Abstract
Transinguinal preperitoneal (TIPP) single-layer mesh herniorrhaphy has been proven effective. Mesh manufacturers make either a single-unit, two-layer mesh design or a separate optional onlay with the pre-peritoneal mesh. For peace of mind, most surgeons still incorporate the optional onlay. This study evaluated any counterproductive effects of adding the onlay to single-layer TIPP mesh herniorrhaphy and compared the long-term efficacy. This prospective, single-surgeon, single-center, randomized trial compared two groups of 50 consecutive patients at a 1 to 1 ratio. The control group received a single-layer modified Kugel mesh in the preperitoneal space, while the study group received the optional onlay mesh in the inguinal canal with preperitoneal mesh placement. A single surgeon performed the same operation to place the preperitoneal mesh in both groups, the only difference being the placement of the optional onlay mesh in the study group. A blinded researcher performed post-operative interviews using a series of questions at 1, 3, 6, and 12 months after surgery, and another unblinded researcher organized and performed statistical analysis of the peri-operative and post-operative data. The primary endpoints included foreign body sensation, pain, and any other discomfort in the inguinal region following surgery; and the secondary endpoints included recurrence and any complications related to surgery. The patient demographics were similar between the two groups. The average follow-up period was 29 months. Two patients in the 1-layer group and one patient in the 2-layer group were lost to follow-up. Postoperative pain, numbness and soreness were similar between groups. No patients experienced a foreign body sensation after 3 months in the 1-layer group, while five patients still had a foreign body sensation at 12 months in the 2-layer group. No recurrence was noted in either group during the follow-up period. Adequate dissection of the preperitoneal space is the key to a successful single-layer TIPP herniorrhaphy. With decreased materials in the inguinal canal, single-layer TIPP has a lower rate of long-term postoperative discomfort without increasing the risk of future recurrence.
Trial registration: ISRCTN 47111213
Similar content being viewed by others
Introduction
Before the introduction of polypropylene mesh, tissue repair was the main form of herniorrhaphy, but a poor long-term efficacy (> 10% recurrence in 10 years) and immediate postoperative pain were the main drawbacks1,2. The invention of polypropylene mesh improved the recurrence rate but increased post-herniorrhaphy chronic pain (> 3 months). Due to simplicity, Lichtenstein mesh repair is easy to learn, but post-herniorrhaphy chronic pain is a major drawback, with some studies reporting an incidence of up to 40%3,4. Explanations for the high rate of chronic herniorrhaphy pain include periosteum damage during mesh positioning, nerve damage (due to stretching, division, compression, electrocautery injury) or inflammation from surrounding mesh materials3,4,5,6. Careful nerve dissection and manipulation during inguinal canal preparation before mesh placement can help to reduce the chance of chronic pain, but mesh placed in the vicinity of inguinal canal nerves can cause inflammation, fibrosis and granuloma formation7,8,9. Mesh manufacturers either make a single-unit, two-layer design (i.e., UHS and PHS mesh by Ethicon US, LLC) or a preperitoneal mesh with a separate optional onlay mesh to strengthen the posterior wall (i.e., PerFix Plug by Davol Inc. or ULTRAPRO Plug by Ethicon US, LLC). Some surgeons performing transinguinal preperitoneal (TIPP) mesh repair routinely place the optional onlay in the inguinal canal for fear of future recurrence. In our retrospective study, we noted that 1-layer preperitoneal mesh placement did not put patients at risk of future recurrence (< 1%). The key to TIPP mesh placement is adequate dissection of the preperitoneal space, which results in flat mesh placement covering the hernia defects. Most recent studies have compared different types of mesh and different mesh placements. In this prospective study, we investigated whether the use of the optional onlay mesh included in the modified Kugel mesh is associated with long-term complications and examined the efficacy of single-layer TIPP herniorrhaphy in terms of preventing recurrence.
Methods/design
Ethics and informed consent
The institutional review board at Changhua Christian Hospital approved the trial protocol (IRB number 140312) of this study. The trial was registered online with ISRCTN (online registry number: 47111213 assigned on: 2017/03/21). In the preoperative outpatient clinic, all participants were informed in detail about the method of randomization and surgical method before informed consent was obtained according to the IRB protocol.
Study population
The study population included patients undergoing elective inguinal hernia surgery using MK mesh at Changhua Christian Hospital. The exclusion criteria included: (1) recurrent hernia, (2) femoral hernia, (3) hernia defects larger than the Posiflex® memory ring diameter, and (4) patients refusing to participate in the randomization protocol. Of the 110 patients eligible during the study period, seven patients did not meet the inclusion criteria and three patients refused to participate in the study. One-hundred patients were allocated into two groups, with 50 patients in each group. During the follow-up period, two patients in the 1-layer group and two in the 2-layer group were lost to follow-up due to changes in telephone number.
Study endpoints
This randomized, prospective clinical trial aimed to investigate the reduction in post-operative complications associated with mesh herniorrhaphy and determine the long-term effectiveness of single-layer preperitoneal mesh placement as compared with two-layer. The primary endpoint was to evaluate patients’ chronic postoperative complications (chronic pain, neuropathy, foreign body sensation). The secondary objective was to determine the overall success of the hernia operation (recurrence, perioperative and early postoperative complications).
Study design, patient randomization and blinding
This was a prospective, double-blind (patients and observer blinded), single-center, randomized study with a two-arm parallel-group design. A series of 100 consecutive patients undergoing elective inguinal hernia surgery were randomized by computer-generated allotment into two groups at a 1:1 ratio (Fig. 1). A research fellow (P.H.C.) handled data collection and concealment of randomization. Once a patient was diagnosed with inguinal hernia at the outpatient clinic, they were assigned a patient number (1–100), which was used in the computer-generated allotment. The patients were blinded as to whether they would receive 1-layer or 2-layer herniorrhaphy. To eliminate bias in surgical techniques, a single surgeon (H.C.C.) performed all the hernia operations during this trial. Before the start of the surgery, the research fellow (P.H.C.) informed the surgeon (H.C.C.) as to whether the patient was assigned to the 1- or 2-layer group. A modified Kugel (MK) 8 × 12 cm mesh (with Posiflex Memory Technology, BARD-Davol Inc., Cranston, RI, USA) was used in this study. The standard packaging comes with an MK mesh and an optional onlay mesh (Fig. 2). The manufacturer recommends using the optional onlay mesh if the hernia defect extends beyond the Posiflex® ring. The control group (1-layer) received a single-layer MK mesh in the preperitoneal space. The study group (2-layer) received the optional onlay patch in the inguinal canal in addition to the preperitoneal placement of the MK mesh. All patients attended a routine outpatient clinic follow-up on postoperative day 7 (POD-7) to assess any early post-op complications. As the surgeon (H.C.C.) did not participate in the follow-up period, a blinded research assistant (Y.C.H.) performed telephone interviews on all patients using a set of questions at regular intervals (at the 1st, 3rd, 6th and 12th months, and every 6 months subsequently post-op). The patient recruitment period ran from 2014/05/22 to 2015/06/30. All patients were followed-up for a minimum duration of 1 year after their operation. The follow-up period ranged from 2014/05/22 to 2017/06/30.
Surgical method
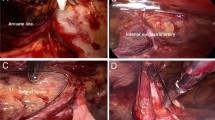
Depending on patient preference and existing contraindications, all patients received either spinal or general anesthesia. After completion of the anesthesia, prophylactic antibiotics (cefazolin 1000 mg) were given at least 30 min prior to incision. The operative field was shaved with electric clippers and disinfected with 2% chlorhexidine + 75% ethanol (Easy Antiseptic liquid 2%, Panion & BF Biotech Inc, Taipei, Taiwan). Operative time was calculated using the digital operating room timer and was recorded from the initial skin incision until the last suture securing the mesh. The surgical method, preperitoneal dissection and mesh placement were as described in our previous retrospective study10. Intraoperative findings, such as depth of subcutaneous fat (distance from the skin to the fascia of the external oblique muscle), perispermatic cord lipomas, nerves identified in the inguinal canal, and type of hernia defect (Nyhus classification) were recorded. After the flat placement of the MK mesh and securing of the positioning strap, the digital timer was stopped, and the time was recorded (Fig. 3). After the placement of the 1st layer, the surgeon was informed if the patient set to receive 1 layer or 2 layers. For the 1-layer group, the fascia and wound were closed with 2–0 and 4–0 braided absorbable sutures (Polysorb, Covidien, USA). For patients randomized into the 2-layer group, a second timer was started to record the time needed for placement of the onlay mesh, and then the wound was closed using the same method. Unless the patient had renal function impairment, routine oral 25 mg diclofenac potassium (Cataflam, Novartis Pharmaceuticals) every 8 h and intravenous nalbuphine hydrochloride 10 mg (Bain, Genovate Biotechnology Co.) every 6 h when necessary were used for postoperative pain management. For patients with renal function impairment, oral 500 mg acetaminophen tablets every 6 h were used instead of diclofenac potassium.
Data collection (long-term patient follow-up and evaluation)
After the initial post-operative clinical examination, a blinded research assistant (Y.C.H.) followed-up with the patients via a series of telephone interviews. A set of questions (Table 1) was designed to serve as a history-taking lesson. If any patients exhibited signs of possible recurrence, they were asked to return to the clinic for a physical examination and imaging (ultrasound and CT scan) to confirm the diagnosis of recurrence. Post-operative complications such as recurrence, pain, foreign body sensation, and infections were recorded and analyzed in the two groups. Our classification of foreign body sensation was any discomfort not described as pain, soreness, or numbness, or any discomfort that deviated from the norm that affected daily activities. The Clavien-Dindo classification for post-operative complications was used to evaluate the severity of the complications. Unless the patient was lost during follow-up, all patients were followed-up for a minimum of 12 months. All gathered data were then passed to the research fellow (P.H.C.) for statistical analysis.
Sample size and statistical analysis
All relevant results in the two groups were analyzed using 1-way ANOVA, the chi-square test, linear regression and the Kruskal–Wallis test with IBM SPSS statistics version 22 software, with a p-value < 0.05 considered statistically significant. Post-operative discomfort and pain after 3 months was reported in 40% of patients11 who received mesh herniorraphy with onlay mesh. We estimated a postoperative rate of less than 10% for single-layer TIPP mesh herniorrhaphy from reported studies and our retrospective study10,12,13,14,15,16. With an α of 0.05 and a power of 0.8, the sample size was estimated to be 47 in each group (total 94 patients). With an expected dropout rate of 10 to 15%, the study was initially estimated to require 110 patients randomized into two groups (Fig. 1).
Results
The patients’ general information, peri-operative and in-hospital data are presented in Tables 2 and 3. There were no discrepancies in terms of age, sex, form of anesthesia, body mass index (BMI), EHS classification, time needed to place mesh in the preperitoneal space, length of stay, or visual analog scale (VAS) score on discharge between the two groups. Without requiring time to place the extra onlay, the single-layer group had a shorter total operative time (22.4 vs. 29.5 min, p < 0.001). During long-term follow-up, two patients in the 1-layer and one patient in the 2-layer group were lost. The average onlay placement time was 451 s (7 min 30 s). Upon reviewing the recorded data, we noted that the average duration for onlay placement in the initial 10 cases was10.9 min, with a range of 10–15 min. After the initial 10 cases, the average onlay placement duration decreased to 6.7 min, with a range of 3–10 min. Increased repetition most likely contributed to the decrease in the onlay placement duration. Pain, soreness and numbness complications were similar between the two groups (Table 4). At the initial post-op day 7 and 1-month follow-up, both groups had very similar numbers and rates of pain, soreness and numbness complications. At the 3-, 6- and 12-month follow-ups, the numbers and percentages of pain, soreness and numbness in the 2-layer group were higher, but these results were not statistically significant (p > 0.5). The number of reported foreign body sensation occurrences was slightly lower in the 1-layer group from POD-7 to the 3-month follow-up, but this was not statistically significant (Table 5, P > 0.3). The 1-layer group had a noticeable decrease in the rate of reported foreign body sensation at the 6- and 12-month follow-ups (n = 0, p = 0.043, p = 0.023, respectively). Although both groups had similar rates of spinal anesthesia and patients with benign prostatic hyperplasia requiring medication, the 2-layer group had a higher rate of post-operative urine retention needing catheterization (N = 2 vs. 8, P = 0.045). When analyzing different variables and the relation to operative time, the experience of the assistant (different years of resident training or surgical technician) and EHS classification were not related to an increased operative time. There was a linear correlation between operative time and skin thickness (Fig. 4, p < 0.05). There was no recurrence in either group.
Discussion
The emphasis of earlier hernia repair studies has been on lowering the operative time, future recurrence rate, wound infection rate, and rates of other morbidities (i.e., incarceration, testicular problems)2,15. Tissue repair lacks long-term efficacy, with a reported recurrence rate of more than 10% at the 10-year follow-up point2, while mesh repair has a recurrence of less than 5%1,11. During our follow-up period (range, 24–39 months), we did not treat any patients for recurrence, and saw no signs of suspected recurrence that needed additional clinical hours for physical examination to confirm the diagnosis. In our previous retrospective experience and clinical experience, one recurrence was noted, and TEP was used to repair the hernia protrusion10,17. In that recurrence case, inadequate preperitoneal dissection resulted in suboptimal mesh placement17,18,19. We have since standardized our dissection with 7 wet surgical gauzes, and no recurrence was noted in the time (2012–2017) spanning from the previous study to this study10. Surgical plane disruption in anterior TIPP mesh herniorrhaphy is a concern regarding future recurrence. Prevention of recurrence should be the priority, with adequate preperitoneal dissection, identification of lipoma and occult peritoneal reflection being important aspects in prevention10,17,18,19. Pre-operative imaging can help identify recurrence and also identify other anatomical anomalies (i.e., lipoma)20,21. In our experience, with recurrence in anterior preperitoneal mesh placements (< 1% of cases), either the anterior or laparoscopic approach are feasible depending on the surgeon’s preference and experience.
With a longer follow-up period, the focus of recent studies has shifted towards evaluating post-operative quality of life (QoL) and minimizing long-term post-operative pain/discomfort1,9,11,22,23,24,25,26,27,28,29,30,31. Surrogate end points such as post-operative discomfort (i.e., pain, foreign body sensation, etc.) have been used to quantify post-operative QoL. Even though the short form-36 (SF-36) questionnaire has been used to evaluate QoL in different diseases and treatments, few hernia studies have incorporated SF-36 to quantify post-operative QoL. Iftikhar et al. used SF-36 to show that post-operative physical and emotional function improved after hernia surgery31. Currently lacking an inguinal hernia-specific QoL questionnaire, Huang et al. proposed the HERQL questionnaire, which aimed to assess post-herniorrhaphy QoL; this needs to be validated in future studies32.
With all the advantages of the Lichtenstein method (i.e., short operative time, low recurrence rate), the debilitating effect of post-herniorrhaphy chronic pain is a major drawback1,11,25,26,27,28. Many factors can influence post-operative pain, such pre-operative pain, intra-operative nerve manipulation/damage, and mesh-related inflammatory processes6,23,29,30,33,34,35. Pre-operative pain and discomfort are good indicators of post-operative discomfort34,35. Mier et al. used the SF-12 questionnaire and pre-operative visual analog SPS score to determine post-operative QoL35. In that study, patients with SPS ≤ 12 had an improved long-term QoL. Manangi et al. showed that 27% of patients without pre-operative pain developed chronic pain, while 77% with pre-operative pain developed chronic pain34. Wright et al. studied the reasons for pain in inguinal hernia patients at the histological level, and found that compression neuropathy of the inguinal nerve is a culprit for pre-operative pain. On a microscopic level, increased pain is correlated with an increased nerve diameter, fascicle count, and more myxoid material within the perineurium and endoneurium36. These factors are all innate to patients and can only be identified during surgery. Some intraoperative factors that surgeons can be wary of include nerve identification/manipulation and minimizing foreign materials around the nerves. Some studies have suggested that placement of the mesh in the pre-peritoneal space while minimizing nerve involvement (i.e., nerve manipulation and damage) are related to a decrease in the rate of chronic herniorrhaphy pain6,13,14,27,30,37,38,39. After implanting prosthetic material, the body will start to undergo acute inflammation and subsequent chronic fibroblastic change, which lead to scarring and fibrosis. In some cases, the inflammatory process can also cause damage to the surrounding tissues7,8. Nienhuijs et al. compared the Prolene Hernia System (PHS), mesh plug, and Lichtenstein methods for open hernia repair, and found that there was no difference in postoperative pain (39.7% at 3 months and 43.3% long-term) between the three groups11. One possible culprit for the high rate of post-operative pain is the presence of foreign materials in the inguinal canal near the inguinal nerves. In a study by Nienhuijs et al., the rate of chronic pain increased from 39 to 43%, which might suggest the presence of “late-onset chronic pain”. Chronic pain is usually defined as post-operative pain for longer than 6 months, while late-onset chronic pain is usually initially asymptomatic but increasingly more symptomatic after 6 months. Reinpold et al. reported the nerve management of all different open herniorrhaphy procedures with a follow-up duration of at least 5 years6. The study concluded that neurolysis, nerve contact with mesh and the Lichtenstein method are related to the development of chronic herniorrhaphy pain6. The study also stated the need to differentiate between short-term and long-term chronic pain in future studies. The hypothesis of the cause of late persistent chronic pain is the presence of foreign materials in the inguinal canal, causing progressive chronic inflammatory irritation and fibrotic changes leading to nerve traction. In our study, the rate of neuropathy (pain, soreness, numbness) at 3-months post-op was similar in both groups, but the complaint of a foreign body sensation was statistically significantly different (no patients in the single-layer group complained of this sensation at the 6-month follow-up point). An additional patient complained of a foreign body sensation at 12 months in the 2-layer group. We hypothesized that chronic inflammation might be the reason for the increase in the number of patients experiencing a foreign body sensation in the 2-layer group.
The first limitation in our study is the initial sample size. The reported postoperative pain/discomfort for MK mesh with onlay is not available, therefore we used the 40% reported rate in Lichtenstein studies. In assuming the rate of 40%, the sample size would be underpowered. Second limitation would be the telephone interview as form of follow-up. In our current clinical practice and healthcare environment, telephone interviews are the most effective way of following these patients for periods of more than 3 months. The follow-up period is also a limitation to determine any late recurrence patients. Single surgeon design of this study is a limitation but also maintain the same quality of surgeon throughout the study period. The single surgeon design limits the generalizability of the study to surgeons with different experience and training.
Conclusions
In this randomized, prospective study, we demonstrated that 1-layer preperitoneal mesh placement is equally effective as 2-layer placement, with a similar recurrence rate. In eliminating the use of onlay mesh, we decreased the manipulation and implantation of foreign material in the inguinal canal. The decreased foreign material also led to a decrease in foreign body sensation (0% after 6 months) and an improved post-operative QoL. Therefore, we strongly believe that properly-placed, single-layer TIPP MK mesh provides the advantage of open mesh repair without the drawback of chronic herniorrhaphy pain associated with Lichtenstein repair.
Abbreviations
- MK:
-
Modified Kugel
- POD:
-
Postoperative day
- TIPP:
-
Transinguinal preperitoneal
References
van Veen, R. N. et al. Randomized clinical trial of mesh versus non-mesh primary inguinal hernia repair: Long-term chronic pain at 10 years. Surgery 142(5), 695–698 (2007).
Beets, G. L., Oosterhuis, K. J., Go, P. M. N. Y. H., Baeten, C. G. M. I. & Kootstra, G. Long-term followup (12–15 years) of Randomized Controlled Trial comparing Bassini-Stetten, Should ice, aqnd High ligation with narrowing of the internal ring for primary inguinal hernia repair. J. Am. Coll. Surg. 185, 352–7 (1997).
Koning, G. G. et al. Randomized clinical trial of chronic pain after the transinguinal preperitoneal technique compared with Lichtenstein’s method for inguinal hernia repair. Br. J. Surg. 99(10), 1365–1373 (2012).
Koning, G. G. et al. The transinguinal preperitoneal hernia correction vs Lichtenstein’s technique; is TIPP top?. Hernia 15(1), 19–22 (2011).
Koning, G. G. et al. The Tilburg double blind randomised controlled trial comparing inguinal hernia repair according to Lichtenstein and the transinguinal preperitoneal technique. Trials 10, 89 (2009).
Reinpold, W. M., Nehls, J. & Eggert, A. Nerve management and chronic pain after open inguinal hernia repair: A prospective two phase study. Ann. Surg. 254(1), 163–168 (2011).
Hirose, T. et al. Randomized clinical trial comparing lightweight or heavyweight mesh for mesh plug repair of primary inguinal hernia. Hernia 18(2), 213–219 (2014).
O’Dwyer, P. J. et al. Randomized clinical trial assessing impact of a lightweight or heavyweight mesh on chronic pain after inguinal hernia repair. Br. J. Surg. 92(2), 166–170 (2005).
Brown, C. N. & Finch, J. G. Which mesh for hernia repair?. Ann. R. Coll. Surg. Engl. 92, 272–278 (2010).
Chiang, H. C. et al. Inguinal hernia repair outcomes that utilized the modified Kugel patch without the optional onlay patch: A case series of 163 consecutive patients. Hernia 19(3), 437–42 (2014).
Nienhuijs, S., van Oort, I., Keemers-Gels, M. E., Strobbe, L. J. A. & Rosman, C. Randomized clinical trial comparing the Prolene Hernia System, mesh plug repair and Lichtenstein method for open inguinal hernia repair. Br. J. Surg. 92, 33–38 (2005).
Neumayer, L. et al. Open mesh versus laparoscopic mesh repair of inguinal hernia. N. Engl. J. Med. 350(18), 1819–1827 (2004).
Suwa, K. et al. Modified Kugel herniorrhaphy using standardized dissection technique of the preperitoneal space: Long-term operative outcome in consecutive 340 patients with inguinal hernia. Hernia 17, 699–707 (2013).
Azeem, M., Ullha, Z., Gorya, S., Bashir, A. & Arshad Cheem, M. Modified kugel versus lichtenstein operation for inguinal hernia repair: A prospective randomized study. APMC 9, 117–123 (2015).
Li, J., Ji, Z. & Cheng, T. Comparison of open preperitoneal and Lichtenstein repair for inguinal hernia repair: A meta-analysis of randomized controlled trials. Am. J. Surg. 204, 769–778 (2012).
Li, J., Ji, Z. & Li, Y. The comparison of self-gripping mesh and sutured mesh in open inguinal hernia repair: The results of meta-analysis. Ann. Surg. 259(6), 1080–1085 (2014).
Kebapcı, E., Ozturk, S. & Unver, M. Outcomes of Endoscopic Totally Extraperitoneal (TEP) repair of clinically occult inguinal hernia diagnosed with ultrasonography. POL PRZEGL CHIR 93(4), 11–14 (2021).
Nahm, C. et al. Suction test to demonstrate the peritoneal edge during laparoscopic extraperitoneal inguinal hernia repair. Surg. Endosc. 27, 4360–3 (2013).
van den Heuvel, B., van den Broek, J. & Dwars, B. J. The incidence and natural course of occult inguinal hernias during TAPP repair. Surg. Endosc. 27, 4142–4146 (2013).
Henriksen, N. A., Thorup, J. & Jorgensen, L. N. Unsuspected femoral hernia in patients with a preoperative diagnosis of recurrent inguinal hernia. Hernia 16, 381–385 (2012).
Shakil, A. et al. Inguinal hernias: Diagnosis and management. Am. Fam. Physician 102(8), 487–92 (2020).
Gutlic, N., Rogmark, P., Nordin, P., Petersson, U. & Montgomery, A. Impact of mesh fixation on chronic pain in total extraperitoneal inguinal hernia repair (TEP): A nationwide register-based study. Ann. Surg. 263(6), 1199–1206 (2016).
Bendavid, R. et al. A mechanism of mesh-related post-herniorrhaphy neuralgia. Hernia 20(3), 357–365 (2016).
Zannoni, M. et al. Wide nervous section to prevent post-operative inguinodynia after prosthetic hernia repair: A single center experience. Hernia 19(4), 565–570 (2015).
Hallen, M. et al. Risk factors for reoperation due to chronic groin postherniorrhaphy pain. Hernia 19(6), 863–9 (2015).
Arslan, K. et al. Minimally invasive preperitoneal single-layer mesh repair versus standard Lichtenstein hernia repair for inguinal hernia: A prospective randomized trial. Hernia 19(3), 373–81 (2015).
Nienhuijs, S., Staal, E., Keemers-Gels, M., Rosman, C. & Strobbe, L. Pain after open preperitoneal repair versus lichtenstein repair: A randomized trial. World J. Surg. 31, 1751–1757 (2007).
Nienhuijs, S. et al. Chronic pain after mesh repair of inguinal hernia: A systematic review. Am. J. Surg. 194(3), 394–400 (2007).
Mitura, K. et al. Factors influencing inguinal hernia symptoms and preoperative evaluation of symptoms by patients: Results of a prospective study including 1647 patients. Hernia 22, 585–91 (2018).
Mitura, K. et al. The change in groin pain perception after transabdominal preperitoneal inguinal hernia repair with glue fixation: A prospective trial of a single surgeon’s experience. Surg. Endosc. 32, 4284–9 (2018).
Iftikhar, N. & Kerawala, A. A. Quality of life after inguinal hernia repair. Pol. Przegl Chir. 93(3), 1–5 (2021).
Huang, C.-C. et al. Quality of life of inguinal hernia patients in Taiwan: The application of the hernia-specific quality of life assessment instrument. PLoS ONE 12(8), e0183138 (2017).
Mitura, K. & Garnysz, K. Long-term follow-up of a randomized controlled trial of Lichtenstein repair vs the Valenti technique for inguinal hernia. Hernia 23, 547–54 (2019).
Manangi, M., Shivashankar, S. & Vijayakumar, A. Chronic Pain after Inguinal Hernia Repair (International Scholarly Research Notices, 2014).
Mier, N. et al. Preoperative pain in patient with an inguinal hernia predicts longterm quality of life. Surgery 163, 578–81 (2018).
Wright, R. et al. Why do inguinal hernia patients have pain? Histology points to compression neuropathy. Am. J. Surg. 213, 975e82 (2017).
Liem, M. S. et al. Comparison of conventional anterior surgery and laparoscopic surgery for inguinal hernia repair. N. Engl. J. Med. 336, 1541–7 (1997).
Schmedt, C. G., Sauerland, S. & Bittner, R. Comparison of endoscopic procedures vs Lichtenstein and other open mesh techniques for inguinal hernia repair: A meta-analysis of randomized controlled trials. Surg. Endosc. 19(2), 188–199 (2005).
Dedemadi, G. et al. Laparoscopic versus open mesh repair for recurrent inguinal hernia: A meta-analysis of outcomes. Am. J. Surg. 200(2), 291–297 (2010).
Author information
Authors and Affiliations
Contributions
Treatment of patients, data collection and assessment: H.C.C., J.T.C., P.H.C. Data collection and statistical analysis: Y.C.H., P.H.C. Manuscript written, discussion, revision: P.H.C., H.C.C., J.L. Study coordination: H.C.C., J.L., P.H.C. Study conception and design: H.C.C., J.L., J.T.C., Y.C.H., P.H.C.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chiang, HC., Lin, J., Chen, JT. et al. Minimizing complications following transinguinal preperitoneal modified Kugel mesh herniorrhaphy: a double blind prospective randomized clinical trial. Sci Rep 12, 16370 (2022). https://doi.org/10.1038/s41598-022-20803-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-20803-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.