSeq platform with Reagent Kit V3 (600 cycles). The bacterial composition of the mock community was estimated on the basis of the results analysed in QIIME257.
Termite gut bacteria
Specimens of the wood-feeding termite Reticulitermes speratus were collected in Tsukuba in Ibaraki Prefecture, Japan. The guts of five worker termites were removed, and the gut contents were suspended in solution U58, which consisted of 9.2 mM NaHCO3, 5.1 mM trisodium citrate dihydrate, 13 mM KH2PO4, 37 mM NaCl, 0.75 mM CaCl2 and 0.4 mM MgSO4. The extracellular DNA and protist DNA were digested using DNase I, and the bacterial cells were collected at 18,000×g for 5 min by centrifugation and washed with water as mentioned above43. The washed termite gut bacteria were suspended in water (10 µL). MDA-in-AGM for the termite gut bacteria was performed using the suspension as the mock community.
Genome sequencing and bioinformatics
AGMs containing amplified DNA, detected with SYBR Green I, were transferred individually to PCR tubes under an inverted fluorescence microscope equipped with a micromanipulator (TransferMan NK2; Eppendorf, Hamburg, Germany). Water (29 µL) was added to each PCR tube with a single AGM and incubated at 60 °C for 5 min to release DNA by solating the agarose shell. To check the degree of contamination, we screened the MDA-in-AGM products by direct Sanger sequencing of 16S rRNA gene as mentioned above. After removing the contaminated samples, sequencing libraries were prepared using the QIAseq FX DNA Library Kit. Since the MDA products in AGM cores were too small to quantify, the fragmentation and ligation times in the library construction were performed using a condition of the < 10 ng input DNA in the manufacturer’s instructions. Genome sequencing was performed on the MiSeq platform with Reagent Kit V3 (600 cycles). Sequence libraries for single cells isolated from the termite gut microbiota using FACS were prepared using the Nextera XT DNA Library Prep Kit (Illumina) and analysed on the Illumina HiSeq 2500 platform (500 cycles).
The generated short reads were trimmed using Cutadapt (https://github.com/marcelm/cutadapt), PRINSEQ (http://prinseq.sourceforge.net/), FASTX Trimmer, FASTQ Quality Trimmer (http://hannonlab.cshl.edu/fastx_toolkit/download.html) and cmpfastq_pe (http://compbio.brc.iop.kcl.ac.uk/software/download/cmpfastq_pe). The trimmed reads were assembled using SPAdes ver. 3.13.0 (k-mer: 21, 33, 55, 77, 99 and 127, options: -sc, -careful)28 into contigs. Only > 1 kb contigs were selected using the SeqKit (https://bioinf.shenwei.me/seqkit/) for the subsequent analyses.
Taxonomic classification of single-cell genomes from termite gut samples was conducted using the Genome Taxonomy Database Tool Kit (GTDB-Tk)44. Genomic sequence data showing > 5% contamination, identified using CheckM29, were sequentially subjected to binning, refinement, and reassembly processes with the BINNING (including metaBAT259, MaxBin260 and CONCOCT61), BIN_REFINEMENT, and REASSEMBLE_BINS modules of metaWRAP31.
For single-cell genome analyses using E. coli DH5α and human gut bacteria with increasing coverage (Fig. 3), adapter-trimmed reads using Trimmomatic62 were randomly chosen using Rasusa63 and assembled de novo using SPAdes28. Genome completeness and numbers of contigs in the assemblies were evaluated using CheckM29 and plotted using R. The sequencing reads corresponding to different coverage were mapped onto known genome sequences using Bowtie264, and the results were visualised as heatmaps using IGV ver. 2.3.26 (http://software.broadinstitute.org/software/igv/). Covered genome (%), which was the genome regions covered with at least one read per 1 kb-bin, was calculated using BBtools (http://jgi.doe.gov/data-and-tools/bbtools/).
The amplification biases of SAGs were evaluated using Gini coefficients and Lorenz curves. After sequencing reads of SAGs were mapped on reference genomes using Bowtie264, the reads were randomly chosen to be 60-fold (E. coli) and 40-fold (human gut bacteria) coverage of their genomes, and sequencing depths per base were calculated from the reads using SAMtools (http://www.htslib.org). Gini coefficients of SAGs were calculated from mean sequencing depths per 50-kb bin using the R ineq package. SAGs, which had Gini coefficients equivalent to their medians, were further plotted on Lorenz curves using the R gglorenz package.
The chimeric read rate was calculated by mapping short reads to the reference genome with the Barrows-Wheeler Aligner (https://sourceforge.net/projects/biobwa/files/) and SAMtools. The reference genomes are listed in Supplementary Table S4. Cross-contaminations of genomic sequencing data with < 5% contamination using CheckM29 (Supplementary Table S7) were calculated by mapping sequencing reads to reference genomes in the mock community16.
Data availability
The raw fastq files (DRR253532–DRR253885) and assemblies for SAGs of termite gut bacteria (BNTM01000000–BOCE01000000) were deposited into DDBJ under BioProject Accession No. PRJDB10679.
References
Woyke, T., Doud, D. F. R. & Schulz, F. The trajectory of microbial single-cell sequencing. Nat. Methods 14, 1045–1054 (2017).
Wrighton, K. C. et al. Fermentation, hydrogen and sulfur metabolism in multiple uncultivated bacterial phyla. Science 337, 1661–1665 (2012).
Albertsen, M. et al. Genome sequences of rare, uncultured bacteria obtained by differential coverage binning of multiple metagenomes. Nat. Biotechnol. 31, 533–538 (2013).
Anantharaman, K. et al. Thousands of microbial genomes shed light on interconnected biogeochemical processes in an aquifer system. Nat. Commun. 7, 1–11 (2016).
Delmont, T. O. et al. Nitrogen-fixing populations of Planctomycetes and Proteobacteria are abundant in surface ocean metagenomes. Nat. Microbiol. 3, 804–813 (2018).
Zhang, K. et al. Sequencing genomes from single cells by polymerase cloning. Nat. Biotechnol. 24, 680–686 (2006).
Kaster, A.-K. & Sobol, M. S. Microbial single-cell omics: The crux of the matter. Appl. Microbiol. Biotechnol. 104, 8209–8220 (2020).
Woyke, T. et al. One bacterial cell one complete genome. PLoS ONE 5, e10314 (2010).
Fu, A. Y., Spence, C., Scherer, A., Arnold, F. H. & Quake, S. R. A microfabricated fluorescence-activated cell sorter. Nat. Biotechnol. 17, 1109–1111 (1999).
Marcy, Y. et al. Nanoliter reactors improve multiple displacement amplification of genomes from single cells. PLoS Genet. 3, 1702–1708 (2007).
Joensson, H. N. & Andersson Svahn, H. Droplet microfluidics—A tool for single-cell analysis. Angew. Chem. Int. Ed. 51, 12176–12192 (2012).
Zong, C., Lu, S., Chapman, A. R. & Xie, X. S. Genome-wide detection of single-nucleotide and copy-number variations of a single human cell. Science 338, 1622–1626 (2012).
Dean, F. B. et al. Comprehensive human genome amplification using multiple displacement amplification. Proc. Natl. Acad. Sci. U.S.A. 99, 5261–5266 (2002).
Stepanauskas, R. et al. Improved genome recovery and integrated cell-size analyses of individual uncultured microbial cells and viral particles. Nat. Commun. 8, 84 (2017).
Gole, J. et al. Massively parallel polymerase cloning and genome sequencing of single cells using nanoliter microwells. Nat. Biotechnol. 31, 1126–1132 (2013).
Xu, L., Brito, I. L., Alm, E. J. & Blainey, P. C. Virtual microfluidics for digital quantification and single-cell sequencing. Nat. Methods 13, 759–762 (2016).
Sidore, A. M., Lan, F., Lim, S. W. & Abate, A. R. Enhanced sequencing coverage with digital droplet multiple displacement amplification. Nucleic Acids Res. 44, e66 (2016).
Nishikawa, Y. et al. Monodisperse picoliter droplets for low-bias and contamination-free reactions in single-cell whole genome amplification. PLoS ONE 10, e0138733 (2015).
Hosokawa, M., Nishikawa, Y., Kogawa, M. & Takeyama, H. Massively parallel whole genome amplification for single-cell sequencing using droplet microfluidics. Sci. Rep. 7, 5199 (2017).
Chijiiwa, R. et al. Single-cell genomics of uncultured bacteria reveals dietary fiber responders in the mouse gut microbiota. Microbiome 8, 5 (2020).
Bigdeli, S., Dettloff, R. O., Frank, C. W., Davis, R. W. & Crosby, L. D. A simple method for encapsulating single cells in alginate microspheres allows for direct PCR and whole genome amplification. PLoS ONE 10, e0117738 (2015).
Rabanel, J. M., Banquy, X., Zouaoui, H., Mokhtar, M. & Hildgen, P. Progress technology in microencapsulation methods for cell therapy. Biotechnol. Prog. 25, 946–963 (2009).
Siltanen, C. et al. One step fabrication of hydrogel microcapsules with hollow core for assembly and cultivation of hepatocyte spheroids. Acta Biomater. 50, 428–436 (2017).
Breguet, V., Gugerli, R., von Stockar, U. & Marison, I. W. CHO immobilization in alginate/poly-L-lysine microcapsules: An understanding of potential and limitations. Cytotechnology 53, 81–93 (2007).
Sakai, S., Hashimoto, I. & Kawakami, K. Production of cell-enclosing hollow-core agarose microcapsules via jetting in water-immiscible liquid paraffin and formation of embryoid body-like spherical tissues from mouse ES cells enclosed within these microcapsules. Biotechnol. Bioeng. 99, 235–243 (2008).
Tamminen, M. V. & Virta, M. P. J. Single gene-based distinction of individual microbial genomes from a mixed population of microbial cells. Front. Microbiol. https://doi.org/10.3389/fmicb.2015.00195 (2015).
Poncelet, D. Production of alginate beads by emulsification/internal gelation. Ann. N. Y. Acad. Sci. 944, 74–82 (2001).
Bankevich, A. et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 19, 455–477 (2012).
Parks, D. H., Imelfort, M., Skennerton, C. T., Hugenholtz, P. & Tyson, G. W. CheckM: Assessing the quality of microbial genomes recovered from isolates, single cells and metagenomes. Genome Res. 25, 1043–1055 (2015).
Gurevich, A., Saveliev, V., Vyahhi, N. & Tesler, G. QUAST: Quality assessment tool for genome assemblies. Bioinformatics 29, 1072–1075 (2013).
Uritskiy, G. V., DiRuggiero, J. & Taylor, J. MetaWRAP—A flexible pipeline for genome-resolved metagenomic data analysis. Microbiome 6, 158 (2018).
Lasken, R. S. & Stockwell, T. B. Mechanism of chimera formation during the multiple displacement amplification reaction. BMC Biotechnol. 7, 19 (2007).
Tu, J. et al. Systematic characteristic exploration of the chimeras generated in multiple displacement amplification through next generation sequencing data reanalysis. PLoS ONE 10, e0139857 (2015).
Hongoh, Y., Ohkuma, M. & Kudo, T. Molecular analysis of bacterial microbiota in the gut of the termite Reticulitermes speratus (Isoptera; Rhinotermitidae). FEMS Microbiol. Ecol. 44, 231–242 (2003).
Ni, J. & Tokuda, G. Lignocellulose-degrading enzymes from termites and their symbiotic microbiota. Biotechnol. Adv. 31, 838–850 (2013).
Brune, A. Symbiotic digestion of lignocellulose in termite guts. Nat. Rev. Microbiol. 12, 168–180 (2014).
Hongoh, Y. et al. Genome of an endosymbiont coupling N2 fixation to cellulolysis within protist cells in termite gut. Science 322, 1108–1109 (2008).
Hongoh, Y. et al. Intra- and interspecific comparisons of bacterial diversity and community structure support coevolution of gut microbiota and termite host. Appl. Environ. Microbiol. 71, 6590–6599 (2005).
Arora, J. et al. The functional evolution of termite gut microbiota. Microbiome 10, 1–22 (2022).
Hongoh, Y. et al. Complete genome of the uncultured termite group 1 bacteria in a single host protist cell. Proc. Natl. Acad. Sci. USA 105, 5555–5560 (2008).
Izawa, K. et al. Comparison of intracellular “Ca. Endomicrobium trichonymphae” genomovars illuminates the requirement and decay of defense systems against foreign DNA. Genome Biol. Evol. 8, 3099–3107 (2016).
Kuwahara, H., Yuki, M., Izawa, K., Ohkuma, M. & Hongoh, Y. Genome of ‘Ca. Desulfovibrio trichonymphae’, an H2-oxidizing bacterium in a tripartite symbiotic system within a protist cell in the termite gut. ISME J. 11, 766–776 (2017).
Yuki, M. et al. Dominant ectosymbiotic bacteria of cellulolytic protists in the termite gut also have the potential to digest lignocellulose. Environ. Microbiol. 17, 4942–4953 (2015).
Chaumeil, P. A., Mussig, A. J., Hugenholtz, P. & Parks, D. H. GTDB-Tk: A toolkit to classify genomes with the genome taxonomy database. Bioinformatics. https://doi.org/10.1093/bioinformatics/btz848 (2019).
Lan, F., Demaree, B., Ahmed, N. & Abate, A. R. Single-cell genome sequencing at ultra-high-throughput with microfluidic droplet barcoding. Nat. Biotechnol. 35, 640–646 (2017).
Leonaviciene, G., Leonavicius, K., Meskys, R. & Mazutis, L. Multi-step processing of single cells using semi-permeable capsules. Lab. Chip 20, 4052–4062 (2020).
Rodrigue, S. et al. Whole genome amplification and de novo assembly of single bacterial cells. PLoS ONE 4, e6864 (2009).
Gasperini, L., Mano, J. F. & Reis, R. L. Natural polymers for the microencapsulation of cells. J. R. Soc. Interface 11, 20140817 (2014).
Zheng, G. X. et al. Massively parallel digital transcriptional profiling of single cells. Nat. Commun. 8, 14049 (2017).
Diamond, R. Real-space refinement of the structure of hen egg-white lysozyme. J. Mol. Biol. 82, 371–391 (1974).
Narayanan, J., Xiong, J. Y. & Liu, X. Y. Determination of agarose gel pore size: Absorbance measurements vis a vis other techniques. J. Phys. 28, 83–86 (2006).
Clingenpeel, S., Clum, A., Schwientek, P., Rinke, C. & Woyke, T. Reconstructing each cell’s genome within complex microbial communities—Dream or reality? Front. Microbiol. 5, 771 (2015).
Cadet, J., Douki, T. & Ravanat, J.-L. Oxidatively generated damage to cellular DNA by UVB and UVA radiation. Photochem. Photobiol. 91, 140–155 (2015).
Nayfach, S., Shi, Z. J., Seshadri, R., Pollard, K. S. & Kyrpides, N. C. New insights from uncultivated genomes of the global human gut microbiome. Nature 568, 505–510 (2019).
Merino, N. et al. Single-cell genomics of novel actinobacteria with the Wood-Ljungdahl pathway discovered in a serpentinizing system. Front. Microbiol. 11, e1031 (2020).
Hermanson, G. T. Bioconjugate Techniques (Academic Press, 1996).
Bolyen, E. et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 37, 852–857 (2019).
Trager, W. The cultivation of a cellulose-digesting flagellate, Trichomonas termopsidis and of certain other termite protozoa. Biol. Bull. 66, 182–190 (1934).
Kang, D. D. et al. MetaBAT 2: An adaptive binning algorithm for robust and efficient genome reconstruction from metagenome assemblies. PeerJ 7, e7359 (2019).
Wu, Y.-W., Simmons, B. A. & Singer, S. W. MaxBin 2.0: An automated binning algorithm to recover genomes from multiple metagenomic datasets. Bioinformatics 32, 605–607 (2016).
Alneberg, J. et al. Binning metagenomic contigs by coverage and composition. Nat. Methods 11, 1144–1146 (2014).
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 (2014).
Hall, M. B. Rasusa: Randomly subsample sequencing reads to a specified coverage. J. Open Source Softw. 7, 3941 (2022).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012).
Acknowledgements
This work was supported by the RIKEN Pioneering Project “Biology of Symbiosis”, JSPS KAKENHI Grants 16K07224 to H. A, and 17H01447 and 19H05679 to M. O., and the Institute of Fermentation, Osaka to M. Y.
Author information
Authors and Affiliations
Contributions
Y.Y. and M.O. supervised the study. H.A., M.Y., Y.H. and M.O. designed the study. H.A., M.Y. and Y.H. wrote the manuscript. H.A. developed the AGM preparation protocol. M.Y. and M.S. performed the next-genome sequencing and subsequent data analyses. All authors contributed to and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aoki, H., Masahiro, Y., Shimizu, M. et al. Agarose gel microcapsules enable easy-to-prepare, picolitre-scale, single-cell genomics, yielding high-coverage genome sequences. Sci Rep 12, 17014 (2022). https://doi.org/10.1038/s41598-022-20923-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-20923-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.