Abstract
In this study, a new heterogeneous magnetic catalytic system based on selenium-functionalized iron oxide nanoparticles is presented and suggested for facilitating amide/peptide bonds formation. The prepared nanocatalyst, entitled as “Fe3O4/SiO2-DSBA” (DSBA stands for 2,2′-diselanediylbis benzamide), has been precisely characterized for identifying its physicochemical properties. As the most brilliant point, the catalytic performance of the designed system can be mentioned, where only a small amount of Fe3O4/SiO2-DSBA (0.25 mol%) has resulted in 89% reaction yield, under a mild condition. Also, given high importance of green chemistry, convenient catalyst particles separation from the reaction medium through its paramagnetic property (ca. 30 emu·g−1) should be noticed. This particular property provided a substantial opportunity to recover the catalyst particles and successfully reuse them for at least three successive times. Moreover, due to showing other excellences, such as economic benefits and nontoxicity, the presented catalytic system is recommended to be scaled up and exploited in the industrial applications.
Similar content being viewed by others
Introduction
Over the time, micro and nanoscale heterogeneous catalytic systems have attracted an increasing attention due to several reasons, such as high-efficiency, convenient separation, well recyclability, biocompatibility, and consistency with the green chemistry’s principles1,2,3. Among various types of the heterogeneous catalysts, the systems based on iron oxide (Fe3O4) magnetic nanoparticles are very interesting because they are easily synthesized. Moreover, their surfaces can be modified, and they can be separated from the reaction medium by using an external magnet. This easy separation from the reaction medium is an important step towards green chemistry because the requirement of the organic solvents use in the separation and purification processes is completely addressed4,5,6,7,8,9,10,11. Surface-coating of the Fe3O4 nanoparticles with different layers increases the surface area ratio and causes their surface to be tightly functionalized with the desired functional groups12.
Organocatalysts, are small organic molecules that can catalyze the synthetic reactions in the absence of the metals or metal ions13,14,15,16,17. One of the major challenges of organocatalysts’ utilization is their separation and reusability. The stabilization of these catalysts onto the nanoparticle surfaces, especially Fe3O4 magnetic nanoparticles, can be an excellent resolution for addressing this challenge18,19,20. In addition to providing a solid support for the organic catalytic sites, utilization of Fe3O4 includes several other advantages in comparison with the other species. From the chemical aspect, since the surface of the Fe3O4 nanoparticles is full of hydroxyl functional groups, it would be quite possible to functionalize that with different species through covalent bonding21,22. So far, there have been several reports about the composition of the organic compounds with the Fe3O4 nanoparticles, through which great upshots in various applications were observed23,24. From the physical aspect, the structural stability and thermal resistance (and also resistance against oxidation and degradation) is one of the main contributors to the wide utilization of the Fe3O4 nanoparticles25. The mentioned excellence has provided this possibility to recycle these materials and reuse them for several times26. Moreover, great paramagnetic property of the Fe3O4 nanoparticles has led to more convenient separation, which is of high importance in the field of catalysis27. Besides, there are biological and environmental justifications (e.g. nontoxicity, biocompatibility and biodegradability) for the use of these materials that are seriously regarded by green chemistry principles28,29. However, in this work, we intend to take advantage from those features that are effective in the catalysis scope.
One of the most challenging synthetic reactions in the scope of organic chemistry, is peptide bond formation in the solution phase30,31. In this way, various expensive substances such as “TBTU” (2-(1H-benzotriazole-1-yl)-1,1,3,3-tetramethylaminium tetrafluoroborate), “HBTU” 2-(1H-benzotriazole-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate), and “HATU” (1-[bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxide hexafluorophosphate) are traditionally used as the peptide coupling reagent32. So far, various methods have been developed, in which the application of the functionalized nanomaterials (nanocatalysts) has been particularly noticed33,34. In the pharmaceutical industry, amide bond formation is one of the most common transformations in medicinal chemistry laboratories35,36. Because of proton exchange between the coupling partners, the ideal approach for amide synthesis, i.e., the direct condensation of a carboxylic acid and an amine group with the production of one equivalent of water (dehydration) as the sole byproduct, is not practicable. This interaction can only occur under forcing conditions (such as high temperatures37 and microwave irradiation38), making it incompatible with the chemical complexity demonstrated by existing therapeutic candidates39. This is why, design and manufacture of different types of amide coupling reagents has always been a topic of interest for many researchers around the world for many years.
Among various types of the functionalized nanomaterials, organoselenium compounds such as ebselen40,41, diphenyl diselenide42, and selenocysteine43 are very important and valuable reagents in the organic synthesis due to their high activity44. Recently, diselenide bonds have been developed as one type of novel dynamic covalent bonds because of their modulable dynamic behaviors similar to disulfide bonds. Since both elements sulfur and selenium belong to the chalcogen of the periodic table, they exhibit the same chemical properties, so the chemical bonds of diselenide and disulfide show similar behaviors. Compared to the disulfide bonds, the bonding energy of a diselenide bond is lower (diselenide bonds: 172 kJ mol−1; disulfide bonds: 240 kJ mol−1), so the diselenide bonds are more labile to be dynamically broken and reconstructed than disulfide bonds45. So far, there have been several reports about the use of diselenide species as a prone center for chemical catalysis purposes. For example, in 2018, Rangraz et al. using the nano-(Fe3O4@SiO2-Se)2, which contained the catalytic site of diselenide (Se-Se), could catalyze the conversion of various aldehydes to their corresponding carboxylic acids with high yield44,46,47.
Based on the above information, in this work, an attempt has been made to develop a novel methodology for the amide bond formation by the amino acids, without any need to the conventional coupling reagents. For the first time, a diselenide aromatic structure is loaded onto a heterogeneous supporting substrate, via covalent attachment. The target diselenide compound (DSBA, 2,2′-diselanediylbis benzamide) was initially synthesized, and then covalently attached onto the amine-functionalized Fe3O4/SiO2 nanostructures. As a brilliant point of this thesis, it should be noticed that the prepared diselenide nanocatalyst is recovered under the air atmosphere, and physically recovered and reused through its paramagnetic feature. From mechanistic aspect that have been previously approved by kinetic studies, a reduction/oxidation (red/ox) process is passed through using a small amount of triethyl phosphite48. Also, the final dehydration is assisted by the silica network (in the structure of catalyst), which acts as a great molecular sieve32. To the best of our knowledge, this is the first time that a magnetic diselenide nanocatalyst (with general formulation of Fe3O4/SiO2-DSBA) is applied in the amide/peptide coupling reactions. High catalytic performance of the proposed system has been clarified by the optimization reactions. Concisely, it was revealed that high reaction yields are obtained in the dipeptide synthesis reactions by using a small amount of the prepared Fe3O4/SiO2-DSBA catalytic system, over a short time (180 min), at room temperature.
Results and discussion
Preparation of Fe3O4/SiO2-DSBA catalytic system
Synthesis of DSBA organic compound
According to Fig. 1, several steps were required to prepare the Fe3O4/SiO2-DSBA catalytic system. First, the metal salt of potassium diselenide was made using selenium, potassium hydroxide, and potassium borohydride. The important point in the synthesis of this salt was high reactivity with oxygen, which resulted in a very foul-smelling gray substance. Therefore, great care was taken to synthesize this salt under the nitrogen atmosphere. The synthesis of potassium diselenide metal salt was performed simultaneously with the synthesis of 2-carboxybenzenediazonium chloride. To synthesize 2-carboxybenzenediazonium chloride, anthranilic acid was dissolved in the hydrochloric acid solution (Fig. 1a). Simultaneously, NaNO2 was dissolved in water and then added to 2-carboxybenzenediazonium chloride solution, and then stirred at zero temperature (Fig. 1b). In this stage, it should be noticed that forming a red color mixture originating from diazonium salt means that the synthesize process is failed. In the next step, the synthesized metal salt of potassium diselenide was added to the solution inside the ice bath, which foamed due to generation of nitrogen gas during the process (Fig. 1c). At the end of this step it was very important to check the pH of the solution. The acidic pH values indicate that there are still some primary reactants in the medium that did not react with the potassium diselenide salt. At this point, by alkalizing the environment, the excess hydrochloric acid of the environment is neutralized leading to a complete consumption of all primary reactants in the environment. Afterward, to eliminate the unreacted selenium and oxidized selenium from the products, the solution was filtered through a thin celite pad. Hydrochloric acid was then added to the filtered solution and then the solid product was filtered through paper filter. In the last step, the resulted sediment was recrystallized in hot methanol to purify the product (see Video #1 Diselenobis Recrystallization)49. The appearance of the obtained products from successive stages of the DSBA synthesis process is illustrated in Fig. 2.
Digital images of: (a) synthesis of K2Se2 metal salt, (b) synthesis of 2-carboxybenzenediazonium chloride, (c) synthesis of 2,2′-diselanediyldibenzoic acid (N2 release creates the bubbles), (d) synthesis of 2,2′-diselanediyldibenzoic acid after stirring for 2 h at 90 °C, (e) filtered unreacted selenium and oxidized selenium by celite pad, and filtrate including 2,2′-diselanediyldibenzoic acid, and (f) 2,2′-diselanediyldibenzoic acid sediment after addition of hydrochloric acid (1 M) (recrystallization has been presented in Video #1).
Preparation of Fe3O4@SiO2-DSBA catalytic system
To turn our nanocatalyst into a heterogeneous magnetic nanocatalyst, as-synthesized 2,2′-diselanediyldibenzoic acid was loaded onto the amine-modified Fe3O4 magnetic nanoparticles. To synthesize the Fe3O4 magnetic nanoparticles, iron (II) and iron (III) chloride salts were used under alkaline conditions provided by concentrated ammonium solution50. The formed dark precipitations were collected by an external magnet and washed several times with deionized water, ethanol, and acetone. To increase hydroxyl groups onto the surface of magnetic nanoparticles (MNPs), they were coated with a silica (SiO2) network using tetraethylorthosilicate (TEOS). Since amine functional groups can form an amide bond with the carboxylic acid functional groups present in the structure of the synthesized catalysts, 3-aminopropyl triethoxysiane (APTES) was used to modify the surface of the Fe3O4@SiO2 nanoparticles51. Figure 3 schematically represents the preparation route of the Fe3O4@SiO2-DSBA catalytic system.
Characterization of Fe3O4@SiO2-DSBA catalytic system
Different equipment and methods were exploited for characterization of the prepared Fe3O4/SiO2-DSBA catalytic system. Fourier-transform infrared (FTIR) spectroscopy was used to examine the functional groups of the new nanocatalyst. Practically, KBr tablets containing the samples were prepared and studied by FTIR spectrometer. Energy-dispersive X-ray (EDX) spectroscopy was used to investigate the presence of different elements in the whole stages of the preparation process. Field-emission scanning-electron microscopy (FESEM) were used to examine the size and morphology of the samples, and electron-transmission microscopy (TEM) was utilized to examine the core–shell structure of the catalyst. To prepare the samples for these imaging methods, the particles were ultrasonicated by a cleaner bath (50 kHz, 100 W L−1) for two minutes, at room temperature. Then, dispersions in ethanol were then poured onto the glass laminates. The magnetic properties of the final catalyst were investigated using a vibrational-sample magnetometer (VSM). The thermal resistance and decomposition state of the prepared nanocatalyst was studied in a thermal range of 50–800 °C, by a thermogravimetric analysis (TGA). To ensure that there would not be any probability of oxidation during the TGA study, argon atmosphere was subjected to the sample during the study. X-ray diffraction (XRD) analysis was performed in order to better understand the properties and structure of the catalyst. The brand and model of the used equipment are listed in the experimental section (Table 3).
FTIR spectroscopy
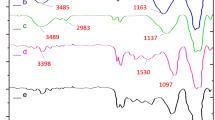
The FTIR spectra were used to study the functional groups present in the various compounds produced during the preparation of the Fe3O4/SiO2-DSBA nanocatalyst. According to Figures S1–S4, given in the Supporting Information (SI) section, the peak at ca. 578 cm−1 (in all the spectra) is related to the stretching vibration of the Fe–O bond confirming the formation of the iron oxide nanoparticles52. In addition, the bands at ca. 803 and 1082 cm−1 (Figures S2–S4, in SI section) are attributed to the stretching vibration of Si–O and the asymmetric stretching vibration of Si–O–Si, respectively53. In the spectrum of Fe3O4@SiO2-NH2 particles (Figure S3), the stretching and bending vibrations of the amine groups have been appeared at ca. 3432 and 1629 cm−1, corroborating that aminopropyl silane (APS) has been successfully placed onto the Fe3O4@SiO2 surfaces54,55,56,57. The formed amide groups in the structure of the Fe3O4/SiO2-DSBA catalytic system verifies the covalent attachment of 2,2′-diselenobis (benzoic acid) (DSBA) onto the surfaces. In this regard, in the spectrum of Fe3O4/SiO2-DSBA (Figure S4), the peaks that appeared at ca. 1629 and 1383 cm−1 correspond to C=O and C–N, respectively58,59. Also, the peaks related to the stretching vibrations of C–H and C–C bonds present in the aromatic rings seem to be overlapped with the other peaks (Figure S4).
EDX analysis
The EDX spectroscopy was utilized to further confirm the existence of elements that are predicted to be present at various stages of nanocatalyst preparation. Figure 4 shows the EDX results of Fe3O4, Fe3O4@SiO2, Fe3O4@SiO2@NH2, and Fe3O4/SiO2-DSBA nanoparticles. Figure 4a is related to the Fe3O4 nanoparticles, which expectedly corroborates the presence of Fe and O in the sample. Figure 4b confirms the presence of Fe, O, and Si elements in the structure of Fe3O4@SiO2 nanoparticles. The presence of C and N elements in addition to Fe, O, and Si in Fig. 4c origins from successful modification of the surface of Fe3O4@SiO2 nanoparticles by APS layer. In Fig. 4d, surface attachment of 2,2′-diselenobis benzoic acid onto the Fe3O4@SiO2@NH2 particles is verified by the appearance of Se element’s peaks. Also, this is observed that the weight ratio (wt%) of the C element has increased to 14.65% after attachment of 2,2′-diselenobis benzoic acid, well confirming the addition of a new ingredient into the structure.
VSM analysis
One of the most important features of the prepared catalyst is its easy separation from the reaction mixture by an external magnet. This property of Fe3O4/SiO2-DSBA catalytic system that origins from the presence of Fe3O4 nanoparticles, has been investigated by vibrating-sample magnetometer (VSM) analysis, as shown in Fig. 560,61. This featured behavior of the Fe3O4/SiO2-DSBA catalytic system is particularly bolded in recyclization process, where the particles can be conveniently separated through holding an external magnet at the bottom of the flask, and reused multiple times. Obviously, the magnetic property of the Fe3O4 nanoparticles is decreased after formation of a successive layers onto the surfaces. More precisely, the magnetic property of Fe3O4/SiO2 nanoparticles is ca. 35 emu·g−1, while this value reduced to ca. 30 emu·g−1 after conversion to Fe3O4/SiO2-DSBA structure. However, this amount of magnetization has demonstrated to be quite adequate for execution of the magnetic separation during the catalytic process.
XRD analysis
The XRD pattern of the prepared Fe3O4/SiO2-DSBA catalytic system is exhibited in Fig. 6. According to this figure, the peaks that appeared at 2θ = 30.4°, 35.7°, 43.4°, 54.0°, 57.3°, 63.9°, 71.7°, and 74.4°, and are respectively signed by Miller indices of (2 2 0), (3 1 1), (4 0 0), (4 2 2), (5 1 1), (4 4 0), (6 2 0), and (5 3 3), are attributed to the Fe3O4 magnetic nanoparticles (MNPs) (JCPDS database: PDF#99–0073)62. The SiO2 network gives a broad peak in a range of 2θ = 11.0°–44.0° that overlaps with one of the Fe3O4 peaks63. The other additional peaks that appeared at 2θ = 26.12°, 79.44°, 87.92°, 90.28°, and 95.24° (marked with NP) are related to the new crystalline phase formed on the surface of the Fe3O4/SiO2 MNPs after functionalization with DSBA.
Thermogravimetric analysis
To evaluate thermal stability and decomposition states of the Fe3O4/SiO2-DSBA catalytic system, thermogravimetric analysis (TGA) was performed on the sample under argon atmosphere, in thermal range of 50–800 °C32. As presented in Fig. 7a, physical adsorption of the moisture in the air caused a partial increase (1.0%) in the weight, which was quickly returned back by hating the sample up to ca. 120 °C. Then, ca. 5.5% of the total weight was lost by increasing the temperature to around 370 °C, which is attributed to removal of the entrapped water molecules in the silica network64. In the next stage, a relatively intense decrease in the weight was occurred through which ca. 6.0% of the total weight was lost. The degradation of the organic structures at this thermal range (300–600 °C) has been confirmed by literature, therefore, this weight loss can be ascribed to decomposition of APS and DSBA organic layer44. In continue, a tangible increase in the weight is observed at 630 °C, which may be due to re-adsorption of the combusted materials or adsorption of the argon gas by a porous structure that formed at this temperature65. Also, the curve of differential thermal analysis (DTA) was provided for the sample, in the same thermal range. As presented by Fig. 7b, totally an endothermic trend is observed for the Fe3O4/SiO2-DSBA sample, which corroborates well integration and high thermal resistance of the structure. As is seen in the DTA curve, the structure and the used components were not affected by the change in temperature, confirming that functional groups on the surfaces are almost stable.
Electron microscopy
The FESEM and TEM methods were utilized to examine morphology, real structure, size, and dispersion state of the prepared Fe3O4/SiO2-DSBA nanoparticles. As shown in Fig. 8a, Fe3O4 MNPs have a uniform spherical morphology and are well dispersed, although they are slightly agglomerated after being coated with TEOS, as illustrated in Fig. 8b53. According to Fig. 8c, Fe3O4/SiO2-DSBA MNPs are well dispersed and have a spherical morphology. It means that these MNPs provide an extremely active surface area that is required for the catalytic intentions. At this state, the chemical active sites (here Se-Se bonds) are quite available to the raw substances. Figure 8d–f confirm successful formation of the core/shell architecture via TEM imaging. In this images, the dark areas are related to the magnetic cores (Fe3O4 MNPs) and gray areas (light) are related to the shell (SiO2-DSBA). The g-series of Fig. 8 is related to the SEM energy-mapping of the prepared Fe3O4/SiO2-DSBA catalytic system, in which each element has been highlighted by a special color. These images better reveal the composition state of the elements and localization of the used ingredients.
Mass spectroscopy
The bond energy of dieselnide is only 172 kJ mol−166, while this value for C=C, C–H, and C–O are 602, 346, and 358 kJ mol−1, respectively. Given these explanations, it is reasonable to expect that the Se-Se bond in 2,2′-diselenobis benzoic acid breaks earlier during the mass process, in comparison with the other bonds of this molecular structure. This claim has been proven by the results of mass analysis (MS) on 2,2′-diselenobis benzoic acid sample. The total molecular weight of the symmetric structure of the synthesized 2,2′-diselenobis benzoic acid is 402 g mol−1. When this structure undergoes through a mass process, it makes sense that its diselenide bond is broken faster than the rest sites, resulting in the appearance of a signal at 201 g mol−1. The mass result of the synthesized 2,2′-diselenobis benzoic acid has been shown in Figure S5 (in SI section), which well confirms breaking of the Se-Se bond upon exposure to the excited electrons within the MS analysis.
1HNMR and 13CNMR analyses on 2,2′-diselenobis benzoic acid compound
For further confirmation of the successful synthesis of 2,2′-diselenobis benzoic acid compound, H- and C-NMR spectroscopy were used. Figure 9 represents the spectral data and the provided NMR spectra that verify successful formation of the synthesized 2,2′-diselenobis benzoic acid structure.
1H-NMR (500 MHz, DMSO-d6) δ = 13.73 (bs, 1H, OH), 8.05 (d, J = 11.0 Hz, 1H, ArCH), 7.69 (d, J = 13.0 Hz, 1H, ArCH), 7.50 (t, J = 11 Hz, 1H, ArCH), 7.37 ppm (t, J = 12 Hz, 1H, ArCH); 13C NMR (125.76 MHz, DMSO-d6) = 169.00, 133.88, 132.02, 129.92, 129.16, 126.99 ppm50.
Catalytic application of Fe3O4@SiO2-DSBA in peptide construction
In this section, the catalytic activity of the prepared Fe3O4@SiO2-DSBA system is investigated in the real peptide coupling reactions. To initiate the process, the optimal condition for the amide bond formation between two protected amino acids in the presence of Fe3O4@SiO2-DSBA catalytic system was investigated through examining different factors. In this way, two different methods such as ultrasonication and magnetic stirring have been monitored for the catalytic process. According to literature, ultrasonication can provide a synergistic effect with the heterogeneous particles and positively affect their dispersion state and surface energy of the Fe3O4@SiO2-DSBA particles67,68. Hence, this method (abbreviated as US) has also been considered in the experimental stages. Moreover, other effective parameters such as reaction medium, temperature, catalyst amount, and reaction time have been precisely screened. For this purpose, the coupling reaction between glycine methyl ester (Gly-COOMe) and N-protected phenylalanine (Fmoc-Phe-OH) was considered as a model reaction. For further assessments, the same process has been applied for N-protected alanine (Fmoc-Ala-OH), cysteine methyl ester (Cys-COOMe), and N-protected arginine (Fmoc-Arg(pbf)-OH), at the obtained optimal conditions. In continue, the recyclability of the used Fe3O4/SiO2-DSBA catalytic system is experimented and discussed in detail, and a plausible mechanism is suggested for the catalytic process implemented by Fe3O4/SiO2-DSBA system. Finally, a quick comparison is made between the suggested catalytic system in this project and the previously reported ones.
Optimization of catalytic values in peptide coupling reactions
In order to determine the optimized conditions for the catalytic process of the Fe3O4/SiO2-DSBA as a coupling reagent for amide bond formation, different experimental conditions including catalyst type and amount, solvent, temperature, time, and the applied method were investigated. For this purpose, the reaction progress was evaluated with thin-layer chromatography (TLC) and ninhydrin spray32. As reported in Table 1, the no traceable reaction yield (%) was obtained in the model reaction of the peptide coupling in the absence of the Fe3O4/SiO2-DSBA catalyst, after three hours of stirring in ethanol solvent (Table 1, entry 1). In the same conditions, the reaction yield increased to 38% only by adding 25 mol% of Fe3O4@SiO2 MNPs to the reaction medium (Table 1, entry 2). It means that the Fe3O4@SiO2 particles have provided a suitable substrate for the raw materials to get approach together and start interactions and bonding. It may origin from tight hydrogen-bond interaction between the amino acids and the present hydroxyl groups onto the surface of the particles. As is observed in Table 1(entry 3), this value reached to 89% through subjection of Fe3O4/SiO2-DSBA catalyst to the reaction, at the same conditions. Obviously, this difference comes from DSBA and its related interactions with the raw materials. Further, the effects of the reaction time, amount of catalyst, and reaction medium were precisely monitored. From the perfumed control experiments, it was disclosed that the highest yield is obtained via using 25 mol% of Fe3O4/SiO2-DSBA particles in ethanol over 180 min. Also, from a comparison between the applied methods, it was revealed that the stirring better works than the ultrasonication. Although, a better dispersion state is obtained for the catalyst’s particles under the ultrasonication conditions, it seems that the Se-Se site is not stable enough to tolerate the ultrasound waves. The water medium and even solvent-free conditions were experimented for the catalytic process. As is observed in Table 1(entries 14 and 15), very low reaction yields were obtained at the mentioned conditions. For the water medium, it may originate from inappropriate dispersion of the particles due to the presence of the propyl groups (as a hydrophobic agent) on the surfaces69. For the solvent-free conditions, a ball-milling equipment was used, and it was found out that the Fe3O4/SiO2-DSBA structure is sensitive to mechanical hitting, and is damaged. As well, it was mentioned in characterization section (MS analysis) that the Se-Se bond is sensitive to the excited electrons, and quickly breaks down. The determined optimal condition was applied in some additional peptide coupling reactions, and the obtained dipeptide structures were recognized with H-NMR spectroscopy, as presented in Figures S6–S8 (in the SI section). According to the above descriptions, other dipeptide structures (reported in the SI section) were synthesized under the optimal catalytic conditions.
Recyclability of Fe3O4/SiO2-DSBA catalytic system
The reusability of the prepared Fe3O4/SiO2-DSBA catalytic system in amid bond formation was evaluated in the model reaction of glycine methyl ester and protected phenylalanine. For this aim, after completion of the reaction, the Fe3O4/SiO2-DSBA nanoparticles were separated from the reaction mixture by an external magnet and then washed with distilled water, and then dried in an oven in order to get ready for the next catalytic run. Then, the recovered catalyst in a constant amount was utilized for additional five subsequent runs. According to Fig. 10a, a partial reduction (7%) in catalytic performance of the recovered Fe3O4/SiO2-DSBA was observed, but a sharp decrease was occurred during the next recycles until 35% of the initial value was lost. As the most probable contributor to this, it can be stated that there was a severe agglomeration in the recovered particles after the third and fourth runs. At the first stages of recyclization, irradiation of the ultrasound waves (50 kHz, 100 W L−1) led to well re-dispersion of the particles, but severe agglomeration after the third run reduced the total performance of the catalyst. The mentioned agglomeration that is occurred due to the paramagnetic behavior of the Fe3O4/SiO2-DSBA particles, causes the active catalytic sites (Se-Se) to be blocked and significantly reduced54. Therefore, the catalytic performance is sharply dropped after several times utilization and recovery, and longer times of ultrasonication is needed. According to literature, long time ultrasonication in the cleaner bath can cause damage to the core/shell structure of Fe3O4/SiO269. Figure 10b, c show the results of the EDX and SEM analyses on the recovered Fe3O4/SiO2-DSBA nanoparticles after six successive usages. According to Fig. 10b, after six consecutive uses, the Fe3O4/SiO2-DSBA nanocatalyst still has the main element of its catalytic site, (selenium), which can be a reason for a yield of 54% after six consecutive uses. According to Fig. 10c, the morphology, uniformity, and size of the Fe3O4/SiO2-DSBA catalytic system have not significantly changed compared to the first use, but particles agglomeration is clearly confirmed by the prepared SEM image. Also, Fig. 10d reveals that no changes in the present functional groups onto the surface of the Fe3O4/SiO2-DSBA particles occurred during the recyclization, as the sharp peaks related to the stretching vibrations of Si‒O‒Si, C=O, and C‒H bonds are still seen in the prepared FTIR spectrum. Based on these results, this is concluded that the presented Fe3O4/SiO2-DSBA catalytic system includes economic benefits in comparison with the homogeneous analogues, as they are not able to be recycled and reused for several times.
(a) Recyclability investigation of Fe3O4/SiO2-DSBA nanoparticles in catalyzed peptide coupling reactions. The results were obtained from the coupling reaction between glycine methyl ester and Fmoc-protected phenyl alanine, per 0.25 mol% of the catalyst at room temperature, (b) EDX data, (c) SEM image, and (d) FTIR spectrum of the recovered Fe3O4/SiO2-DSBA nanoparticles after six times recycles.
Suggested mechanism
A plausible mechanism for the catalyzed amide/peptide bond formation by the prepared Fe3O4/SiO2-DSBA system is schematically presented in Fig. 1148. As is observed, totally five stages should be passed to achieve the intended amide/peptide bond and the recovered Fe3O4/SiO2-DSBA. The first stage of this mechanism begins by insertion of triethyl phosphite as an initial reducing agent70. At this stage, the attachment of phosphorus atom to one of the involved selenium atoms creates a phosphonium structure which is an active intermediate. In stage 2, the carboxylate group in the structure of the first amino acid is attached to the phosphonium center. In the third stage, a selenide attacks to the carbonyl group, and a triethyl phosphate (O = P(OEt)3) is subsequently released71. At this state, the first amino acid is active and ready for the attachment of the amine group from second amino acid. The next stage involves the attack of the amine group of the second amino acid to the carbonyl group of the first amino acid leading to the formation of a peptide bond. In the final stage (stage 5), the negatively charged selenium is oxidized by the oxygen in the air72, and the initial structure of DSBA is recovered through elimination of a water molecule.
Comparisons
So far, several heterogeneous catalytic systems have been suggested for facilitating the amide/peptide bond formation, because this type of chemical couplings is of high importance in the current pharmaceutical researches73. Hence, it would be essential to highlight the advantageous of these catalytic systems for further consideration by the researchers in the field. As discussed in the introduction section, high heterogeneity and paramagnetic behavior of the designed Fe3O4/SiO2-DSBA catalytic system can be mentioned as the foremost merits that provide this great opportunity to conveniently separate and recover the particles for successive utilization. Therefore, in comparison with the homogenous species (Table 2, entries 1–4), the proposed Fe3O4/SiO2-DSBA catalytic system includes merit for utilization and recyclization. Form economic aspect, it was clearly presented in this report that inexpensive materials were used that are quite available in the laboratories. So, preparation of the presented catalytic system would be reasonable for large-scale utilization. In comparison with the similar systems that include magnetic property (Table 2, entry 5), exploitation of diselenide compounds are safer than the isothiazolone (IT) derivatives, which can cause severe side effects such as skin irritations and allergies74. As well, the used amount of the catalyst particles is less in the case of Fe3O4/SiO2-DSBA system, confirming higher efficiency than the other similar systems. Table 2 provides information on several catalysts that are capable of catalyzing the formation of amide bonds. This table can be used to compare the performance of the Fe3O4/SiO2-DSBA catalytic system with the other catalysts with a quick glance. Given the yield percentage and reaction condition of the method presented in this study, it seems that this method deserves much attention.
Experimental section
Materials and equipment
All the chemicals, reagents, and equipment used in this study are listed in Table 3.
Preparation methods
Preparation of K2Se2
Initially, 4.38 mmol of selenium element powder was transferred into a round-bottom flask (50 mL), and the reflux system was set up at room temperature, under N2 atmosphere. Then, 6.6 mmol of KOH and 0.55 mmol of KBH4 were poured into a beaker which was in the ice bath, and then 4.0 mL of deionized water was added and then stirred with a glass stirrer to obtain a clear solution. The resulting clear solution was added into the selenium-containing flask using a syringe. Next, the content of the flask was stirred vigorously under reflux conditions (90 °C) for an hour to obtain a red–black solution.
Synthesis of DSBA
For the synthesis of 2-carboxybenzenediazonium chloride, in a round-bottom flask (50 mL), anthranilic acid (4.38 mmol) was dissolved in deionized water (8.0 mL) via stirring. Then, 0.5 mL of HCl was added into the flask through a dropwise manner to obtain a clear solution. After complete dissolution, the flask was transferred into an ice bath including salt and acetone (0 °C). Then, NaNO2 (5.27 mmol) was dissolved in 1.5 mL of deionized water in a separate beaker. The NaNO2 solution was then added dropwise into the anthranilic acid-containing flask, which had been placed in an ice bath. Next, the resulting solution was stirred at 0 °C for 45 min. In the next step, the metal salt solution of K2Se2, which was synthesized in the previous step, was added dropwise to the solution in the ice bath. The flask was then cooled down to room temperature. Afterward, it was stirred vigorously at 90 °C for 2 h until a dark red solution was precipitated at the end of the reaction flask. Again, the flask was cooled down to room temperature. Next, to separate the unreacted and oxidized selenium from the products, the resulting solution was filtered by a thin celite pad. The presence of a small amount of the unreacted selenium on the celite pad indicated that the majority of the primary material has been converted to Se2− form. In the last step, HCL (7.0 mL, 1.0 M) was added to the filtrate and then the resulting precipitates were filtered through a paper filter. The resulting precipitate was recrystallized with hot methanol for purification79.
Preparation of Fe3O4 nanoparticles
In a three-necked round-bottom flask (500 mL), 20.0 mmol of FeCl3·6H2O and 20.0 mmol of FeCl2·4H2O were dissolved in 200.0 mL of deionized water, via ultrasonication for 20 min. The flask was then placed in an oil bath and heated up to 45 °C, and stirred under N2 atmosphere. After complete dissolution, the temperature was slowly raised to 85 °C and the mixture was vigorously stirred at the same condition for additional 2 h. Then, 30.0 mL of concentrated ammonia solution (25%) was dropwise added into the mixture for 1 h. Finally, after cooling the solution, the magnetic nanoparticles were collected by an external magnet and washed several times with deionized water, ethanol and acetone, and dried in oven (60 °C).
Preparation of Fe3O4@SiO2 nanoparticles
In a round-bottom flask (50 mL), 1.0 g of Fe3O4 NPs was placed and 10.0 mL of deionized water, 5.0 mL of ethanol, 5.0 mL of PEG-300, and 1.0 mL of ammonia were added and stirred at room temperature. Next, 2.0 mL of tetraethylorthosilicate (TEOS) was dissolved in 10.0 mL of ethanol in a separate flask, and the resulted solution was dropwise added into the flask containing Fe3O4 NPs, and the content was stirred for 12 h at room temperature. In the next step, the obtained Fe3O4@SiO2 nanoparticles were collected by an external magnet and washed several times with deionized water as well as ethanol, and ultimately dried in oven (60 °C).
Preparation of Fe3O4@SiO2-NH2 nanoparticles
In a round-bottom flask (100 mL), 2.0 g of Fe3O4@SiO2 that was prepared in the previous stage, was dispersed in toluene (50.0 mL) via ultrasonication for 15 min. Then, 4.0 mL of 3-aminopropyltriethoxysilane (APTES) was added into the flask, and the reflux was set up at 110 °C under N2 atmosphere. The content was vigorously stirred for 12 h. Finally, after cooling down to room temperature, the obtained Fe3O4@SiO2-NH2 nanoparticles were collected by an external magnet and washed several times with toluene.
Preparation of Fe3O4/SiO2-DSBA catalytic system
In a round-bottom flask (25 mL), 0.7 g of Fe3O4@SiO2-NH2 was dispersed in 3.0 mL of dimethylsulfoxide (DMSO), under N2 atmosphere. Then, in a separate flask, 0.1 g of the synthesized DSBA was dissolved in 3.0 mL of DMSO, and in another flask, 0.2 mL of diisopropylethylamine (DIEA) was mixed with 2.0 mL of DMSO and 0.095 g of TBTU. Then, these two solutions were simultaneously added into the main flask containing Fe3O4@SiO2-NH2 using a syringe (drop-by-drop), and stirred at room temperature for 2 h. Finally, the Fe3O4/SiO2-DSBA nanoparticles were collected by an external magnet and washed several times with water and ethanol, and dried at room temperature.
General procedure of amide/peptide bond formation catalyzed by Fe3O4/SiO2-DSBA system
In a round-bottom flask (25 mL), Fe3O4/SiO2-DSBA particles (0.1 g, 0.25 mol%) were dispersed in ethanol (10.0 mL) using an ultrasound bath (50 kHz, 100 W L−1), and P(OEt)3 (0.1 mol) was added into the mixture. Next, 4.0 mmol of the first amino acid (N-protected) was added into the flask, and the resulting mixture was stirred for 30 min at room temperature. Then, 4.6 mmol of acid-protected amino acid was added and the resulting mixture was stirred at room temperature, for 3 h under air atmosphere. After this time, the nanoparticles of Fe3O4/SiO2-DSBA were separated from the reaction medium by an external magnet and washed several times with ethanol and then dried in an oven at 60 °C to be reused if necessary. For the purification of the synthesized dipeptide compound, 10.0 mL dichloromethane (DCM) and 5.0 mL of deionized water were added to the solution, and the mixture was transferred into a separatory funnel (100 mL) and well mixed. The phosphate compound and unreacted amino acid (acid-protected) are removed via separation of DCM from aqueous phase. Then, the DCM phase was dehydrated through addition of magnesium sulfate powder (0.5 g). After 30 min, the swollen magnesium sulfate crystals were separated via paper filtration, and the remained solution was dried by rotary evaporator. To have a clean NMR spectrum of the synthesized Cys-Arg dipeptide structure, removal of the protecting groups was essential to be performed. For this purpose, 2.0 mmol of the obtained Cys-Arg (protected) was dissolved in DCM (4.0 mL), and then piperidine (2.0 mL, 0.25% in DCM) was added into the solution, and stirred for 30 min at room temperature. Then, the flask was put into an ice bath and cold diethyl ether was gradually added to the solution during gentle stirring. The obtained white powder was separated via filtration with a sintered glass filter, and dried in the vacuum oven. The powder was then dissolved in ethyl acetate (2.0 mL) and trifluoroacetic acid (8.0 mL, 95% in water) and stirred for 30 min, at 10 °C in an ice bath. Finally, the solution was concentrated by rotary evaporator, and cold diethyl ether was gradually added to the solution during gentle stirring until the color of the solution turned into white. The obtained white powder was separated via filtration with a sintered glass filter, and dried in the vacuum oven.
Conclusion
In continuing our previous efforts in preparation of the heterogeneous peptide coupling reagents, a nanoscale catalytic system has been designed and successfully applied in rapid formation of the amide/peptide bond between the amino acids in the solution phase. In this regard, a simple core/shell structure of Fe3O4/SiO2 nanoparticles has been constructed and functionalized with 2,2′-diselenobis(benzoic acid) (DSBA), as the main catalytic site for amide/peptide bonding. The DSBA structure has been synthesized through organic synthesis techniques, and then identified by NMR and MS spectroscopic methods. After full characterization of the catalyst’s structure, its capability in assisting the amide bond formation was investigated in the solution-phase dipeptide constructions, wherein ca. 89% reaction yield was obtained at optimal conditions (180 min, room temperature). The protected amino acids including Fmoc-Ala-OH, Fmoc-Phe-OH, Fmoc-Arg(pbf)-OH, and glycine methyl ester were purchased and experimented to screen the catalytic process. In this account, a plausible mechanism has been suggested for the catalytic process in which sensitive role of the diselenide bond was highlighted, based on the supportive resources. Concisely, a red/ox process is driven by triethyl phosphine through which the diselenide bond in the structure of DSBA is opened, and the carboxylate group of the amino acid is activated. The structure of DSBA is then recovered through oxidation by the air. Due to showing a substantial paramagnetic behavior, the Fe3O4/SiO2-DSBA particles were conveniently collected, revived, and reused in three successive catalytic runs with only 7% reduction in the total performance. Overall, due to all mentioned brilliant points for the presented nanocatalyst, large-scale fabrication and utilization is the industrial applications is recommended. As a point that may be focused in future practices, the preparation method of the proposed catalyst can be modified, since the active diselenide bond may be affected to some extent within the covalent attachment onto the particles surfaces. Hence, it can be a challenging suggestion for the next efforts in the same field of research.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information file.
References
Liu, X. H., Ma, J. G., Niu, Z., Yang, G. M. & Cheng, P. An efficient nanoscale heterogeneous catalyst for the capture and conversion of carbon dioxide at ambient pressure. Angew. Chem. Int. Ed. 54(3), 988–991 (2015).
Pathak, G., Das, D., Rajkumari, K. & Rokhum, L. Exploiting waste: Towards a sustainable production of biodiesel using Musa acuminata peel ash as a heterogeneous catalyst. Green Chem. 20(10), 2365–2373 (2018).
Ahmadizadeh Shendy, S., Babazadeh, M., Shahverdizadeh, G. H., Hosseinzadeh-Khanmiri, R. & Es’haghi, M. Synthesis of the quinazolinone derivatives using an acid-functionalized magnetic silica heterogeneous catalyst in terms of green chemistry. Mol. Divers. 25(2), 889–897 (2021).
Taheri-Ledari, R., Hashemi, S. M. & Maleki, A. High-performance sono/nano-catalytic system: CTSN/Fe3O4–Cu nanocomposite, a promising heterogeneous catalyst for the synthesis of N-arylimidazoles. RSC Adv. 9(69), 40348–40356 (2019).
Zhang, W. et al. Nanoscale Bioconjugates: A review of the structural attributes of drug-loaded nanocarrier conjugates for selective cancer therapy. Heliyon 8, e09577 (2022).
Taheri-Ledari, R., Rahimi, J., Maleki, A. & Shalan, A. E. Ultrasound-assisted diversion of nitrobenzene derivatives to their aniline equivalents through a heterogeneous magnetic Ag/Fe3O4-IT nanocomposite catalyst. New J. Chem. 44(45), 19827–19835 (2020).
Varzi, Z., Esmaeili, M. S., Taheri-Ledari, R. & Maleki, A. Facile synthesis of imidazoles by an efficient and eco-friendly heterogeneous catalytic system constructed of Fe3O4 and Cu2O nanoparticles, and guarana as a natural basis. Inorg. Chem. Commun. 125, 108465 (2021).
Taheri-Ledari, R. et al. Highly porous copper-supported magnetic nanocatalysts: Made of volcanic pumice textured by cellulose and applied for the reduction of nitrobenzene derivatives. RSC Adv. 11(41), 25284–25295 (2021).
Zolfigol, M. A., Kiafar, M., Yarie, M., Taherpour, A. A. & Saeidi-Rad, M. Experimental and Theoretical Studies of the nanostructured {Fe3O4@SiO2@(CH2)3 Im}C(CN)3 catalyst for 2-amino-3-cyanopyridine preparation via an anomeric based oxidation. RSC Adv. 6(55), 50100–50111 (2016).
Taheri-Ledari, R. & Maleki, A. Magnetic nanocatalysts utilized in the synthesis of aromatic pharmaceutical ingredients. New J. Chem. 45(9), 4135–4146 (2021).
Rahimi, J., Taheri-Ledari, R., Niksefat, M. & Maleki, A. Enhanced reduction of nitrobenzene derivatives: Effective strategy executed by Fe3O4/PVA-10% Ag as a versatile hybrid nanocatalyst. Catal. Commun. 134, 105850 (2020).
Taheri-Ledari, R. et al. High-performance sono/nano-catalytic system: Fe3O4@Pd/CaCO3-DTT core/shell nanostructures, a suitable alternative for traditional reducing agents for antibodies. Ultrason. Sonochem. 61, 104824 (2020).
Isac-García, J., Dobado, J. A., Calvo-Flores, F. G., & Martínez-García, H. Chapter 13-Green Chemistry Experiments. In Experimental Organic Chemistry, 417–484 (2016).
Oliveira, V. G. D., Cardoso, M. F. D. C. & Forezi, L. D. S. M. Organocatalysis: A brief overview on its evolution and applications. Catalysts 8(12), 605 (2018).
Yarie, M., Zolfigol, M. A. & Saeidi-Rad, M. Tributyl (3-sulfopropyl) phosphonium hydrogen sulfate (TBSPHS) as a novel task-specific phosphonium ionic liquid: A robust catalyst for the synthesis of 1, 5-dihydro-2H-pyrrol-2-ones. J. Mol. Liq. 249, 144–152 (2018).
Sharma, H., Sharma, S. & Paul, S. 2-(5-phenyl-4H-1, 2, 4-triazol-3-ylthio) acetic acid: Greener and efficient organocatalyst for multicomponent reactions under aqueous media. Curr. Res. Green Sustain. Chem. 4, 100181 (2021).
Wang, Z. H., You, Y., Chen, Y. Z., Xu, X. Y. & Yuan, W. C. An asymmetric organocatalytic vinylogous Mannich reaction of 3-methyl-5-arylfuran-2 (3H)-ones with N-(2-pyridinesulfonyl) imines: Enantioselective synthesis of δ-amino γ, γ-disubstituted butenolides. Org. Biomol. Chem. 16(10), 1636–1640 (2018).
Dam, B., Saha, M., Jamatia, R. & Pal, A. K. Nano-ferrite supported glutathione as a reusable nano-organocatalyst for the synthesis of phthalazine-trione and dione derivatives under solvent-free conditions. RSC Adv. 6(60), 54768–54776 (2016).
Gleeson, O. et al. The immobilisation of chiral organocatalysts on magnetic nanoparticles: The support particle cannot always be considered inert. Org. Biomol. Chem. 9(22), 7929–7940 (2011).
Fattahi, N., Ramazani, A., Ahankar, H., Asiabi, P. A. & Kinzhybalo, V. Tetramethylguanidine-functionalized Fe3O4/Chloro-Silane core-shell nanoparticles: An efficient heterogeneous and reusable organocatalyst for aldol reaction. SILICON 11(3), 1441–1450 (2019).
Salman, D. et al. Synthesis, surface modification and characterization of magnetic Fe3O4@ SiO2 core-shell nanoparticles. J. Phys. Conf. Ser. 1773, 012039 (2021).
Ganjali, F., Kashtiaray, A., Zarei-Shokat, S., Taheri-Ledari, R. & Maleki, A. Functionalized hybrid magnetic catalytic systems on micro-and nanoscale utilized in organic synthesis and degradation of dyes. Nanoscale Adv. 4, 1263–1307 (2022).
Maleki, A., Valadi, K., Gharibi, S. & Taheri-Ledari, R. Convenient and fast synthesis of various chromene pharmaceuticals assisted by highly porous volcanic micro-powder with nanoscale diameter porosity. Res. Chem. Intermed. 46(9), 4113–4128 (2020).
Salman, A. D. et al. Synthesis and surface modification of magnetic Fe3O4@SiO2 core-shell nanoparticles and its application in uptake of scandium (III) ions from aqueous media. Environ. Sci. Pollut. Res. 28(22), 28428–28443 (2021).
Nguyen, M. D., Tran, H. V., Xu, S. & Lee, T. R. Fe3O4 Nanoparticles: Structures, synthesis, magnetic properties, surface functionalization, and emerging applications. Appl. Sci. 11(23), 11301 (2021).
Ashraf, M. A., Liu, Z., Peng, W. X. & Zhou, L. Glycerol Cu (II) complex supported on Fe3O4 magnetic nanoparticles: A new and highly efficient reusable catalyst for the formation of aryl-sulfur and aryl-oxygen bonds. Catal. Lett. 150(4), 1128–1141 (2020).
Mallakpour, S., Tukhani, M. & Hussain, C. M. Sustainable plant and microbes-mediated preparation of Fe3O4 nanoparticles and industrial application of its chitosan, starch, cellulose, and dextrin-based nanocomposites as catalysts. Int. J. Biol. Macromol. 179, 429–447 (2021).
Sharma, R. K. et al. Fe3O4 (iron oxide)-supported nanocatalysts: Synthesis, characterization and applications in coupling reactions. Green Chem. 18(11), 3184–3209 (2016).
Kainz, Q. M. & Reiser, O. Polymer-and dendrimer-coated magnetic nanoparticles as versatile supports for catalysts, scavengers, and reagents. Acc. Chem. Res. 47(2), 667–677 (2014).
Wang, X. Challenges and outlook for catalytic direct amidation reactions. Nat. Catal. 2, 98–102 (2019).
Isidro-Llobet, A. et al. Sustainability challenges in peptide synthesis and purification: From R&D to production. J. Org. Chem. 84, 4615–4628 (2019).
Maleki, A., Taheri-Ledari, R., Rahimi, J., Soroushnejad, M. & Hajizadeh, Z. Facile peptide bond formation: Effective interplay between isothiazolone rings and silanol groups at silver/iron oxide nanocomposite surfaces. ACS Omega 4(6), 10629–10639 (2019).
Kalantari, F., Ramazani, A., Poor Heravi, M. R., Aghahosseini, H. & Ślepokura, K. Magnetic nanoparticles functionalized with copper hydroxyproline complexes as an efficient, recoverable, and recyclable nanocatalyst: Synthesis and its catalytic application in a tandem Knoevenagel-Michael cyclocondensation reaction. Inorg. Chem. 60, 15010–15023 (2021).
Veisi, H., Taheri, S. & Hemmati, S. Preparation of polydopamine sulfamic acid-functionalized magnetic Fe3O4 nanoparticles with a core/shell nanostructure as heterogeneous and recyclable nanocatalysts for the acetylation of alcohols, phenols, amines and thiols under solvent-free conditions. Green Chem. 18(23), 6337–6348 (2016).
Philpott, H. K., Thomas, P. J., Tew, D., Fuerst, D. E. & Lovelock, S. L. A versatile biosynthetic approach to amide bond formation. Green Chem. 20(15), 3426–3431 (2018).
Sabatini, M. T., Boulton, L., Sneddon, H. F. & Sheppard, T. D. A green chemistry perspective on catalytic amide bond formation. Nat. Catal. 2(1), 10–17 (2019).
Gooßen, L. J., Ohlmann, D. M. & Lange, P. P. The thermal amidation of carboxylic acids revisited. Synthesis 2009(1), 160–164 (2009).
Perreux, L., Loupy, A. & Volatron, F. Solvent-free preparation of amides from acids and primary amines under microwave irradiation. Tetrahedron 58(11), 2155–2162 (2002).
Dunetz, J. R., Magano, J. & Weisenburger, G. A. Large-scale applications of amide coupling reagents for the synthesis of pharmaceuticals. Org. Process Res. Dev. 20, 140–177 (2016).
Müller, A., Cadenas, E., Graf, P. & Sies, H. A novel biologically active seleno-organic compound—1: Glutathione peroxidase-like activity in vitro and antioxidant capacity of PZ 51 (Ebselen). Biochem. Pharmacol. 33(20), 3235–3239 (1984).
Chaudhary, S., Chauhan, P., Kumar, R. & Bhasin, K. K. Toxicological responses of surfactant functionalized selenium nanoparticles: A quantitative multi-assay approach. Sci. Total Environ. 643, 1265–1277 (2018).
Nogueira, C. W. & Rocha, J. B. T. Diphenyl diselenide a janus-faced molecule. J. Braz. Chem. Soc. 21, 2055–2071 (2010).
Satheeshkumar, K., Raju, S., Singh, H. B. & Butcher, R. J. Reactivity of selenocystine and tellurocystine: Structure and antioxidant activity of the derivatives. Chem. Eur. J. 24(66), 17513–17522 (2018).
Rangraz, Y., Nemati, F. & Elhampour, A. Diphenyl diselenide immobilized on magnetic nanoparticles: A novel and retrievable heterogeneous catalyst in the oxidation of aldehydes under mild and green conditions. J. Colloid. Interface Sci. 509, 485–494 (2018).
Chen, L. et al. Healable and rearrangeable networks of liquid crystal elastomers enabled by diselenide bonds. Angew. Chem. Int. Ed. 133(30), 16530–16534 (2021).
Chaudhary, S., Umar, A. & Mehta, S. K. Selenium nanomaterials: An overview of recent developments in synthesis, properties and potential applications. Prog. Mater. Sci. 83, 270–329 (2016).
Chauhan, P. & Chaudhary, S. Role of surface modification on selenium nanoparticles: Enumerating the optical, thermal and structural properties. Opt. Mater. 97, 109380 (2019).
Handoko, S. S., Panigrahi, N. R. & Arora, P. S. Rational design of an organocatalyst for peptide bond formation. J. Am. Chem. Soc. 141(40), 15977–15985 (2019).
Begini, F. et al. Continuous flow synthesis of 2,2′-diselenobis (benzoic acid) and derivatives. React. Chem. Eng. 5(4), 641–644 (2020).
Taheri-Ledari, R., Rahimi, J. & Maleki, A. Method screening for conjugation of the small molecules onto the vinyl-coated Fe3O4/silica nanoparticles: Highlighting the efficiency of ultrasonication. Mater. Res. Express 7(1), 015067 (2020).
Fatahi, H., Jafarzadeh, M. & Pourmanouchehri, Z. Synthesis of α-aminonitriles and 5-substituted 1 H-tetrazoles using an efficient nanocatalyst of Fe3O4@ SiO2–APTES-supported trifluoroacetic acid. J. Heterocycl. Chem. 56(8), 2090–2098 (2019).
Hajizadeh, Z., Valadi, K., Taheri-Ledari, R. & Maleki, A. Convenient Cr (VI) removal from aqueous samples: Executed by a promising clay-based catalytic system, magnetized by Fe3O4 nanoparticles and functionalized with humic acid. ChemistrySelect 5(8), 2441–2448 (2020).
Maleki, A., Taheri-Ledari, R., Ghalavand, R. & Firouzi-Haji, R. Palladium-decorated o-phenylenediamine-functionalized Fe3O4/SiO2 magnetic nanoparticles: A promising solid-state catalytic system used for Suzuki-Miyaura coupling reactions. J. Phys. Chem. Solids 136, 109200 (2020).
Yang, L. et al. Modification and characterization of Fe3O4 nanoparticles for use in adsorption of alkaloids. Molecules 23(3), 562 (2018).
Shendy, S. A., Shahverdizadeh, G. H., Babazadeh, M. & Hosseinzadeh-Khanmiri, R. Preparation and characterization of acetic acid-functionalized Fe3O4@SiO2 nanoparticles as an efficient nanocatalyst for the synthesis of nitrones in water. SILICON 12(7), 1735–1742 (2020).
Karade, V. C. et al. APTES monolayer coverage on self-assembled magnetic nanospheres for controlled release of anticancer drug Nintedanib. Sci. Rep. 11(1), 5674 (2021).
Ghasemzadeh, M. A., Abdollahi-Basir, M. H. & Babaei, M. Fe3O4@ SiO2–NH2 core-shell nanocomposite as an efficient and green catalyst for the multi-component synthesis of highly substituted chromeno [2, 3-b] pyridines in aqueous ethanol media. Green Chem. Lett. Rev. 8, 40–49 (2015).
Hassanzadeh-Afruzi, F. et al. Efficient removal of Pb (II)/Cu (II) from aqueous samples by a guanidine-functionalized SBA-15/Fe3O4. Sep. Purif. Technol. 291, 120956 (2022).
Taheri-Ledari, R. et al. Plasmonic photothermal release of docetaxel by gold nanoparticles incorporated onto halloysite nanotubes with conjugated 2D8-E3 antibodies for selective cancer therapy. J. Nanobiotechnol. 19(1), 1–21 (2021).
Zhang, W. et al. Enhanced activity of vancomycin by encapsulation in hybrid magnetic nanoparticles conjugated to a cell-penetrating peptide. Nanoscale 12(6), 3855–3870 (2020).
Maleki, A., Taheri-Ledari, R. & Soroushnejad, M. Surface functionalization of magnetic nanoparticles via palladium-catalyzed Diels-Alder approach. ChemistrySelect 3(46), 13057–13062 (2018).
Nasrollahzadeh, M., Sajadi, S. M., Rostami-Vartooni, A., Bagherzadeh, M. & Safari, R. Immobilization of copper nanoparticles on perlite: Green synthesis, characterization and catalytic activity on aqueous reduction of 4-nitrophenol. J. Mol. Catal. A Chem. 400, 22–30 (2015).
Hosseini Mohtasham, N. & Gholizadeh, M. Nano silica extracted from horsetail plant as a natural silica support for the synthesis of H3PW12O40 immobilized on aminated magnetic nanoparticles (Fe3O4@ SiO2-EP-NH-HPA): A novel and efficient heterogeneous nanocatalyst for the green one-pot synthesis of pyrano [2, 3-c] pyrazole derivatives. Res. Chem. Intermed. 46, 3037–3066 (2020).
Taheri-Ledari, R. et al. Cefixime-containing silica nanoseeds coated by a hybrid PVA-gold network with a Cys-Arg dipeptide conjugation: Enhanced antimicrobial and drug release properties. Langmuir 38(1), 132–146 (2021).
Taheri-Ledari, R. et al. Multi-stimuli nanocomposite therapeutic: Docetaxel targeted delivery and synergies in treatment of human breast cancer tumor. Small 16(41), 2002733 (2020).
Xu, H., Cao, W. & Zhang, X. Selenium-containing polymers: Promising biomaterials for controlled release and enzyme mimics. Acc. Chem. Res. 46(7), 1647–1658 (2013).
Taheri-Ledari, R., Rahimi, J. & Maleki, A. Synergistic catalytic effect between ultrasound waves and pyrimidine-2, 4-diamine-functionalized magnetic nanoparticles: Applied for synthesis of 1, 4-dihydropyridine pharmaceutical derivatives. Ultrason. Sonochem. 59, 104737 (2019).
Taheri-Ledari, R. & Maleki, A. Antimicrobial therapeutic enhancement of levofloxacin via conjugation to a cell-penetrating peptide: An efficient sonochemical catalytic process. J. Pept. Sci. 26(10), e3277 (2020).
Saedi, S., Shokri, M., Priyadarshi, R. & Rhim, J. W. Silver ion loaded 3-aminopropyl trimethoxysilane-modified Fe3O4 nanoparticles for the fabrication of carrageenan-based active packaging films. Colloids Surf. B 208, 112085 (2021).
Aegurla, B. et al. Triethyl phosphite/benzoyl peroxide mediated reductive dealkylation of O-benzoylhydroxylamines: A cascade synthesis of secondary amides. Eur. J. Org. Chem. 27, 4235–4238 (2020).
Liebeskind, L. S., Gangireddy, P. & Lindale, M. G. Benzoisothiazolone organo/copper-cocatalyzed redox dehydrative construction of amides and peptides from carboxylic acids using (EtO)3P as the reductant and O2 in air as the terminal oxidant. J. Am. Chem. Soc. 138(21), 6715–6718 (2016).
Akondi, S. M., Gangireddy, P., Pickel, T. C. & Liebeskind, L. S. Aerobic, diselenide-catalyzed redox dehydration: Amides and peptides. Org. Lett. 20(3), 538–541 (2018).
Lundberg, H., Tinnis, F., Selander, N. & Adolfsson, H. Catalytic amide formation from non-activated carboxylic acids and amines. Chem. Soc. Rev. 43(8), 2714–2742 (2014).
Silva, V. et al. Isothiazolinone biocides: Chemistry, biological, and toxicity profiles. Molecules 25(4), 991 (2020).
Koshizuka, M., Makino, K. & Shimada, N. Diboronic acid anhydride-catalyzed direct peptide bond formation enabled by hydroxy-directed dehydrative condensation. Org. Lett. 22, 8658–8664 (2020).
El Dine, T. M., Erb, W., Berhault, Y., Rouden, J. & Blanchet, J. Catalytic chemical amide synthesis at room temperature: One more step toward peptide synthesis. J. Org. Chem. 80(9), 4532–4544 (2015).
Filip, S. V., Lejeune, V., Vors, J. P., Martinez, J. & Cavelier, F. Peptide bond formation using polymer-bound BOP. Eur. J. Org. Chem. 2004(9), 1936–1939 (2004).
Muramatsu, W., Hattori, T. & Yamamoto, H. Substrate-directed lewis-acid catalysis for peptide synthesis. J. Am. Chem. Soc. 141, 12288–12295 (2019).
Krasowska, D. et al. Ultrasound-assisted synthesis of alkali metals diselenides (M2Se2) and their application for the gram-scale preparation of 2,2’-diselenobis (benzoic acid). ARKIVOC 2019, 24–37 (2019).
Acknowledgements
The authors appreciate partial support of Iran University of Science & Technology (IUST).
Author information
Authors and Affiliations
Contributions
R.T.-L., F.S.Q. and M.S. have drafted the work, writing—review and editing, analysis and interpretation of data and wrote the main manuscript text and all figures. A.M.: the corresponding (submitting) author of current study, substantial contributions to the conception, design of the work, have drafted the work, writing—review and editing, substantively revised it. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary Video 1.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Taheri-Ledari, R., Qazi, F.S., Saeidirad, M. et al. A diselenobis-functionalized magnetic catalyst based on iron oxide/silica nanoparticles suggested for amidation reactions. Sci Rep 12, 14865 (2022). https://doi.org/10.1038/s41598-022-19030-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-19030-w
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.