Abstract
Alpha-1 antitrypsin deficiency (AATD, OMIM #613490) is a rare metabolic disorder affecting lungs and liver. The purpose of this study is to assess the impact of the US orphan drug act on AATD by providing a quantitative clinical-regulatory insight into the status of FDA orphan drug approvals and designations for compounds intended to treat AATD. This is across-sectional analysis of the FDA database for orphan drug designations. Primary endpoint: orphan drug approvals. Secondary endpoint: orphan drug designations by the FDA. Close of database was 16 July 2021. STROBE criteria were respected. Primary outcome: one compound, alpha-1-proteinase inhibitor (human) was approved as an orphan drug in 1987 with market exclusivity until 1994. Secondary outcome: sixteen compounds received FDA orphan drug designation including protein, anti-inflammatory, mucolytic, gene, or cell therapy. Drug development activities in AATD were comparable to other rare conditions and led to the FDA-approval of one compound, based on a relatively simple technological platform. The current unmet medical need to be addressed are extrapulmonary manifestations, in this case the AATD-associated liver disease. Orphan drug development is actually focusing on (1) diversified recombinant AAT production platforms, and (2) innovative gene therapies, which may encompass a more holistic therapeutic approach.
Similar content being viewed by others
Introduction
Alpha-1 antitrypsin deficiency (OMIM #613490) is a rare metabolic disorder caused by mutations in the SERPINA1 gene1,2. The estimated prevalence based on European data is 20/100,0003. The disease is inherited in an autosomal codominant manner. In addition to the deficiency mutant protease inhibitor (Pi) Z, which results from the substitution of a single amino acid, Glu342Lys responsible for the majority of clinically apparent cases, several genetic variants have been described that can be associated with severe AATD deficiency4,5,6,7,8. Alpha-1 antitrypsin (AAT) is mainly synthesized in the endoplasmic reticulum of the liver2. PiZ allele carriers produce a misfolded but basically functional protein, that tend to form polymers which accumulate in the hepatocytes resulting in only a small amount of protein being secreted. AAT is an antiprotease that protects lung tissue from proteolytic damage by inactivating neutrophil elastase (NE) released by neutrophil granulocytes in response to respiratory infections. Due to their antiprotease deficiency, homozygous PiZ allele carriers show accelerated loss of lung tissue, particularly in association with smoking1. The protein has also several immunomodulatory properties9. AATD is associated with different autoimmune diseases10,11 and other pulmonary conditions such as bronchiectasis, caused by the inflammatory burden of overwhelming NE12. The accumulation of AAT polymers in the liver via proteotoxic stress can lead to severe hepatopathy and hepatocellular carcinoma13,14. Patients with AATD usually present with respiratory symptoms mainly cough and dyspnoea due to the progressive development of emphysema, often interpreted as non-hereditary emphysema, so that several years usually elapse between symptom onset and diagnosis2.
There is a great need for easy-to-use or sustainable treatment options, possibly tailored to the individual disease phenotype that address extrapulmonary manifestations especially AATD liver disease in addition to lung disease. Therefore, the orphan drug research pipeline for AATD is of interest.
The U.S. Orphan Drug Act passed in 1983 and provides various incentives to pharmaceutical companies with the intention to stimulate drug development for the treatment of rare diseases. These incentives include a seven years' marketing exclusivity, tax credit for 50% of clinical trial costs, protocol assistance, Food and Drug Administration fee waiver, and orphan products grants program15. By 16 July 2021 (close of database for this analysis), a total of 998 orphan drugs were approved by the FDA16. Similar orphan drug policies were subsequently introduced in other jurisdictions worldwide, e.g., Singapore 1991, Japan 1993, Australia 1998, the European Union 200017,18.
Because the U.S. orphan drug legislation is the oldest worldwide, and the United States is an important market for the pharmaceutical industry, the purpose of this study is to assess the impact of the U.S. orphan drug act on AATD by providing a quantitative clinical-regulatory insight into the status of FDA orphan drug approvals and designations for compounds intended to treat AATD. This analysis gives an insight into dynamics, productivity, innovation, timelines, opportunities and limitations of orphan drug development for AATD from an FDA-centered perspective. It provides transparency, raises awareness of gaps, and has the potential to inspire further research and development activities worldwide.
Methods
This is a cross-sectional observational study. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) criteria were respected in design, execution, analysis, and reporting19.
Endpoints
The primary endpoint of this analysis was numbers and nature of orphan drug approvals by the FDA intended to treat AATD. The secondary endpoint was numbers and nature of orphan drug designations by the FDA intended to treat AATD.
Data sources
In order to search for orphan drug designations and approvals, accessed the FDA Orphan Drug Product designation database at https://www.accessdata.fda.gov/scripts/opdlisting/oopd/ on 16 July 2021. Search terms for the field "orphan designation" was "antitrypsin" and "proteinase". This search delivered 14 results and two results, respectively, all specific to alpha-1-antitrypsin deficiency. The results were downloaded in excel format. The following available variables were analysed: generic name of compound, year of FDA orphan drug designation, orphan designation (intended treatment indication), orphan designation status (designated, withdrawn, or approved), name and country of sponsor company.
In addition, we verified whether there were approved drugs for the treatment of alpha-1-antitrypsin deficiency, that were not listed in the U.S. Food and Drug Administration Orphan Drug Product database. We therefore performed a full text search in the FDA drug label database20. Search terms were “antitrypsin” and “proteinase” in the section “indications and usage”. Identified drugs from the search in FDALabel were juxtaposed to the approved compounds identified from the search in Orphan Drug Product designation database21. The search term “antitrypsin” delivered four, the search term “proteinase” one result. In addition, we cross-validated whether or not the compounds identified from the search in FDALabel were registered as orphan drugs in the U.S. Food and Drug Administration Orphan Drug Product database21.
Definitions
Compounds were classified into functionally meaningful types based on their biochemical properties, molecular mechanisms of action, or therapy platforms22. These types included: protein therapy, anti-inflammatory, mucolytic, gene therapy, and cell therapy.
Statistical analysis
Standard techniques of descriptive statistics were applied. Analysed variables were: generic name, classification, year of orphan drug designation, orphan designation (i.e., intended therapeutic indication), orphan designation status (designated, withdrawn, approved), FDA orphan approval status, approved label indication, year of marketing approval, year of exclusivity end date, sponsor company and country21,22.
Missing data were not imputed.
Results
Primary endpoint: orphan drug approvals by the FDA
One compound, alpha-1-proteinase inhibitor (human) was approved as an orphan drug in 1987 with market exclusivity until 1994, after having received orphan drug designation in 1984.
Secondary endpoint: FDA orphan drug designations
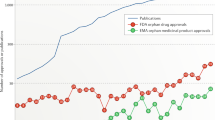
Between 1984 and 16 July 2021 (close of database), sixteen compounds intended to treat alpha-1 antitypsin deficiency received FDA orphan drug designation (Fig. 1). Five compounds were subsequently withdrawn (Table 1). Of interest, another alpha1-proteinase inhibitor (human) received orphan drug designation in 1999 which was subsequently withdrawn and not approved under the U.S. orphan drug legislation, whereas alpha-1-proteinase inhibitor human sponsored by the same manufacturer was granted FDA approval in 2003 (Table 2).
Type of compound intended to treat alpha-1 antitrypsin deficiency by year of FDA orphan drug designation. Full circles: designated compound. Open circles: withdrawn orphan drug designation. Alpha1-proteinase inhibitor (human) received orphan drug designation in 1984 and was approved by the FDA in 1987, the arrow indicates the development time between orphan drug designation and FDA orphan drug approval. The grey area shows the period of marketing exclusivity for this compound between 1987 and 1994.
Protein therapies with orphan drug designations differed in pharmaceutical manufacturing methods, i.e. their origins were either human, recombinant DNA, transgenic, made in Oryza sativa (rice), or CHO cell lines (Table 1). Gene therapies with orphan drug designations included a synthetic double-stranded RNA oligonucleotide, double stranded oligomer ADS-001 RNA interference-based liver targeted therapeutic, and a recombinant adeno-associated virus alpha-1 antitrypsin vector. An orphan drug designation for a cell therapy (three-dimensional bioprinted therapeutic liver tissue) was granted in 2017, but withdrawn subsequently. The mucolytic hyaluronic acid received orphan drug designation in 2002 and 2017, respectively, but no FDA approval. The anti-inflammatory recombinant secretory leucocyte protease inhibitor received orphan drug designation in 1991 which was later withdrawn. Fourteen orphan drug designation sponsors were pharmaceutical companies, ranging from big pharma to smaller, specialized biopharmaceutical companies. One sponsor was an academic institution, and one sponsor an individual physician. Thirteen sponsors were based in the United states, and one, respectively, in China, Israel, Switzerland, and the United Kingdom.
Currently, i.e. by 5 August 2021, there are five versions of alpha-1-proteinase inhibitor drug products approved by the FDA. They differ in galenic formulation, three of them are delivered as lyophilized powder and two as a solution (Table 2). Three alpha-1-proteinase inhibitor drug products were approved by the FDA after the end of market exclusivity of the original alpha-1-proteinase inhibitor in 1994 (Table 2).
Discussion
The objective of this study was to analyse the FDA approvals and orphan drug designations for compounds to treat alpha-1 antitypsin deficiency (AATD) in order to gain a comprehensive overview of the status of current therapy developments for this rare hereditary disease.
Primary endpoint: orphan drug approvals by the FDA
Since 1984, only one compound out of 16 orphan drug designations (= 6%) currently received FDA approval for the treatment of alpha-1-antitrypsin deficiency. In general, drug development in orphan indications appear to have a higher success rate than non-orphan indications. In a recent report from 2021, the likelihood of success from phase I to approval was 5.9% for chronic high prevalence diseases compared with a success rate of 17% for rare diseases23. As drug development in AATD currently is ongoing, it is possible that the current success rate of 6% increases if the drug development programs prove to be successful. A few non-orphan drug licenses listed in Table 2 appear to reflect a commercial strategy where the company may have felt that the orphan drug legislation did not offer additional advantages. This phenomenon was rather an exception than the rule in the overall AATD drug development portfolio which heavily relied on the orphan drug development pathway. The precise reasons for these choices remain, unfortunately, unknown. We speculate that, in general, the non-orphan regulatory drug development approach in rare diseases is chosen only in exceptional, infrequent circumstances, because of the various tangible incentives and benefits that the U.S. orphan drug act provides to the pharmaceutical industry.
Administration of purified human AAT is the only specific treatment; prospective randomized-controlled and open-label studies have shown a slowed decline in lung density with weekly infusions of 60 mg AAT/kg24,25. Whether AAT augmentation therapy has a positive effect on the extrapulmonary manifestations of the disease is currently unproven; moreover, weekly i.v. therapy is time-consuming and the treatment costs are high. In addition to preventive measures such as abstinence from nicotine, alcohol and the prompt treatment of respiratory infections, various symptomatic therapies for emphysema e.g. bronchodilators, inhaled steroids, the administration of oxygen or non-invasive ventilation are available if needed26.
Of note, alpha-1-proteinase inhibitors of human origin are subjected to availability of plasma donors and, despite manufacturing precautions, the risks related to plasma donations, e.g., virus contamination or Creutzfeldt-Jakob disease. Interestingly, the development of a recombinant alpha-1-proteinase inhibitor, which would mitigate these risks, has never been successful until close of database. One example for successful transition from donor-based protein sources to recombinant production is Gaucher disease, a rare neurogenetic disorder due to the inherited deficiency of the enzyme glucocerebrosidase. Here, the missing enzyme was initially produced from collected placenta tissue (alglucerase, approved by the FDA in 1994) and subsequently switched swiftly towards recombinant production in Chinese hamster ovary cell cultures (imiglucerase, approved by the FDA in 1994)22,27.
Early identification of gene carriers and education about necessary preventive measures such as nicotine abstinence and vaccination are essential for all individuals with severe AAT deficiency (genotype PI ZZ, ZNull or NullNull and other rarer gene variants) to avoid premature loss of lung function28. AAT augmentation therapy, as the only available therapeutic option with a causal approach, is usually administered in the late stages of the disease and therefore does not contribute to the prevention of lung disease, but only serves to slow the progression of the disease. An important characteristic of the disease that can be insufficiently addressed by early detection and appropriate lifestyle changes such as alcohol abstinence and weight reduction is AATD liver disease. Approximately one third of adults with PI ZZ AAT deficiency develop clinically significant liver fibrosis during the course of the disease29,30. Despite more than three decades of intensive research activity, no drug has yet been approved for this manifestation of AATD.
Major limitations of alpha-1-proteinase inhibitor augmentation therapy are its inconvenient application and lack of effect on extrapulmonary manifestations, which defines the current unmet medical need. The attempt of alternative alpha-1-proteinase inhibitor applications as well as the efforts to develop inhaled therapies31 have not yet led to a marketing approval. The possibility of intravenous self-administration at home allows suitable patients some freedom despite fixed weekly treatment schedules, at least because of the time savings and flexibility of self-administration32.
With respect to liver manifestation, emerging RNAi-based therapeutics targeting hepatic Z-AAT production currently in development may, if proven to be safe and efficacious, benefit patients with AATD-associated liver disease in the future33,34.
Secondary endpoint: orphan drug designations by the FDA
Alpha-1-proteinase inhibitors of human origin were the first compounds intended to treat AATD that received an orphan drug designation in 1984 (Fig. 1).
Only one new compound (an anti-inflammatory) received an orphan drug designation during the marketing exclusivity period of the protein therapy alpha1-proteinase inhibitor (human) (Fig. 1), whereas thirteen orphan drug designation were granted after expiration of this marketing exclusivity in 1994. This could mean that exclusivity protected the pharmaceutical market whereas the absence of marketing exclusivity in a drug development environment with previously demonstrated success in orphan drug approval may stimulate innovation.
There are both commercial as well as economical aspects that play a role in drug development for rare diseases. From a patient’s perspective, the driver should be the unmet medical need. From a pharmaceutical company’s perspective, the main leitmotiv is returns on investment influenced by likelihood of success in the clinical development program and commercialization27. Therefore, as further explained in the introduction, in 1983, the US Orphan Drug Act passed, in order to provide incentives to pharmaceutical companies to invest into the development of drugs for rare diseases15. The output and impact can vary: medicines developed under the US orphan drug act in the very active field of rare cancers were in general very innovative, and had shifted from non-cytotoxic agents to targeted therapies21,35. Likewise, in lysosomal storage diseases, orphan drug development was very innovative with “a drug made for a disease”22,27. In lysosomal storage disorders, success factors for approval included disease prevalence, the choice of endpoints, regulatory precedence and technology platform22,27. In contrast to this, the development output in rare epilepsies and rare rheumatological diseases delivered, in general, little innovation (although there were notable exceptions such as stiripentol for Dravet’s syndrome), or focused mainly on compounds with other indications or similar molecular pathways, but further research and development activities are ongoing36,37. A better understanding of the genetics of epilepsy may improve pharmacological management and further inform and stimulate drug development in this field38. Overall, the drug development activities in AATD were quantitively similar to the spectrum observed within the family of lysosomal storage diseases, but the technological approach of the FDA-approved therapy for AATD remains rather simple and is still based on human protein sources. Of interest, four of 17 sponsoring institutions (24%) were based outside the U.S. in industrialized countries. Commercial sponsors ranges from big pharma to smaller, specialized biopharmaceutical companies39. The low number of orphan drug designations from academic institutions is not surprising, as there are no incentives specifically encouraging academics to seek orphan designation which is a well-known but neglected problem. Barriers may include inadequate structures, funding issues, career perspectives, and a lack of cross-functional capabilities (e.g., regulatory, intellectual property, clinical operations, translational medicine, or biostatistics support).
Limitations and directions for future research
As pointed out previously, this analysis has some limitations that have to be taken into account for the correct interpretation of the present findings21,22,27,35,36,37. Guided by strategic and patent related considerations manufacturers may choose not to reveal the intent to develop a drug in a publicly visible way at an early stage which may have influenced the timeline to approval in Fig. 1. This analysis focusses intentionally on the FDA perspective40 and did therefore not consider regulatory jurisdictions elsewhere. Because in general drug development for rare diseases is a global endeavour, we regard the findings of the present analysis generalizable within the context of the limitations described21. This report serves as a baseline for future progress.
Conclusion
Alpha-1 antitrypsin deficiency is a rare disease. Drug development activities in the field were comparable to other rare, treatable conditions (e.g., lysosomal storage diseases), and led to the FDA-approval of one compound, based on a relatively simple technological platform, meanwhile available in different galenic versions, which renders the condition treatable. However, alpha-1-proteinase inhibitor augmentation therapy is dependent on human donors and theoretically subjected to infectious risks. The current unmet medical need to be addressed are extrapulmonary manifestations, in this case the AATD-associated liver disease. Development is actually focussing on (1) diversified recombinant protein production platforms for alpha-1-proteinase inhibitor augmentation therapy, and (2) innovative gene therapies, which may, if successfully, encompass a more holistic therapeutical approach.
Data availability
The full data set supporting the conclusions of this article is available upon request from Franziska C Trudzinski.
References
Crystal, R. G. Alpha 1-antitrypsin deficiency, emphysema, and liver disease. Genetic basis and strategies for therapy. J. Clin. Invest. 85, 1343–1352. https://doi.org/10.1172/JCI114578 (1990).
Strnad, P., McElvaney, N. G. & Lomas, D. A. Alpha1-antitrypsin deficiency. N. Engl. J. Med. 382, 1443–1455. https://doi.org/10.1056/NEJMra1910234 (2020).
Orphanet Report Series, Prevalence and incidence of rare diseases, Bibliographic data, January 2022: available at https://www.orpha.net/orphacom/cahiers/docs/GB/Prevalenceof_rarediseasesby_alphabetical_list.pdf. Accessed 28 April 2022.
Curiel, D. T., Vogelmeier, C., Hubbard, R. C., Stier, L. E. & Crystal, R. G. Molecular basis of alpha 1-antitrypsin deficiency and emphysema associated with the alpha 1-antitrypsin Mmineral springs allele. Mol. Cell Biol. 10, 47–56. https://doi.org/10.1128/mcb.10.1.47-56.1990 (1990).
Fraizer, G. C., Harrold, T. R., Hofker, M. H. & Cox, D. W. In-frame single codon deletion in the Mmalton deficiency allele of alpha 1-antitrypsin. Am. J. Hum. Genet. 44, 894–902 (1989).
Kalsheker, N., Hayes, K., Weidinger, S. & Graham, A. What is Pi (proteinase inhibitor) null or PiQO?: A problem highlighted by the alpha 1 antitrypsin Mheerlen mutation. J. Med. Genet. 29, 27–29. https://doi.org/10.1136/jmg.29.1.27 (1992).
Takahashi, H. et al. Characterization of the gene and protein of the alpha 1-antitrypsin “deficiency” allele Mprocida. J. Biol. Chem. 263, 15528–15534 (1988).
Presotto, M. A. et al. Clinical characterization of a novel alpha1-antitrypsin null variant: PiQ0Heidelberg. Respir. Med. Case Rep. 35, 101570. https://doi.org/10.1016/j.rmcr.2021.101570 (2022).
Weitz, J. I., Silverman, E. K., Thong, B. & Campbell, E. J. Plasma levels of elastase-specific fibrinopeptides correlate with proteinase inhibitor phenotype. Evidence for increased elastase activity in subjects with homozygous and heterozygous deficiency of alpha 1-proteinase inhibitor. J. Clin. Invest. 89, 766–773. https://doi.org/10.1172/JCI115654 (1992).
Geddes, D. M. et al. alpha 1-antitrypsin phenotypes in fibrosing alveolitis and rheumatoid arthritis. Lancet 2, 1049–1051. https://doi.org/10.1016/s0140-6736(77)91883-9 (1977).
Fortin, P. R., Fraser, R. S., Watts, C. S. & Esdaile, J. M. Alpha-1 antitrypsin deficiency and systemic necrotizing vasculitis. J. Rheumatol. 18, 1613–1616 (1991).
Gramegna, A. et al. Neutrophil elastase in bronchiectasis. Respir. Res. 18, 211. https://doi.org/10.1186/s12931-017-0691-x (2017).
Wu, Y. et al. A lag in intracellular degradation of mutant alpha 1-antitrypsin correlates with the liver disease phenotype in homozygous PiZZ alpha 1-antitrypsin deficiency. Proc. Natl. Acad. Sci. USA 91, 9014–9018. https://doi.org/10.1073/pnas.91.19.9014 (1994).
Fargion, S. et al. Alpha 1-antitrypsin in patients with hepatocellular carcinoma and chronic active hepatitis. Clin. Genet. 19, 134–139. https://doi.org/10.1111/j.1399-0004.1981.tb00684.x (1981).
Haffner, M. E., Torrent-Farnell, J. & Maher, P. D. Does orphan drug legislation really answer the needs of patients?. Lancet 371, 2041–2044. https://doi.org/10.1016/S0140-6736(08)60873-9 (2008).
FDA. Search Orphan Drug Designations and Approvals. Available from: https://www.accessdata.fda.gov/scripts/opdlisting/oopd/listResult.cfm. Accessed 16 July 2021.
Orphanet. About Orphan Drugs. Available from: https://www.orpha.net/consor/cgi-bin/Education_AboutOrphanDrugs.php?lng=EN&stapage=ST_EDUCATION_EDUCATION_ABOUTORPHANDRUGS_SIN. Accessed 04 August 2022.
Orphanet. About Orphan Drugs. Available from: https://www.orpha.net/consor/cgi-bin/Education_AboutOrphanDrugs.php?lng=EN. Accessed 04 August 2022.
Vandenbroucke, J. P. et al. Strengthening the reporting of observational studies in epidemiology (STROBE): Explanation and elaboration. PLoS Med. 4, e297. https://doi.org/10.1371/journal.pmed.0040297 (2007).
FDA. FDALabel. Available from: https://nctr-crs.fda.gov/fdalabel/ui/search. Accessed 05 August 2021.
Johann, P., Lenz, D. & Ries, M. The drug development pipeline for glioblastoma—A cross sectional assessment of the FDA Orphan Drug Product designation database. PLoS ONE 16, e0252924. https://doi.org/10.1371/journal.pone.0252924 (2021).
Garbade, S. F. et al. FDA orphan drug designations for lysosomal storage disorders—a cross-sectional analysis. PLoS ONE 15, e0230898. https://doi.org/10.1371/journal.pone.0230898 (2020).
Clinical Development Success Rates and Contributing Factors 2011–2020. Available from: https://pharmaintelligence.informa.com/~/media/informa-shop-window/pharma/2021/files/reports/2021-clinical-development-success-rates-2011-2020-v17.pdf. Accessed 04 August 2022.
Chapman, K. R. et al. Intravenous augmentation treatment and lung density in severe alpha1 antitrypsin deficiency (RAPID): A randomised, double-blind, placebo-controlled trial. Lancet 386, 360–368. https://doi.org/10.1016/S0140-6736(15)60860-1 (2015).
McElvaney, N. G. et al. Long-term efficacy and safety of alpha1 proteinase inhibitor treatment for emphysema caused by severe alpha1 antitrypsin deficiency: An open-label extension trial (RAPID-OLE). Lancet Respir. Med. 5, 51–60. https://doi.org/10.1016/S2213-2600(16)30430-1 (2017).
Vogelmeier, C. F. et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am. J. Respir. Crit. Care Med. 195, 557–582. https://doi.org/10.1164/rccm.201701-0218PP (2017).
Mechler, K., Mountford, W. K., Hoffmann, G. F. & Ries, M. Pressure for drug development in lysosomal storage disorders—a quantitative analysis thirty years beyond the US orphan drug act. Orphanet. J. Rare Dis. 10, 46. https://doi.org/10.1186/s13023-015-0262-5 (2015).
Miravitlles, M. et al. European Respiratory Society statement: diagnosis and treatment of pulmonary disease in alpha1-antitrypsin deficiency. Eur Respir J 50, 1. https://doi.org/10.1183/13993003.00610-2017 (2017).
Clark, V. C. et al. Clinical and histologic features of adults with alpha-1 antitrypsin deficiency in a non-cirrhotic cohort. J. Hepatol. 69, 1357–1364. https://doi.org/10.1016/j.jhep.2018.08.005 (2018).
Hamesch, K. et al. Liver fibrosis and metabolic alterations in adults with alpha-1-antitrypsin deficiency caused by the Pi*ZZ mutation. Gastroenterology 157, 705–719. https://doi.org/10.1053/j.gastro.2019.05.013 (2019).
Brand, P. et al. Lung deposition of inhaled alpha1-proteinase inhibitor in cystic fibrosis and alpha1-antitrypsin deficiency. Eur. Respir. J. 34, 354–360. https://doi.org/10.1183/09031936.00118408 (2009).
Herth, F. J. F. et al. Alpha 1 antitrypsin therapy in patients with alpha 1 antitrypsin deficiency: Perspectives from a Registry Study and practical considerations for self-administration during the COVID-19 pandemic. Int. J. Chron. Obstruct. Pulmon. Dis. 16, 2983–2996. https://doi.org/10.2147/COPD.S325211 (2021).
Wooddell, C. I. et al. Development of an RNAi therapeutic for alpha-1-antitrypsin liver disease. JCI Insight 5. https://doi.org/10.1172/jci.insight.135348 (2020).
Turner, A. M. et al. Hepatic-targeted RNA interference provides robust and persistent knockdown of alpha-1 antitrypsin levels in ZZ patients. J. Hepatol. 69, 378–384. https://doi.org/10.1016/j.jhep.2018.03.012 (2018).
Stockklausner, C., Lampert, A., Hoffmann, G. F. & Ries, M. Novel treatments for rare cancers: The U.S. Orphan drug act is delivering-a cross-sectional analysis. Oncologist 21, 487–493. https://doi.org/10.1634/theoncologist.2015-0397 (2016).
Döring, J. H., Lampert, A., Hoffmann, G. F. & Ries, M. Thirty years of orphan drug legislation and the development of drugs to treat rare seizure conditions: A cross sectional analysis. PLoS ONE 11, e0161660. https://doi.org/10.1371/journal.pone.0161660 (2016).
Lutz, T., Lampert, A., Hoffmann, G. F. & Ries, M. Novel treatments for rare rheumatologic disorders: Analysis of the impact of 30 years of the US orphan drug act. Orphanet J. Rare Dis. 11, 60. https://doi.org/10.1186/s13023-016-0443-x (2016).
Ademuwagun, I. A., Rotimi, S. O., Syrbe, S., Ajamma, Y. U. & Adebiyi, E. Voltage gated sodium channel genes in epilepsy: Mutations, functional studies, and treatment dimensions. Front. Neurol. 12, 600050. https://doi.org/10.3389/fneur.2021.600050 (2021).
Dunleavy, K. The top 20 pharma companies by 2021 revenue. Available from: https://www.fiercepharma.com/special-reports/top-20-pharma-companies-2021-revenue. Accessed 04 Aug 2022 (2022).
FDA. Public Law 97–414, 97th Congress, An Act To amend the Federal Food, Drug, and Cosmetic Act to facilitate the development of drugs for rare diseases and conditions, and for other purposes 1983. Available from: https://www.fda.gov/media/99546/download. Accessed 11 Feb 2022.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
F.C.T. and M.R. were involved in the design of the study, the interpretation of the data, drafting and finalization of the manuscript, approved the final submitted version, and agreed to be accountable for all aspects of the work. M.A.P., E.B. and F.J.F.H. were involved in the interpretation of the data, drafting and finalization of the manuscript, approved the final submitted version, and agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
FCT received lecture fees from CSL Behring and Grifols Deutschland GmbH. EB received a travel grant from CSL Behring. FJFH received lecture fees from CSL Behring and Grifols Deutschland GmbH. MAP and MR have nothing to disclose.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Trudzinski, F.C., Presotto, M.A., Buck, E. et al. Orphan drug development in alpha-1 antitypsin deficiency. Sci Rep 12, 15497 (2022). https://doi.org/10.1038/s41598-022-19707-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-19707-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.