Abstract
Due to several centuries of ongoing mining activities, Middle Spiš (Slovakia) is one of the areas with a damaged environment. The contents of Fe, Mn, Zn, Cu, Ni, Pb, Cd, and Hg were determined in the soils and home-grown vegetables (potatoes, carrots, tomatoes). Except for Pb, the contents of heavy metals in the soils of some plots were higher than the limit values. Based on the values of Contamination factor (Cf), Degree of contamination (Cdeg), Geo-accumulation index (Igeo), and Pollution load index (PLI), very high Fe, Cd, and Hg contamination (Cf ≥ 6), very high soil contamination (Cdeg ≥ 20), extremely heavy Fe and Hg contamination (Igeo > 5), resp. moderately pollution to non-pollution (1 < PLI ≤ 2) was found in all plots. In vegetable samples, the maximum levels were exceeded for Cu, Pb, Hg (potato), Pb (carrot, tomato), and Hg (carrot, plot E). Bioaccumulation factor values BAF > 1 were for Cu (carrots, potatoes). Estimated daily intake values for all heavy metals were lower than their tolerable daily intake. Chronic daily intake of heavy metals ranged 2.495E−06 (Hg)—0.1416 (Fe) mg/kg/day. Based on Hazard index values, potato consumption poses a risk (0.8068–1.3057). The results showed that the monitoring of soils and cultivated production is necessary for the investigated area.
Similar content being viewed by others
Introduction
Environmental pollution is a global problem closely linked to the content of toxic chemicals in the environment1. The mining and subsequent processing of complex iron and copper ores in Slovakia have a negative effect on the area of Middle Spiš. The most important mining sites include Krompachy, Spišská Nová Ves, Mlynky, Novoveská Huta, Rudňany, Poráč, and Slovinky. Exceedances of the limit values of Hg, Cu, Zn, As, Cd, and Pb were found in the soils2.
Heavy metals (HMs) can accumulate in the edible parts of plants, enter the food chain, and cause adverse toxicological effects to consumers3,4. Their accumulation in vital organs such as the liver, kidneys, and bones can lead to many serious health disorders5.
Iron (Fe), as an essential mineral, plays an important role in basic biological processes. Fe is also a cofactor of many enzymes involved in the photosynthesis of plant hormones6. Although iron (Fe) is essential for most life forms and is widely used in various proteins to perform many functions7, there is a presumption that the interaction of iron and cholesterol is a crucial mechanism in promoting oxidative damage that causes atherosclerosis and neurodegeneration8.
Manganese (Mn), the most abundant trace element, is closely related to Fe. In plants, it is involved in the structure of photosynthetic proteins and enzymes. It is also essential for their defence system as an enzyme antioxidant cofactor9 and plays a key role in cell division10. Consumption of high manganese (Mn) concentrations can cause neurodegenerative disorders, cardiovascular toxicity, and liver damage8.
Zinc (Zn), one of the most mobile HMs, is present in the soils in free and complex ionic forms. Zn plays an important role in plant metabolism. It is also important in the activation of many enzymes included in the protein synthesis, cell membrane stabilization, auxin synthesis, and pollen formation9. Ingestion of high zinc levels results in neurotoxic11. Zinc is not generally considered to cause cancer12.
Copper (Cu) occurs naturally as a pure metal13. In plants, Cu is a component of several enzyme systems involved in oxidative stress responses, is involved in photosynthetic electron transport, mitochondrial respiration, and helps in the metabolism of lignin, carbohydrates, and proteins in plants9. Toxic effects of copper are associated with neurodegenerative diseases, diabetes8, acne, alopecia, autism, cystic fibrosis, hypothyroidism14.
Nickel (Ni) is essential for proper plant growth and development. However, at high levels, nickel alters plant metabolic activities, inhibits enzymatic activity, photosynthetic electron transport, and chlorophyll biosynthesis. Human exposure to nickel (Ni) mainly concerns oral ingestion through water and food that may be contaminated with nickel. Ni as an immunotoxic and carcinogenic substance can cause various health effects such as contact dermatitis, cardiovascular disease, asthma, pulmonary fibrosis, and respiratory cancer15.
Lead (Pb) is a highly toxic element, bioaccumulative, and degraded or easily metabolized in the environment16. The plants have only about 0.005 to 0.13% of lead in the soil solution. Nevertheless, food is a significant source of Pb exposure, and the potential risk to the population may be due to the bioaccumulation of Pb in the edible vegetable4. Lead exposure can cause plumbism, anaemia, nephropathy, gastrointestinal colic, and central nervous system symptoms5. Neurological symptoms include ataxia, encephalopathy, seizure, swelling of the optic nerve, disorder of consciousness17.
Cadmium (Cd) generally has a high soil bioavailability. It has higher mobility in plants compared to other heavy metals18, and in addition to reduced nutrient intake, causes chlorosis, necrosis, and growth retardation of roots and shoots19, can inhibit seed germination, and reduce the number of leaves per plant. It is toxic to plants even at low concentrations20,21. Cadmium is primarily toxic to the kidneys and can cause them to fail22. Another target organ for Cd is the liver23. In addition, it is involved in bone diseases, lung edema, liver damage, anaemia, and hypertension and is the cause of Itai-itai disease18. Cadmium is classified as a human carcinogen based on working studies (group 1)24.
Mercury (Hg) is recognized as a toxic, persistent, and mobile contaminant that does not degrade in the environment and is the only element in the periodic table with its own environmental convention, i.e., the Minamata Convention on Mercury25. Hg exposure can also reduce photosynthesis, transpiration rate, and water absorption, and chlorophyll synthesis26. Mercury is toxic to humans in all its primary forms, with the most toxic being methylmercury. Mercury is considered by WHO to be one of the top ten chemicals or groups of chemicals of major public health concern27.
The aim of this study was to (i) assess the impact of old environmental burdens on soil quality by soil indicators using soil risk contamination factors, (ii) determine the ability of Fe, Mn, Zn, Cu, Ni, Pb, Cd, and Hg to accumulate in crops (Solanum tuberosum, L., Solanum esculentum, L., and Daucus carota), and finally (iii) assess the risk of consuming crops grown in soils with increased content of these risk elements.
Material and methods
Study area
The Spiš region is one of the most burdened and hygienically defective areas due to mining activities that have been expanded in the past. The predominant contaminants are heavy metals2.
The area of interest is situated in the locality of the village Poráč, in the Slovak Ore Mountains (Volovské Hills–Hnilecké Hills). In terms of geological construction, the area is built by a diverse range of rocks. It is situated in the boundary of massifs formed by a complex of Mesolithic rocks (Gutenstein limestones) and the occurrence of sandstones, conglomerates, clayey shales, phyllites, and volcanics. Limestone complexes occur mainly in the eastern part of the territory, and other rocks make up a substantial majority of the area28. This diverse geological structure is followed by land cover. Modal rendzinas have been formed in the area of limestones, and cambisol-like rendzina has been formed in places where limestones pass into other rocks. The other area consists exclusively of modal acid cambisols29.
Rendzinas have a shallow humus horizon, but the humus content tends to be high, as calcium carbonate and humic substances form complexes. Below the humus horizon, a compact rock immediately occurs. The soil reaction of these soils is alkaline, the sorption capacity is high. Cambisol is characterized by a Cambic Bv horizon, which has a distinct brown color caused by Fe oxidation. The Cambic horizon is below the shallow humus horizon. Different types of rocks are the substrate for these soils, and therefore, the properties of these soils tend to be very diverse. Under forest stands, there is usually a higher amount of humus, but the values of the soil reaction range from weakly acidic to strongly acidic soil reaction. The sorption capacity is medium, the soil profile is skeletal and permeable to rainwater30.
Sampling and sample processing
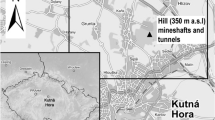
Soil samples were collected in the cadastre of Poráč, which is a part of the Spiš region (Fig. 1). Map with sample sites was made with ArcView 3.2. Potato (Solanum tuberosum, L.), tomato (Solanum esculentum, L.), and carrot (Daucus carota) samples were also taken from a pre-determined five plots (A: 48.883396, 20.728739; B: 48.884139, 20.731591; C: 48.883378, 20.723065; D: 48.883471, 20.716939, E: 48.884733, 20.719931). Each soil and plant sample consisted of an average sample from 3 points (approx. 0.5 kg from one sampling point) from an area of approx. 400–700 m2. Soil samples were taken at a horizon of 0–0.1 m into pedological probe GeoSampler by Fisher.
Organic impurities (leaves, roots) and debris were removed from the soil samples before drying. After drying, the samples were ground (grinding machine VEB Thurm ZG 1) to fine earth (average particle size 0.125 mm), in which the contents of risk metals were determined. The soil samples were stored in polyethylene bags until analysis.
Samples of plant material were mechanically cleaned from organic and inorganic impurities immediately after collection. Subsequently, they were washed with distilled water, sliced, and dried to constant weight at 45 °C. After drying, the samples were homogenized (IKA A10 basic, 30 s., 25,000 rpm). All samples were stored in polyethylene bags until analysis.
Chemical analysis
The contents of risk elements were determined in soil samples using Flame AAS method (Fe, Mn, Zn, Cu, Ni) and Graphite Furnace AAS method (Cd, Pb) (VARIAN AASpectr DUO 240FS/240Z/UltrAA equipped with a D2 lamp background correction system, using an air-acetylene flame, Varian, Ltd., Mulgrave, VIC, AUS). Total Hg content was determined using the Cold Vapour AAS method (AMA 254, Altec s.r.o, Prague, CZE). After microwave digestion (70 min, MARS X-Press 5, CEM Corp., Matthews, NC, USA), the total contents of risk elements, including all metal forms with exception of silicate forms in soil extract by aqua regia (1 g fine earth + 10 mL aqua regia, HNO3, HCl; Merck, Germany) were determined. In soil extract by NH4NO3 (c = 1 mol/L, NH4NO3; Merck, Germany), the contents of risk metals in their bioavailable form were determined.
Mineralization of plant samples was performed using a closed microwave digestion system (Mars X-Press 5) with conc. HNO3. The contents of risk elements in the plant material were determined by F-AAS method (Fe, Mn, Zn, Cu, Ni), GF-AAS method (Cd, Pb), and CV-AAS method (Hg). Fe (Mn, Zn, Cu, Ni, Cd, Pb, and Hg) were determined at wavelength 241.8 (279.5, 213.9, 324.8, 232.0, 228.8, 217.0, and 253,65) nm, Limits of detection LOD were 0.2978 (0.0253, 0.0868, 0.0880, 0.3582, 0.0617, 0.0891, and 0.0190–6) mg/L and Limits of quantification LOQ were 0.4703 (0.0302, 0.1736, 0.0917, 0.5652, 0.1192, 0.1405, and 0.0391) mg/L. Repeatability of determination during analysis—deviation max. 3%. Gas flow: air 13.5 L/min, acetylene 2.0 L/min. The measured results were compared with multielemental standard for F-AAS and GF-AAS, resp. singleelemental standard for CV-AAS (Merck, Germany).
The contents of risk elements determined in soil samples were compared with limit and critical values according to31.
The contents of risk elements determined in plant samples were evaluated according to the maximum allowed amounts given by the valid legislation32,33,34,35. There are no maximum levels for other risk elements.
Soil contamination was assessed using indicators of soil contamination by risk elements, the risk of heavy metals entering the food chain by a bioaccumulation factor, and the impact of risk elements on human health was assessed on the basis of human health risk indicators.
Indicators of soil contamination by risk elements
-
1.
Contamination factor (\({\mathrm{C}}_{\mathrm{f}}^{\mathrm{i}},\) single element contamination factor, Eq. 1):
$${\mathrm{C}}_{\mathrm{f}}^{\mathrm{i}}=\frac{{\mathrm{C}}^{\mathrm{i}}}{{\mathrm{B}}^{\mathrm{i}}}$$(1)is given by the ratio of the concentration of the given risk element in the soil (\({\mathrm{C}}^{\mathrm{i}}\)) and its background concentration (level of geochemical background; \({\mathrm{B}}^{\mathrm{i}}\))36.
-
2.
Degree of contamination (Cdeg, Eq. 2):
is the sum of contamination factors for all examined risk elements and represents the integrated pollution degree of the environment37.
$${\mathrm{C}}_{\mathrm{deg}}= \sum \frac{{\mathrm{C}}^{\mathrm{i}}}{{\mathrm{B}}^{\mathrm{i}}}= \sum {\mathrm{C}}_{\mathrm{f}}^{\mathrm{i}}$$(2) -
3.
Geo-accumulation index (Igeo, Eq. 3):
$${\mathrm{I}}_{\mathrm{geo}}= {\mathrm{log}}_{2}\left(\frac{{\mathrm{C}}^{\mathrm{i}}}{\mathrm{1,5}\times {\mathrm{B}}^{\mathrm{i}}}\right)$$(3) -
4.
Pollution load index (PLI, Eq. 4):
$$\mathrm{PLI}={(\mathrm{C}}_{\mathrm{f}1}^{\mathrm{i}}\times {\mathrm{C}}_{\mathrm{f}2}^{\mathrm{i}}\times {\mathrm{C}}_{\mathrm{f}3}^{\mathrm{i}}\times \dots \times {\mathrm{C}}_{\mathrm{fn}}^{\mathrm{i}}{)}^{\frac{1}{\mathrm{n}}}$$(4)is defined as the n-th root of the multiplications of the contamination factor (\({\mathrm{C}}_{\mathrm{f}}^{\mathrm{i}}\)) of metals, is it an integrated approach of pollution load index of the hazardous elements41.
-
5.
Bioaccumulation factor (BAFi, Eq. 5):
$${\mathrm{BAF}}_{i}= \frac{{\mathrm{C}}_{\mathrm{p}}^{\mathrm{i}}}{{\mathrm{C}}_{\mathrm{s}}^{\mathrm{i}}}$$(5)is calculated from the ratio of the concentration of risk element in the plant (\({\mathrm{C}}_{\mathrm{p}}^{\mathrm{i}}\)) and in the soil (\({\mathrm{C}}_{\mathrm{s}}^{\mathrm{i}}\))3,5.
-
6.
Estimated daily intake (EDI, Eq. 6):
is given by the concentration of the element in the food (C, mg/kg FW), the daily food intake (FIR, g/day), and the reference body weight (BWa, 70 kg)42.
$$\mathrm{EDI}= \frac{\mathrm{C}\times {\mathrm{F}}_{\mathrm{IR}}}{\mathrm{BWa}}$$(6) -
7.
Chronic Daily Intake (CDI, Eq. 7):
was determined by a modified method according to Antoine et al.42, and Onyele and Anyanwu43. CDI is the daily dose of risk elements (mg/kg/day), C is the concentration of heavy metals in the food (mg/kg FW), EFR is the exposure frequency to the trace element (daily/year), ED is the duration of exposure (70 years), AT is the average time (daily for 70 years), and 10-3 is the unit conversion factor
$$\mathrm{CDI}= \frac{\mathrm{C}\times {\mathrm{F}}_{\mathrm{IR}}\times {\mathrm{E}}_{\mathrm{FR}}\times {\mathrm{E}}_{\mathrm{D}}}{\mathrm{BWa}\times \mathrm{AT}}\times {10}^{-3}$$(7) -
8.
Target hazard quotient (THQ, Eq. 8):
is given by the ratio of the daily dose of risk elements to which the consumer may be exposed, and the reference dose of risk elements that can be taken daily for a long period without health risk (RfD, mg/g/day)5.
$$\mathrm{THQ}= \frac{\mathrm{CDI}}{\mathrm{RfD}}$$(8) -
9.
Hazard index (HI, Eq. 9):
$$\mathrm{HI}= \sum \mathrm{THQ}$$(9)
Hazard index (HI) is the sum of the individual target hazard quotient (THQ) of elements evaluated for each type of food42.
Statistical analyses
Results were evaluated using descriptive statistical analysis (Microsoft Excel, Redmond, WA, USA) and analysis of variance (One-Way ANOVA multi-range tests, method: 95.0 percent LSD) using Statgraphics statistical software (Centurion XVI.I, USA).
Results and discussion
Soil
Content of heavy metals in soil
The heavy metal contents in the soil were determined in the soil extract by aqua regia (Table 1). Mineralization with aqua regia is a method of determining the content of metals in the soil, which dissolves most of the soil constituents except those strongly bound in silicate minerals. In this way, all elements that are likely to become bioavailable in the long term are determined. Their content is sometimes referred to as "pseudototal" (determined in aqua regia)44.
The heavy metal contents have been compared to their limit values (the maximum permissible contents of hazardous substances in agricultural land31). It is possible to state that the limit value was exceeded several times in the case of Fe (Cd and Hg). The contents of these elements, even at their lowest concentrations, were higher than limit values 36.5 (34.4 and 8.3, respectively). The highest concentrations of Fe (Zn, Cu, Ni, Cd, and Hg) exceeded limit values 80.3 (2.6, 4.2, 1.3, 61.9, and 175.8, respectively) times. The lead concentration in its pseudototal form was lower than its limit value in all forms in soil. There is no limit value for Mn according to Slovak legislation. There are statistically significant differences between the contents of heavy metals in the soils of individual plots. Based on the obtained results, plot B is the most contaminated (Cu, Ni, Hg).
Many studies pointed to the extraction and processing of heavy metals as one of the most important anthropogenic sources of soil contamination. Critical heavy metal pollution is caused by mining activities in the Guiyang area (China). The average contents of Zn, Pb, Cd, and Cu in the soil were 508.6, 384.8, 7.53, and 356 mg/kg, respectively45. Due to mining activities and inadequate disposal of waste materials in mining areas of Gifurwe, Burera district of Northern Rwanda (also known as the tungsten belt), high concentrations of heavy metals, especially As (531 mg/kg), Cr (130 mg/kg), and Pb (56 mg/kg), are present in agricultural soils46. High concentrations of Mn (1008–2007), Zn (63–140), Cu (76.3–252), Ni (44–84), Pb (44 mg/kg), and Sr (46–51) mg/kg were recorded in soil samples in the Falansa mining area and the Olode area (Pegmatite mining area) in Nigeria47. Due to the mining of lead and zinc ore in Kishnica (Kosovo), the concentrations of heavy metals in the soil samples exceed the standard values. The average content of Fe (6009.81), Pb (3106.49), Ni (277.07), and Cd (3.49) mg/kg was significantly (p < 0.01) higher than in the soil from the uncontaminated area of Koliq48.
Mobile (bioavailable) forms of heavy metals are crucial for assessing soil hygiene (biotoxicity). Their concentrations are given in Table 2.
Critical values for bioavailable forms of heavy metals were exceeded in the case of Pb. Its lowest content was 2.1 times higher than the specified critical value (0.1 mg/kg). The highest contents of Zn (Cd) exceeded the set critical values by 5.6 (1.7) many times. No critical values are set for Fe and Mn.
There are statistically significant differences between the contents of heavy metals in the soils of individual plots (Tables 1, 2).
Indicators of soil contamination by risk elements
The pollution indices can be divided into six groups for different calculation purposes and can provide information on: (i) the individual pollution levels from each of the heavy metals analyzed (Igeo, PI, Cf); (ii) the scale of the total pollution (PIsum, PINemerow, PLI, PIave, mCd, PIVector, Cdeg, PIN and SCI); (iii) the heavy metal sources (EF and MEC); (iv) the potential environmental risk (RI and MERMQ); (v) areas with the highest potential risk of heavy metal accumulation (ExF); and (vi) the ability of the horizon surface to accumulate heavy metals (BGI)49.
We evaluated the degree of soil contamination by analysis of Contamination factor, Degree of contamination, Pollution load index, and Geo-accumulation index.
Contamination factor (Cf), Degree of contamination (Cdeg):
Background values according to Linkeš et al.50 were used to calculate \({\mathrm{C}}_{\mathrm{f}}^{\mathrm{i}}\). The value of \({\mathrm{B}}^{\mathrm{i}}\) for manganese and iron is not stated in this publication, therefore the value according to He et al.51 and Demková et al.52, respectively was used (Table 3).
The values of Cf and Cdeg for individual heavy metals are given in Table 4.
The average Cf values for Fe, Cd, and Hg ranged from 8.58 (Cd, plot D) to 1091 (Hg, plot B). Based on these data, very high contamination of each of the mentioned elements can be stated for all plots. Plot C and B also showed a very high contamination degree of Cu, soils of plots A, D, E are slightly (1 ≤ \({\mathrm{C}}_{\mathrm{f}}^{\mathrm{i}}\)<3) to substantially (3 ≤ \({\mathrm{C}}_{\mathrm{f}}^{\mathrm{i}}\)<6) contaminated. Also, in the case of Mn, Zn, and Ni, Cf is in the range of 1 – 6. In the case of Pb, the Cf value was from 1.78 to 1.99, which indicates slight contamination with this element. The classification, according to Hakanson53, was used to assess the degree of contamination by individual heavy metals (Table 5), which was also used by other authors in their work36,54,55. In China, the Cf has been accepted as a pollution index (PI), which is often assessed by comparing metal concentrations with relevant environmental directives or concerning relevant background values. The contamination level is classified as follows: uncontaminated (PI < 1), Moderately contaminated (1 ≤ PI < 3), Considerable contamination (3 ≤ PI < 6), high contaminated (6 ≤ PI < 12), very high contaminated (12 < PI)56.
Based on the assessment of soil contamination using the degree of contamination Cdeg, the soils of all plots can be classified as very high contaminated (Table 4). This degree of contamination would also be if soils were assessed only on the basis of the presence of Fe and Hg, in the case of Cd, it would be considerable contamination. That means that these three elements contributed most significantly to soil contamination on individual plots. On the other hand, if only the presence of Mn, Zn, Cu, Ni, and Pb were evaluated, plot B could also be classified as very high contaminated (Cdeg = 22.2). A scale, according to Luo et al.57 was used to evaluate the degree of contamination (Cdeg) (Table 5). Even if a wider range scale was used in the evaluation (Cdeg ≥ 32: very high contamination53, resp. Cdeg > 32: high contamination1, the soils of all plots would be classified as very high contaminated. Even if the soils were evaluated only on the basis on the presence of Fe and Hg.
Geo-accumulation index (Igeo), pollution load index (PLI)
Geo-accumulation index is used as a quantitative index for the degree of heavy metal contamination in the deposit or other materials58. Pollution load index provides information on the total toxicity level of trace elements in a given sample and indicates how many times trace element concentrations in soil exceed background concentrations. This parameter can be used to determine the level of environmental pollution40. PLI was calculated as the eight root of the product (multiple) of the Cf concentration of the eight analyzed heavy metals. Igeo and PLI values are given in Table 6.
A seven-level scale of contamination was used to assess soil contamination based on Igeo58 (Table 7). Seven Igeo classes, but with different expressions of the level of contamination are given by other authors38,40,56. According to the classification by Yakun et al.58, individual plots can be classified as not contaminated with Mn and Cu (plot D), lightly contaminated with Pb and Zn (all plots except plot E with Zn, which was slightly moderate contaminated), Mn (plot B), and Ni (plot D), slightly moderate contaminated with Mn (plots A, C, E), Cu (plots A, E), and Ni (all plots except D), moderate contaminated with Cu (plots B, C), and Cd (plots A, B, D), slightly heavy contaminated with Cd (plots C, E), and extremely heavy contaminated with Fe and Hg (except plot D, which was heavily contaminated with Fe). Even though 57.5% of Igeo were ≤ 2, these soils pose a risk mainly due to mercury. The soils around the mining areas are seriously contaminated with heavy metals emitted from mining activities. Similar results were reported by Li et al.38.
The application of PLI was used to evaluate environmental risk caused by contaminated soil. Pollution load index ranged from 1.58 (plot D) to 1.71 (plots C, E). The results showed that all five plots were from moderately polluted to unpolluted. This assessment is based on a six-level classification of pollution levels, according to Abowaly et al.40 (Table 7). Qarri et al.1 added PLI class 0—background level to the classification. On the other hand, Varol87 mentioned a simple assessment of the level of heavy metal pollution, resp. deterioration of soil conditions due to the accumulation of heavy metals. If PLI < 1, no metal contamination occurs, and PLI > 1 indicates deterioration of soil quality. According to this assessment, all plots were deteriorated in terms of heavy metal contamination.
Plant
The content of heavy metals in soil
The contents of Fe, Zn, Mn, Cu, Ni, Cd, Pb, and Hg were determined in potatoes, carrots, and tomatoes. Samples of the given crops were taken from all investigated plots, except for tomatoes, which were not grown on plots D and E. Heavy metal concentrations are expressed in mg/kg dry weight (DW), for comparison with maximum levels for contaminants in foodstuff (according to Slovak/European regulations) the concentration values are given converted to mg/kg fresh weight (FW) (Table 8). No maximum levels are set for Fe, Mn, and Zn.
The maximum levels (ML) set for Cu, Ni, Pb Cd, and Hg have been exceeded for Cu, Pb, and Hg. The copper content was higher in all potato samples than ML (3 mg/kg FW). The lowest Cu content was in potatoes from plot B, the highest in potatoes from plot E, where the ML was exceeded by 97%. The lead content exceeded the limit value in potatoes and carrots (ML = 0.10 mg/kg FW) from all plots except carrots from plot E and tomatoes (ML = 0.05 mg/kg FW) from plots A and C. The highest exceedance of the ML was in the case of potatoes, while lead content in potatoes from plot E exceeded the ML up to 8.25 times. Lead has the second-lowest mobility in the soil horizon after mercury44. Nevertheless, the effect of a high concentration of its mobile form in the soil was demonstrated (Table 2). The mercury content was lower than ML (0.03 mg/kg FW) in tomatoes and carrots from all plots. In only one case (carrots from plot E), the ML was exceeded—by almost 400%. On the other hand, the lowest Hg concentration was determined in potatoes from this plot. In the samples from plot D (A, B, C), the concentration of Hg was 1.25 (1.78, 2.25, 5.25, respectively) times higher than the determined ML of mercury for potatoes (0.02 mg/kg FW).
Similarly, increased Pb, Zn, Hg, Cu, and Cd levels in carrots and potatoes were reported by Miller et al.59. Water and agricultural land contamination are caused by mining activity in Cerro Rico de Potosí (Southern Bolivia). Higher contents of Ni (0.1–2.5 mg/kg FW) and Hg (0.001–0.116 mg/kg FW) were determined by Yeganeh et al.88 in potatoes grown in Hamedan province (northwest of Iran). The Ni (Hg) content in soil was 26–140 (0.054–0.316) mg/kg. In vegetable samples grown locally in the suburban of Isfahan city (Iran), the content of Pb in carrots was up to 7.14 (Cd up to 2.91) mg/kg DW and in potatoes—Pb up to 7.14 (Cd up to 0.67) mg/kg DW60. Rayhan Khan et al.61, based on selected publications from 2015 to 2020, assessed heavy metal levels in more than 20 types of vegetables available in Bangladesh. The Mn (Fe, Ni, Cu, Zn, Cd, Pb) content was up to 210.70 (2362.56, 37.52, 45.00, 174.60, 240.00, 31.1, respectively) mg/kg. Compared to the FAO/WHO standard, the safe limit was exceeded for all listed elements except Mn.
Bioaccumulation factor (BAF)
Bioaccumulation factor refers to the ratio of heavy metal content in the plant to its content in the soil62, which reflects the plant’s ability to absorb heavy metal58. The BAF values, minimum and maximum values calculated for potatoes, carrots, and tomatoes grown on individual plots, are given in Table 9.
For plant and animal health, BAF > 1 is considered to be a hazardous value for heavy metals63. In the evaluation, we considered maximum BAF values for individual metals and crops. This value has been exceeded in the case of Cu in carrots and potatoes from plot D, which may already pose a risk to the consumer. In carrots, the highest BAF value was for Zn. Overall, these two metals (Cu and Zn) had the highest BAF values and, for other elements, there was no clear tendency to decrease BAF, but the lowest value was for Fe (carrot > potato > tomato). In this order, the BAF value decreased for Mn and Cu. Potatoes had the highest ability to accumulate Pb and tomatoes had the lowest. Authors5,36,64 confirmed different absorption capacities for different crops. However, Hu et al.5 further stated that Cd was most easily absorbed by crops, while Pb was identified as having the lowest accumulation in crops. In our case, the cadmium contents in all potato and tomato samples (and three carrot samples) were below the detection limit. Therefore, the BAF value for Cd was not evaluated.
Human health risk assessment
Estimated daily intake (EDI)
The daily intake of metals depends on the concentration of metals in the food and the daily food consumption. In addition, a person's body weight can affect contaminant tolerance65. The estimated daily intake (EDI) of the heavy metals of interest (Fe, Mn, Zn, Cu, Ni, Pb, Cd, and Hg) were calculated based on their average concentration in the three vegetables (potato, carrot, tomato) and daily intake of the vegetable (Table 11). Data on the consumption of individual vegetables were obtained from Meravá66 (Table 10).
The data obtained on the estimated daily intake showed that, in terms of heavy metal intake, potatoes are the riskiest of the monitored crops. The highest EDI values were in the case of Fe and decreased in the order of Fe > Zn = Cu > Mn > Pb > Hg. In the case of Ni and Cd, the highest EDI is through a carrot. EDI values were compared with TDI (TWI, PTWI, PMTDI, UL), which were expressed as tolerable daily intake from the baseline data for easier comparison (Table 11). For zinc, the value determined for pregnant and lactating women was used, and for lead, the PTWI value of 25 μg/kg bw/week was used for comparison. This data was used even though the CONTAM Panel concluded that the current PTWI of 25 μg/kg bw is no longer appropriate as there is no evidence for a threshold for critical lead-induced effects. In adults, children, and infants, the margins of exposures were such that the possibility of an effect from lead in some consumers, particularly in children from 1 to 7 years of age, cannot be excluded. Protection of children against the potential risk of neurodevelopmental effects would be protective for all other adverse effects of lead in all populations67. Furthermore, the CONTAM Panel set the TWI for inorganic mercury at 4 µg/kg body weight (bw), which is in accordance with EFSA68.
Although the heavy metal contents were increased in all three crops and the limit values for these elements were exceeded for Cu, Pb, and Hg, the EDI values for all heavy metals were several times lower (min. TDI/EDI(Pb) = 2.06, max TDI/EDI(Cu) = 40), as determined by the tolerable daily intake. In thirteen Jamaican-grown food crops in which Al, As, Cd, and Pb were determined, the EDI for Pb (Cd) had lower values compared to our results: 0.004 (0.032), 0.002 (0.137), and 0.009 (0.116) μg/day/kg body weight42. Higher EDI values for cadmium (0.61–1.13) and, on the contrary, lower for Pb (0.27–0.47) calculated based on consumption of rice seeds of paddy fields in southwest of Iran reported Chamannejadian et al.65.
Chronic daily intake (CDI)
Chronic daily intake is the exposure expressed as the weight of the contacted substance per unit body weight per unit time, averaged over a long period (seven years to a lifetime)69. In our calculation, the period was expressed as 70 years. CDI values (Table 12) ranged from 2.495E−06 (Hg) to 0.1416 (Fe). The highest CDI values for all heavy metals except Ni and Cd were calculated for potatoes and corresponded to the highest EDI values (Table 11).
Based on estimates of current iron intake in European countries, the risk of side effects of high Fe intake from food sources, including fortified foods in some countries (but without supplements), is considered low for the general population. Foods rich in total iron include liver and offal, game, and beef; medium to high amounts of Fe also contain cereals, cereal products, and legumes. For the general population, food is the most important source of manganese exposure, but its concentrations vary considerably70. Cereals make up the major proportion of Mn in the diet (57%), followed by fruit, vegetables, nuts, and tea71,72. The main food groups contributing to zinc intake are meat and meat products, cereals and cereal-based products, milk, and dairy products70,73. Foods that contribute most to copper intake include cereals and cereal-based products, meat and meat products74, seafood, nuts, and seeds70. The main sources of Ni in the diet are cereals and cereal-based products, soft drinks (except milk-based beverages), sugar and confectionery, legumes, nuts and oilseeds, vegetables, and vegetable products. The CONTAM Panel concluded that dietary exposure probably represents the most important contribution to the overall Ni exposure in the general population75. The food groups that contributed to most of the dietary exposure to cadmium, mainly due to high consumption, were cereals and cereal-based products, vegetables, nuts, legumes, potatoes, meat, and meat products (EFSA 2009). Cereals and cereals, potatoes, vegetables, and tap water contributed to lead food exposure in the general European population but found no necessary data on bioaccumulation in the food chain67,76.
A survey of raw food in Germany in 1986 found that cereals, potatoes, vegetables, and fruits had average mercury concentrations of 0.005–0.05 mg/kg (ppm fresh weight), cocoa beans, tea leaves, and coffee beans contained average mercury concentrations of 0.005, 0.025, and 0.04 mg/kg, respectively77.
Target hazard quotient (THQ), hazard index (HI)
THQ is defined as the ratio of exposure to a toxic element and reference dose, which is the highest level at which no adverse health effects are expected. Reference dose (RfD) is specific for each trace element examined. The RfD values used in the calculation of THQ are given in Table 13. Hazard index (HI) is the sum of the individual target hazard quotients of the elements evaluated for each type of food42.
THQ values were calculated for each element and each vegetable as well as, HI values were calculated for all three vegetables.
Significantly higher THQ values were in potatoes from all five plots. Apart from Cd (where the Cd content was below the detection limit in the samples from all plots and Ni in the sample from plot A), the lowest values of Ni (from 3.521E−05 plot D to 0.01373 plot B) < Zn (0.0207 plot A–0.0417 plot E) < Fe (0.0518 plot D–0.2023 plot C, except plot B) (Figs. 2, 3, 4, 5 and 6). For other heavy metals, their order is ambiguous. The highest THQ values were for Pb (plot A: 0.3818, plot D: 0.3916, plot E: 0.4808), Mn (plot B: 0.3345), and Hg (plot C: 0.7363). Based on the results, it can be stated that the THQ of Ni, Zn, and Fe through vegetable consumption were much lower than THQ values of other metals. The THQ values of all analyzed metals were < 1.
The target hazard quotient was recognized as a useful parameter to assess the risk associated with the consumption of metal-contaminated foods78. THQ describes the non-carcinogenic health risk posed by exposure to the relevant toxic element. If THQ < 1, no non-carcinogenic health effects are expected. However, if THQ > 1, there is a possibility that adverse health effects may occur. THQ exceeding 1 statistically does not represent a probability of occurrence of adverse non-carcinogenic health effects5,38,79.
Hazard index represents the cumulative effect of consuming several potentially hazardous elements. HIs for the eight heavy metals (Fe, Mn, Zn, Cu, Ni, Pb, and Hg) were evaluated in terms of the consumption of three vegetables (potato, carrot, and tomato). It is potatoes that can be characterized as a hazardous crop, as HI > 1 (for all plots) (Fig. 7). Other HIs ranged from 0.1001 (tomato, plot A) to 0.54.14 (carrot, plot E). Pb (25.6–39.3%), Hg (29.8–46.1%), and Mn (27.9–31.4%) contribute the most to the HI values.
HI anticipates that the consumption of a particular type of food would result in simultaneous exposure to several potentially toxic elements. Although the individual THQs for elements in food are less than one individual, the cumulative effect of consumption can have an adverse health effect. If HI > 1, there is a possibility of adverse non-carcinogenic effects on health42.
Increased intake of monitored heavy metals can have a negative impact on the health or even the life of the consumer. Environmental factors that may increase cancer risk in humans are identified in the monograph program, which is a key part of The International Agency for Research on Cancer (IARC). The IARC is a specialized agency for cancer of the World Health Organization (WHO). Metallic mercury and inorganic mercury compounds are not classifiable as to their carcinogenicity to humans (Group 3)24. IARC evaluated Ni (metallic)80 and Pb81 as possibly carcinogenic to humans (Group 2B), and Cd (cadmium compounds) as carcinogenic to humans (Group 1)82.
Presented results point to potential risks for the consumer resulting from soil contamination caused by old mining activities. For a more accurate evaluation, it would be suitable to extend the research by involving other crops, increasing the number of sampling points, and focusing on other elements (As, Mn, Se, etc.) that also may represent a risk for the consumer.
Conclusion
One of the factors that adversely affect the environment is the negative impact of old environmental burdens. The Spiš region is one of the burdened areas in Slovakia due to the old, in many cases, already terminated mining activities. Concentrations of heavy metals in the soil have, in many cases, exceeded their maximum permissible levels in their pseudototal (Fe, Zn, Cu, Cd, and Hg) or bioavailable (Pb, Cd) form, compared to current legislation. The risk of soil contamination by monitored heavy metals is also confirmed by high values of soil pollution indices. All plots can be classified as very high contaminated with cadmium and mercury (based on Cf), resp. all heavy metals (according to Cdeg). The riskiest element for all plots, according to Igeo, is mercury. Based on the PLI, the pollution level can be characterized as moderately polluted to unpolluted. Increased levels of heavy metals in the soil were reflected in their accumulation in vegetables, especially potatoes. This crop also had the highest Estimated daily intake, Chronic daily intake, Target hazard quotient, and Hazard index. The obtained results showed the importance of soil and crop monitoring, especially in high-risk areas such as the Spiš region.
Data availability
All basic data supporting the results of this study are available from the corresponding author.
References
Qarri, F. et al. The evaluation of air quality in Albania by moss biomonitoring and metals atmospheric deposition. Archiv. Environ. Contam. Toxic. 76, 554–571. https://doi.org/10.1007/s00244-019-00608-x (2019).
Takáč, P., Kozáková, L., Vaľková, M. & Zeleňák, F. Heavy metals in soils in middle Spish. Acta Montan. Slovaca. 13, 82–86. https://actamont.tuke.sk/pdf/2008/n1/11takac.pdf (2008).
Ali, H., Khan, E. & Ilahi, I. environmnetal chemistry and ecotoxicology of hazardous heavy metals: Environmental persistence, toxicity, and bioaccumulation. J. Chem. 2019, 6730305. https://doi.org/10.1155/2019/6730305 (2019).
Beccaloni, E., Vanni, F., Beccaloni, M. & Carere, M. Concentrations of arsenic, cadmium, lead and zinc in homegrown vegetables and fruits: Estimated intake by population in an industrialized area of Sardinia Italy. Microchem. J. 107, 190–195. https://doi.org/10.1016/j.microc.2012.06.012 (2013).
Hu, B. et al. Assessment of heavy metals pollution and health risks in the soil-plant-human system in the Yangtze River Delta, China. Int. J. Environ. Res. Public Health 14, 28891954. https://doi.org/10.3390/ijerph14091042 (2017).
Le Briat, J. F. fer du sol aux produits végétaux. Bull. Acad. Natl. Med. 189, 1609–1621. https://doi.org/10.1016/S0001-4079(19)33419-3 (2005).
Eid, R., Arab, N. T. & Greenwood, M. T. Iron mediated toxicity and programmed cell death: A review and a re-examination of existing paradigms. Biochim. Biophys. Acta Mol. Cell Res. 1864, 399–430. https://doi.org/10.1016/j.bbamcr.2016.12.002 (2017).
Brewer, G. J. Risks of copper and iron toxicity during aging in humans. Chem. Res. Toxicol. 23, 319–326. https://doi.org/10.1021/tx900338d (2010).
Murtić, S., Zahirović, Ć., Jurković, J., Karić, L. & Koleška, I. Essential heavy metals accumulation and distribution pattern in cucumber plants. Studia Univ. Vasile Goldis Arad, Ser. Stiintele Vietii. 29, 177–183. http://www.studiauniversitatis.ro/pdf/29-2019/29-4-2019/Art.%204%20Essential%20heavy%20metals%20accumulation%20and%20distribution%20pattern%20in%20cucumber%20plants.pdf (2019).
Queiroz, H. M. et al. Manganese: The overlooked contaminant in the world largest mine tailings dam collapse. Environ. Int. 146, 106284. https://doi.org/10.1016/j.envint.2020.106284 (2021).
Nriagu, J. O. Zinc Toxicity in Humans in Encyclopedia of environmental health. (ed. Nriagu, J. O.) 801–807 (Elsevier, 2011).
Plum, L. M., Rink, L. & Haase, H. The essential toxin: Impact of zinc on human health. Int. J. Environ. Res. Public Health 7, 1342–1365. https://doi.org/10.3390/ijerph7041342 (2010).
Borah, P. et al. Assessment of mobility and environmental risks associated with copper, manganese and zinc in soils of a dumping site around a Ramsar site. Chemosphere 254, 126852. https://doi.org/10.1016/j.chemosphere.2020.126852 (2020).
Anant, J. K., Inchulkar, S. R. & Bhagat, S. An overview of copper toxicity relevance to public health. EJPMR, 5, 232–237. https://storage.googleapis.com/journal-uploads/ejpmr/article_issue/1540969737.pdf (2018).
Genchi, G., Carocci, A., Lauria, G., Sinicropi, M. S. & Catalano, A. Nickel: Human health and environmental toxicology. Int. J. Environ. Res. Public Health. 17, 679. https://doi.org/10.3390/ijerph17030679 (2020).
Matović, V., Buha, A., Ðukić-Ćosić, D. & Bulat, Z. Insight into the oxidative stress induced by lead and/or cadmium in blood, liver and kidneys. Food Chem. Toxicol. 78, 130–140. https://doi.org/10.1016/j.fct.2015.02.011 (2015).
NORD (National Organization for Rare Disorders). Rare Disease Database. Heavy Metal Poisoning. Lead Poisoning. https://rarediseases.org/rare-diseases/heavy-metal-poisoning/. Accessed 23 Feb 2022. (2021).
Baldantoni, D., Morra, L., Zaccardelli, M. & Alfani, A. Cadmium accumulation in leaves of leafy vegetables. Ecotoxicol. Environ. Saf. 123, 89–94. https://doi.org/10.1016/j.ecoenv.2015.05.019 (2016).
Irfan, M., Hayat, S., Ahmad, A. & Alyemeni, M. N. Soil cadmium enrichment: Allocation and plant physiological manifestations. Saudi J. Biol. Sci. 20, 1–10. https://doi.org/10.1016/j.sjbs.2012.11.004 (2013).
Ali, E. et al. Cadmium phytotoxicity: issues, progress, environmental concerns and future perspectives. Rev Fac Cienc Agrar. 52, 391–405. https://revistas.uncu.edu.ar/ojs/index.php/RFCA/article/view/3068/2224 (2020).
Rasafi, E. T. et al. Cadmium stress in plants: A critical review of the effects, mechanisms, and tolerance strategies. Crit. Rev. Environ. Sci. Technol. 52, 1–52. https://doi.org/10.1080/10643389.2020.1835435 (2020).
EC (European Commission). Food Safety. Cadmium. https://ec.europa.eu/food/safety/chemical-safety/contaminants/catalogue/cadmium_en Accessed 23 Feb 2022.
Ismael, M. A. et al. Cadmium in plants: Uptake, toxicity, and its interactions with selenium fertilizers. Metallomics 11, 255–277. https://doi.org/10.1039/c8mt00247a (2019).
IARC (International Agency for Research on Cancer). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Beryllium, Cadmium, Mercury, and Exposures in the Glass Manufacturing Industry. https://publications.iarc.fr/76 (1993).
Gworek, B., Dmuchowski, W. & Baczewska-Dąbrowska, A. H. Mercury in the terrestrial environment: A review. Environ. Sci. Eur. 32, 1–19. https://doi.org/10.1186/s12302-020-00401-x (2020).
Azevedo, R. & Rodriguez, E. Phytotoxicity of mercury in plants: a review. J. Bot. https://doi.org/10.1155/2012/848614 (2012).
WHO (World Health Organization). Mercury and health. https://www.who.int/news-room/fact-sheets/detail/mercury-and-health. Accessed 23 Feb 2022. (2017).
Lapin, M., Fasko, P., Melo, M., Štastný, P. & Tomlain, J. Landscape Atlas of the Slovak Republic. 1st ed. Bratislava: Ministry of Environment of the Slovak Republic; Banská Bystrica: Slovak Environmental Agency. 344 p. (in Slovak) (2002).
Bedrna, Z. & Jenčo, M. Spatial Laws Pedosphere Differentiation. Published by Comenius University in Bratislava at Charles University Publishing House. (in Slovak) (2016).
Societas pedologica slovaca. Morphogenetic Classification System of Slovak Soils. Basal Reference Taxonomy. NPPC-VUPOP Bratislava. (in Slovak) (2014).
Act No. 220/2004. On the Protection and Use of Agricultural Land. National Council of Slovak Republic, Bratislava. https://www.lecol.sk/images/stories/lecol/220_2004.pdf (in Slovak) (2004).
EU 2021/1323. Commission Regulation (EU) 2021/1323 of 10 August 2021 amending Regulation (EC) No 1881/2006 as regards maximum levels of cadmium in certain foodstuffs. Official Journal of the European Union. https://eur-lex.europa.eu/eli/reg/2021/1323/oj (2021).
EU 2021/1317. Commission Regulation (EU) 2021/1317 of 9 August 2021 amending Regulation (EC) No 1881/2006 as regards maximum levels of lead in certain foodstuffs. Official Journal of the European Union. https://eur-lex.europa.eu/eli/reg/2021/1317/oj (2021).
Regulation SR 18558/2006. Maximum level of contaminants in food. Annex to Decree no. 18558/2006-SL. Regulation of the Ministry of Agriculture of the Slovak Republic. 7 p. https://www.svps.sk/dokumenty/legislativa/18558_2006.pdf (in Slovak) (2006).
Regulation SR 414/2003–100. Contaminants in food. Annex no. 1 to the third title of the second part of the Food Codex. Regulation of the Ministry of Agriculture of the Slovak Republic. https://www.mpsr.sk/resources/bulletin/vest8_1_2003.pdf (in Slovak) (2003).
Antoniadis, V. et al. Trace elements in the soil-plant interface: Phytoavailability, translocation, and phytoremediation—A review. Earth Sci. Rev. 171, 621–645. https://doi.org/10.1016/j.earscirev.2017.06.005 (2017).
Islam, Md. S., Ahmed, Md. K., Habibullah-Al-Mamun, Md. & Masunaga, S. Potential ecological risk of hazardous elements in different land-use urban soils ob Bangladesh. Sci. Total Environ. 512–513, 94–102. https://doi.org/10.1016/j.scitotenv.2014.12.100 (2015).
Li, Z., Ma, Z., van der Kuijp, T. J., Yuan, Z. & Huang, L. A review of soil heavy metal pollution from mines in China: Pollution and health risk assessment. Sci. Total Environ. 468–469, 843–853. https://doi.org/10.1016/j.scitotenv.2013.08.090 (2014).
Mgbemena, N. M., Ilechukwu, I., Ubadi, J. O. & Ndukwe, G. I. Status of Heavy Metals Pollution in the Sediments of the New Calabar River in Rivers State, Nigeria. J. Chem. Soc. Nigeria 42, 84–87. https://www.researchgate.net/publication/319755630 (2017).
Abowaly, M. E. et al. Assessment of soil pollution levels in North Nile Delta, by integrating contamination indices, GIS, and multivariate modeling. Sustainability. 13, 8027. https://doi.org/10.3390/su1314802 (2021).
Rashed, M. N. Monitoring of contaminated toxic and heavy metals, from mine tailings through age accumulation, in soil and some wild plants at Southeast Egypt. J. Hazard. Mater. 178, 739–746. https://doi.org/10.1016/j.jhazmat.2010.01.14 (2010).
Antoine, J. M., Fung, L. A. H. & Grant, C. N. Assessment of the potential health risks associated with the aluminium, arsenic, cadmium and lead content in selected fruits and vegetables grown in Jamaica. Toxicol. Rep. 4, 181–187. https://doi.org/10.1016/j.toxrep.2017.03.006 (2017).
Onyele, O. G. & Anyanwu, E. D. Human health risk assessment of some heavy metals in a rural spring, southeastern Nigeria. AJENSR. 1, 15–23. https://abjournals.org/ajensr/wp-content/uploads/sites/15/journal/published_paper/volume-1/issue-1/AJENSR_xUItsoHS.pdf (2018).
Alloway, B. J. Sources of Heavy Metals and Metalloids in Soils in Heavy Metals in Soils (eds. Alloway, B. J. & Trevors, J. T.) 12–15 (Springer, 2010).
Liao, G. L., Liao, D. X. & Li, Q. M. Heavy metals contamination characteristics in soil of different mining activity zones. Trans. Nonferrous Met. Soc. China. 18, 207–211. https://doi.org/10.1016/S1003-6326(08)60037-0 (2008).
Hirwa, H. et al. Evaluation of soil contamination in mining areas of Rwanda. AJWSE 5, 9–15. https://doi.org/10.11648/j.ajwse.20190501.12 (2019).
Okonkwo, S. I., Idakwo, S. O. & Ameh, E. G. Heavy metal contamination and ecological risk assessment of soils around the pegmatite mining sites at Olode area, Ibadan southwestern Nigeria. Environ. Nanotechnol. Monit. Manag. 15, 100424. https://doi.org/10.1016/j.enmm.2020.100424 (2021).
Bislimi, K., Halili, J., Sahiti, H., Bici, M. & Mazreku, I. Effect of Mining Activity in Accumulation of Heavy Metals in Soil and Plant (Urtica dioica L). J. Ecol. Eng. 22, 1–7. https://doi.org/10.12911/22998993/128691 (2021).
Kowalska, J. B., Mazurek, R., Gąsiorek, M. & Zaleski, T. Pollution indices as useful tools for the comprehensive evaluation of the degree of soil contamination–A review. Environ Geochem Health. 40, 2395–2420. https://doi.org/10.1007/s10653-018-0106-z (2018).
Linkeš, V. et al. Soil monitoring of Slovak republic present state of monitored soil properties. https://www.enviroportal.sk/uploads/2011/08/page/informacny-system-zp/cms_3/PODA/monitoringpod.pdf (1997).
He, T. L., Shentu, J. & Yang, X. E. Manganese and Selenium in Trace Elements in Soil (ed. Hooda, P. S.) 481–495 (Wiley, 2010).
Demková, L. et al. Evaluation of soil and ambient air pollution around un-reclaimed mining bodies in Nižná Slaná (Slovakia) post-mining area. Toxics 8, 33137994. https://doi.org/10.3390/toxics8040096 (2020).
Hakanson, L. An ecological risk index for aquatic pollution control: A sedimentological approach. Water Res. 14, 975–1001. https://doi.org/10.1016/0043-1354(80)90143-8 (1980).
Fazekašová, D. et al. Soil contamination in the problem areas of agrarian Slovakia. Land 10, 1248. https://doi.org/10.3390/land10111248 (2021).
Ullah, R. & Muhammad, S. Heavy metals contamination in soil and plants along with the mafic–ultramafic complex (Ophiolites), Baluchistan, Pakistan: Evaluation for the risk and phytoremediation potential. Environ. Technol. Innov. 19, 100931. https://doi.org/10.1016/j.eti.2020.100931 (2020).
Wu, J., Teng, Y., Lu, S., Wang, Y. & Jiao, X. Evaluation of soil contamination indices in a mining area of Jiangxi China. PLoS ONE 9, e112917. https://doi.org/10.1371/journal.pone.0112917 (2014).
Luo, W. et al. Effects of land use on concentrations of metals in surface soils and ecological risk around Guanting Reservoir China. Environ. Geochem. Health. 29, 459–471. https://doi.org/10.1007/s10653-007-9115-z (2007).
Yakun, S., Xingmin, M., Kairong, L. & Hongbo, S. Soil characterization and differential patterns of heavy metal accumulation in woody plants grown in coal gangue wastelands in Shaanxi China. Environ. Sci. Pollut. Res. 23, 13489–13497. https://doi.org/10.1007/s11356-016-6432-8 (2016).
Miller, J. R., Hudson-Edwards, K. A., Lechler, P. J., Preston, D. & Macklin, M. G. Heavy metal contamination of water, soil and produce within riverine communities of the Rıo Pilcomayo basin Bolivia. Sci. Total Environ. 320, 189–209. https://doi.org/10.1016/j.scitotenv.2003.08.011 (2004).
Jafarian-Dehkordi, A. & Alehashem, M. Heavy metal contamination of vegetables in Isfahan, Iran. Res. Pharm. Sci. 8, 51. https://pubmed.ncbi.nlm.nih.gov/24459476/ (2013).
Rayhan Khan, M. & Hosna Ara, M. A review on heavy metals in vegetables available in Bangladesh. J. Hum. Environ. Health Promot. 7, 108–119. https://doi.org/10.52547/jhehp.7.3.108 (2021).
Fan, Y., Li, Y., Li, H. & Cheng, F. Evaluating heavy metal accumulation and potential risks in soil-plant systems applied with magnesium slag-based fertilizer. Chemosphere 197, 382–388. https://doi.org/10.1016/j.chemosphere.2018.01.055 (2018).
Bhatti, S. S. et al. Physico-chemical Properties and Heavy Metal Contents of Soils and Kharif Crops of Punjab India. Proc. Environ. Sci. 35, 801–808. https://doi.org/10.1016/j.proenv.2016.07.096 (2016).
Kacholi, D. S. & Sahu, M. Levels and health risk assessment of heavy metals in soil, water, and vegetables of Dar es Salaam Tanzania. J. Chem. 2018, 1402674. https://doi.org/10.1155/2018/1402674 (2018).
Chamannejadian, A., Sayyad, G., Moezzi, A. & Jahangiri, A. Evaluation of estimated daily intake (EDI) of cadmium and lead for rice (Oryza sativa L.) in calcareous soils. Iran. J. Environ. Health Sci. Eng. 10, 1–5. https://doi.org/10.1186/1735-2746-10-28 (2013).
Meravá, E. Situation and outlook reports—Fruit ansd vegetables (Situačná a výhľadová správa – Ovocie a zelenina). Research institute of agricultural and food economics. https://www.vuepp.sk/04_komodity.htm (in Slovak) (2021).
EFSA (European Food Safety Authority). Scientific opinion on lead in food. EFSA J. https://doi.org/10.2903/j.efsa.2010.1570 (2010).
EFSA (European Food Safety Authority). Scientific Opinion on the risk for public health related to the presence of mercury and methylmercury in food. EFSA J. https://www.efsa.europa.eu/en/efsajournal/pub/2985 (2012).
EPA (United States Environmental Protection Agency). Risk Assessment Guidance for Superfund (RAGS): Part A. 12/1989, EPA/540/1-89/002. https://www.epa.gov/risk/risk-assessment-guidance-superfund-rags-part (1989).
EFSA (European Food Safety Authority). Tolerable upper intake levels for vitamins and minerals. Scientific Panel on Dietetic Products, Nutrition and Allergies. https://www.efsa.europa.eu/sites/default/files/efsa_rep/blobserver_assets/ndatolerableuil.pdf2 (2006).
EFSA (European Food Safety Authority). Scientific Opinion on Dietary Reference Values for manganese. EFSA J. https://www.efsa.europa.eu/en/efsajournal/pub/3419 (2013).
VKM Report. Assessment of dietary intake of manganese in relation to tolerable upper intake level. Norwegian Scientific Committee for Food and Environment (VKM). VKM Rep. 02, 32 (2018).
EFSA (European Food Safety Authority). Scientific Opinion on Dietary Reference Values for zinc. EFSA J. https://www.efsa.europa.eu/en/efsajournal/pub/3844 (2014).
EFSA (European Food Safety Authority). Scientific Opinion on Dietary Reference Values for copper. EFSA Journal. 2015. https://www.efsa.europa.eu/en/efsajournal/pub/4253 (2015).
EFSA (European Food Safety Authority). Scientific Opinion on the risks to public health related to the presence of nickel in food and drinking water. EFSA J. https://doi.org/10.2903/j.efsa.2015.4002 (2015).
ATSDR (Agency for Toxic Substances and Disease Registry). Toxicological profile for lead. 2020. https://www.atsdr.cdc.gov/toxprofiles/tp13.pdf (2020).
ATSDR (Agency for Toxic Substances and Disease Registry). Toxicological profile for mercury. 1999. https://www.atsdr.cdc.gov/ToxProfiles/tp46.pdf (1999).
Dee, K. H. et al. Health risk assessment of heavy metals from smoked Corbicula fluminea collected on Roadside Vendors at Kelantan, Malaysia. Biomed Res. Int. 2019. https://doi.org/10.1155/2019/9596810 (2019).
Yang, L. et al. The impact of greenhouse vegetable farming duration and soil types on phytoavailability of heavy metals and their health risk in eastern China. Chemosphere 103, 121–130. https://doi.org/10.1016/j.chemosphere.2013.11.047 (2014).
IARC (International Agency for Research on Cancer). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Chromium, Nickel and Welding. 1990. https://publications.iarc.fr/67 (1990).
IARC (International Agency for Research on Cancer). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. OveraIl Evaluations of Carcinogenicity: An Updating of IARC Monographs. 1987. 1–42. 449 p. https://monographs.iarc.who.int/wp-content/uploads/2018/06/Suppl7.pdf (1987).
WHO (World Health Organization). Preventing disease through healthy environments exposure to cadmium: A major public health concern. WHO: Geneva, Switzerland. 2010. https://www.who.int/ipcs/features/cadmium.pdf (2010).
EFSA (European Food Safety Authority). Risk evaluation of chemical contaminants in food in the context of RASFF notifications. 2019. https://www.efsa.europa.eu/en/supporting/pub/en-1625 (2019).
Mohamed, H., Haris, P. I. & Brima, E. I. Estimated dietary intake of essential elements from four selected staple foods in Najran City Saudi Arabia. BMC Chem. 13, 1–10. https://doi.org/10.3390/ijerph14121575 (2019).
FRAMEWORK. For the Management of Contaminated Land. Environmental Affairs, Republic of South Africa. 2010. http://sawic.environment.gov.za/documents/562.pdf (2010).
EPA (United States Environmental Protection Agency). Provisional Peer Reviewed Toxicity Values for Iron and Compounds (CASRN 7439-89-6). 2006. https://cfpub.epa.gov/ncea/pprtv/documents/IronandCompounds.pdf (2006).
Varol, M. Assessment of heavy metals contamination in sediments of the Tigris River (Turkey) using pollution indices and multivariate statistical techniques. J. Hazard. Mater. 195, 21890271. https://doi.org/10.1016/j.jhazmat.2011.08.051 (2011).
Yeganeh, M. et al. Mapping of human health risks arising from soil nickel and mercury contamination. J. Hazard. Mater. 244, 225–239. https://doi.org/10.1016/j.jhazmat.2012.11.040 (2013).
Acknowledgements
This work was financially supported by project VEGA 1/0722/19. The authors would like to thank MSc. Ďuršová (Poráč) for the provided samples.
Funding
Experimental research and field studies on plants, complies with relevant national guidelines and legislation (Decree of the Ministry of Agriculture and Rural Development of the Slovak Republic, which establishes the details of agrochemical testing of soils and storage and use of fertilizers 151/2016).
Author information
Authors and Affiliations
Contributions
Conceptualization: J.M., J.C.h.; methodology: A.V., J.L.; validation: J.A., J.U.; formal analysis: H.F., Ľ.H.; writing-original draft preparation, review, and editing: J.M., H.F.; funding acquisition: J.M., T.T. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Musilová, J., Franková, H., Lidiková, J. et al. Impact of old environmental burden in the Spiš region (Slovakia) on soil and home-grown vegetable contamination, and health effects of heavy metals. Sci Rep 12, 16371 (2022). https://doi.org/10.1038/s41598-022-20847-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-20847-8
This article is cited by
-
Mercury in the feathers of Golden eagle (Aquila chrysaetos) from Western Carpathian, Slovakia
Environmental Science and Pollution Research (2024)
-
Evaluation of cadmium uptake and consumption of parsley in Lebanese diet
International Journal of Environmental Science and Technology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.