Abstract
Patients with SARS-CoV-2 infection present with different lung compliance and progression of disease differs. Measures of lung mechanics in SARS-CoV-2 patients may unravel different pathophysiologic mechanisms during mechanical ventilation. The objective of this prospective observational study is to describe whether Electrical Impedance Tomography (EIT) guided positive end-expiratory pressure (PEEP) levels unravel changes in EIT-derived parameters over time and whether the changes differ between survivors and non-survivors. Serial EIT-measurements of alveolar overdistension, collapse, and compliance change in ventilated SARS-CoV-2 patients were analysed. In 80 out of 94 patients, we took 283 EIT measurements (93 from day 1–3 after intubation, 66 from day 4–6, and 124 from day 7 and beyond). Fifty-one patients (64%) survived the ICU. At admission mean PaO2/FiO2-ratio was 184.3 (SD 61.4) vs. 151.3 (SD 54.4) mmHg, (p = 0.017) and PEEP was 11.8 (SD 2.8) cmH2O vs. 11.3 (SD 3.4) cmH2O, (p = 0.475), for ICU survivors and non-survivors. At day 1–3, compliance was ~ 55 mL/cmH2O vs. ~ 45 mL/cmH2O in survivors vs. non-survivors. The intersection of overdistension and collapse curves appeared similar at a PEEP of ~ 12–13 cmH2O. At day 4–6 compliance changed to ~ 50 mL/cmH2O vs. ~ 38 mL/cmH2O. At day 7 and beyond, compliance was ~ 38 mL/cmH2O with the intersection at a PEEP of ~ 9 cmH2O vs. ~ 25 mL/cmH2O with overdistension intersecting at collapse curves at a PEEP of ~ 7 cmH2O. Surviving SARS-CoV-2 patients show more favourable EIT-derived parameters and a higher compliance compared to non-survivors over time. This knowledge is valuable for discovering the different groups.
Similar content being viewed by others
Introduction
Since the first report of the unusual cases of types of pneumonia attributed to Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) in December 2019 in Wuhan, China; COVID-19, cases have surged across the world, causing considerable strain on the healthcare systems and unprecedented financial recession across the globe1,2. Mechanically ventilated SARS-CoV-2 patients admitted to the Intensive Care Unit (ICU) often require prolonged ventilator support and have a high risk of unfavourable outcome3,4. Studies have tried to unravel the underlying pathophysiology driving the lengthy need for ventilation support and investigate how to optimise ventilation to reduce duration and complications related to mechanical ventilation and favourable outcome5,6.
Since SARS-CoV-2 patients with similar oxygenation efficiency present with diverse lung compliance, pulmonary phenotypes have been proposed7,8. However, vigilance for premature phenotyping has been advocated since disease and pulmonary interaction might change the appearance of phenotype over time9. Nevertheless, heterogeneity of SARS-CoV-2 infection affecting the lung warrants tailored ventilation strategies. Most importantly, disease progression over time requires repeated titration of mechanical ventilation. Patient survival improves with higher positive end-expiratory pressure (PEEP) through reduction of atelectasis and recruitment of collapsed alveoli resulting in a decrease of driving pressure10, whereas excessive PEEP causes alveolar overdistension (OD) and ventilator-induced lung injury11, which is associated with lower patient survival12.
Electrical impedance tomography (EIT) is a clinical bedside technique to tailor mechanical ventilation by visualising aerated lung regions. EIT enables optimisation of PEEP by calculating both percentage OD and percentage alveolar collapse (CL). Optimisation of PEEP improves dynamic lung compliance (Cdyn) and may reduce the length of mechanical ventilation, complications, and mortality13,14,15,16. A case series of 15 mechanically ventilated patients with SARS-CoV-2 infection showed that a high PEEP level was required to reach low OD and low CL as determined by EIT17. However, whether a higher EIT-guided PEEP and a higher PaO2/FiO2-ratio over time are associated with survival remains unknown. Furthermore, case reports and case series of patients with SARS-CoV-2 infection have suggested that EIT can be used to indicate the presence of regional lung strain18, to decide on prone positioning or additional diagnostics in case of clinical deterioration19,20,21,22,23,24,25,26. These studies suggest that Cdyn, OD, and CL change over the course of mechanical ventilation during SARS-CoV-2 infection. How these pulmonary features behave over the course of mechanical ventilation remains uncertain.
We hypothesise that EIT-guided PEEP, OD, CL, and Cdyn change over the course of mechanical ventilation during SARS-CoV-2 infection. Moreover, EIT may identify heterogeneity of pulmonary pathophysiology that differs between survivors and non-survivors over time. Therefore, we conducted an observational study primarily focusing on serial measurements and report on EIT-derived pulmonary parameters over the course of mechanical ventilation in patients with SARS-CoV-227,28.
Methods
The patients’ characteristics, data collection, and the protocol for serial EIT measurements for this cohort have been described previously27.
Participants
In the prospective MaastrICCht cohort, all consecutive COVID-19 patients at a tertiary teaching hospital in the Netherlands were included during the first pandemic wave (25.03.2020 until 12.06.2020). For the present study, we included invasive mechanically ventilated patients with a polymerase chain reaction positive for SARS-CoV-2 and a chest CT-scan scored positive based on a CORADS-score of 4–5 by a radiologist29. Patients were admitted via our emergency department, the non-ICU wards, and referral from other ICUs. Patients with a pacemaker or a body mass index ≥ 50 were excluded.
Our ICU has a capacity of 27 beds, divided over three subunits. To provide care for patients during the first wave of the COVID-19 pandemic, our ICU extended its capacity to a maximum of 64 beds. The medical ethical review board of the Maastricht UMC+ approved the study (review number 2020-1565/300523), which was performed in accordance with the declaration of Helsinki. During the pandemic, the board of directors of Maastricht UMC+ adopted a policy to inform patients or their legal representative and ask their consent to use their data for COVID-19 research purposes27. Informed consent was obtained for every patient. The study is registered in the Netherlands Trial Register (NL8613; 12/05/2020).
Clinical protocol
All patients were ventilated in a pressure-controlled mode with tidal volumes around 6 ml/kg predicted body weight. No strict institutional guidelines on PEEP setting had been in use, and PEEP was set at the discretion of the treating physician based on clinical information and guidance according to a lung-protective ventilation strategy until the first EIT assessment.
Daily clinical, physiological, and laboratory measurements
In addition to EIT measurements, arterial blood gas (ABG) analyses and ventilator settings before and after the PEEP trial were recorded in the daily clinical medical report form. Specifically, an ABG was collected before the PEEP trial (at 6 a.m. and if ventilator settings (besides FiO2) were changed between 6 a.m. and the EIT measurement) and half an hour after the PEEP trial. The ABG information combined with the FiO2 settings enable calculating the PaO2/FiO2-ratio to evaluate the effect of PEEP optimisation30,31,32,33.
Serial chest electrical impedance tomography
An EIT belt was placed around the patients’ chest at the fourth or fifth intercostal space at the parasternal line and connected to an EIT monitor (Pulmovista® 500, Dräger Medical GmbH, Lübeck, Germany). Measurements were done in both prone and supine position. When a patient was turned from a supine to a prone position (or vice versa), the initiation of measurements was postponed at least 30 min to allow for stable mechanical ventilation conditions. To obtain accurate serial measurements, the position of the electrode belt was marked on the patients’ skin to ensure that each subsequent measurement was performed at the same position. The EIT monitor was connected to the ventilator to import all respiratory variables (e.g. tidal volume and Cdyn). The PEEP level was then increased until there was no further alveolar recruitment and solely OD, followed by a decremental PEEP trial, which was performed with steps of 2 cmH2O PEEP, preferably starting at 24 cmH2O. Each PEEP level was maintained for 30–60 s, and driving pressure was kept constant during the PEEP trial. However, the number of steps performed and the starting PEEP level could vary between patients. Hence, we present some data for PEEP levels beyond 24 cmH2O. Chest EIT measurements were recorded continuously during the decremental PEEP trial. The PEEP trial was discontinued if a significant CL was shown on the chest EIT images, a reduction of Cdyn, or a SpO2 < 88%. In addition to the chest EIT images, the percentage of CL and OD, tidal volume and Cdyn for each PEEP step were collected27,34. Importantly, chest EIT measurements were performed serially over the course of mechanical ventilation.
For the present study, any EIT data measured during Pressure support (PS) and Continuous positive airway pressure (CPAP) were excluded. The support ventilation modes increase the chance of informative missing data as serial EIT data on PS and CPAP are likely related to survival since these modes are indicative for weaning from mechanical ventilation. In addition, the EIT algorithm for calculating the level of OD and CL is not validated for PS and CPAP.
Post-measurement
Optimal EIT-guided PEEP was determined at a level of CL ≤ 5% (supplementary Figure S1). If that level of PEEP simultaneously resulted in a high level of OD, driving pressure was decreased if possible. If a decrease in driving pressure was not possible, PEEP was set, allowing more CL closer to the intersection of OD and CL.
Data analysis
Quality control and cleaning of chest EIT data
The EIT data quality is divided into good, intermediate, and poor quality (supplementary Table S1).
Supine vs. prone data: the maximum number of EIT measurements taken per day per patient was two (due to logistical reasons). This approach was applied if patients changed from supine to prone position or vice versa. For the present study, we report the main results of patients with good and intermediate quality EIT data in the supine position.
The data were processed to extract the data for each PEEP-step and prepared in MATLAB (Release 2019b, The MathWorks, Inc., Natick, Massachusetts, United States).
Statistical analyses
All participants eligible for the study in the cohort during the first pandemic wave were included. The sample characteristics were described as mean and standard deviation (SD), median and interquartile range (IQR), or percentage, as appropriate. The statistical analysis was analysed with R version 3.6.1 using the R-studio interface.
Prior to the primary analyses, the course of PaO2/FiO2-ratios and PEEP levels over time was determined for those discharged from the ICU alive and those who died separately. Next, we used linear mixed-effects regression with a random intercept and random slope with time to compute differences in average PaO2/FiO2-ratio and PEEP level and differences in the slope over time between both groups. We used an unstructured variance–covariance matrix for the random effects and an autoregressive correlation structure of the first order for longitudinal measures. To assess non-linear change over time, we added polynomials of time. The best-fitting model for change over time was selected using the Akaike Information Criterion. The crude models were adjusted for age, sex, and APACHE II score.
For the primary analyses, after organising the serial EIT data into three levels, i.e., patient, time (days after intubation), and PEEP steps, we first used local polynomial regression fitting and cubic splines to construct population-based curves, with 95% confidence limits, for Cdyn mL/cmH2O, %CL, %OD for each of the PEEP steps from 24 to 8 cmH2O. We then categorised the serial EIT data into days after intubation: 1–3, 4–6, 7, and more, and used cubic splines to construct population-based curves for Cdyn, CL, and OD for these categories. Finally, with the categories days 1–3, days 4–6, and days 7 and more, and ICU survivors and ICU non-survivors, we used cubic splines to construct population-based curves for Cdyn, CL, and OD for these time categories for ICU survivors and ICU non-survivors. We chose this method to analyse the 3 level data, in contrast to the previously proposed mixed-models, as polynomial regression with cubic splines appeared to be a more flexible model for the 3 level data and used the previously proposed categories27.
We present means (solid line) with 95% CI (dashed lines) of %OD, %CL and Cdyn per PEEP step. p-value < 0.05 were considered statistically significant.
Results
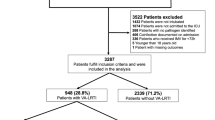
The MaastrICCht cohort comprises 94 patients included in the first pandemic wave (Fig. 1). Fourteen patients did not undergo EIT measurements due to a shorter length of stay (p < 0.001), see supplementary Table S2, while population characteristics were similar to those 80 patients who underwent EIT measurements. The mean age was 64.1 ± 11.6 years; 18 (22.5%) were female. The mean APACHE II score on admission was 16.1 ± 5.0 (Table 1). The mean duration of mechanical ventilation was 19.7 ± 13.0 days. After EIT data cleaning, 279 good quality and 55 intermediate quality EIT observations were included in the present study. Thirty-one poor observations were excluded (8.5% of total). Of all good and intermediate quality EIT observations, 283 (84.7%) were measured in the supine position and 51 (15.3%) in the prone position. Of the supine position EIT measurements, we included 93 observations from day 1 to 3 after intubation, 66 observations from day 4 to 6, and 124 observations beyond day 7. 51 (63.7%) participants survived the ICU. We experienced no significant haemodynamic side effects during the PEEP trials.
Study sample. Patients and good and intermediate quality serial EIT measurements during pressure control mode ventilation. Observations > 7 may include a range of 8 to 17 EIT observations, resulting in a total of 334. ICU, intensive care unit; EIT, electrical impedance tomography; PC, pressure control mode ventilation; PS, pressure support mode ventilation; CPAP, continuous positive airway pressure mode ventilation.
The mean PaO2/FiO2-ratio at baseline was 184.3 (SD 61.4) for ICU-survivors compared to 151.3 (SD 54.4) for those who died in the ICU (p = 0.017). The PEEP level at baseline was 11.8 (SD 2.8) cmH2O for ICU-survivors compared to 11.3 (SD 3.4) cmH2O for those who died in the ICU (p = 0.475). Figure 2 shows the observed PaO2/FiO2-ratio (panel A), PEEP level (panel B), and driving pressure (panel C) for ICU-survivors and ICU-non-survivors throughout follow-up with lines superimposed showing the best-fitting overall trajectories over time, unadjusted for confounders. On average, ICU-survivors had a higher overall PaO2/FiO2-ratio during their ICU stay (regression coefficient: 47.4, 95% CI: 26.6; 68.3, p < 0.001) after adjustment for age, sex, and APACHE II score. A model including an interaction-term between mortality and time revealed no significant interaction by time (p = 0.471). On average, ICU-survivors had a non-statistically significant lower overall mean PEEP during their ICU stay (regression coefficient: − 0.53, 95% CI: − 1.98; 0.92, p = 0.473) after adjustment for age, sex, and APACHE II score. A model including an interaction-term between mortality and time revealed a significant interaction by time (p = 0.026), meaning a steeper decrease for survivors compared to non-survivors over time. There was no evidence of an average difference in overall mean driving pressure between ICU-survivors and non-survivors during their ICU stay (regression coefficient: − 1.37, 95% CI: − 2.98; 0.23, p = 0.096) after adjustment for age, sex, and APACHE II score.
PaO2/FiO2-ratio (mmHg) and PEEP (cmH2O) for survivors and non-survivors over time. (A): PaO2/FiO2-ratio for survivors (black) and non-survivors (red) over time; (B): positive end-expiratory pressure (PEEP) for survivors and non-survivors over time; (C): driving pressure for survivors and non-survivors over time.
EIT population curves
Figure 3 shows OD decreased with a lesser slope over time, and CL decreased from ~ 10 to ~ 7% over time (panel A: day 1–3; panel B: day 4–6; panel C: day ≥ 7, respectively). The combination of these changes shifted the intersection between OD and CL curves to the right (lower PEEP) over time. Maximal Cdyn decreased from ~ 50 mL/cmH2O (panel A: day 1–3) to ~ 35 mL/cmH2O (panel C: day ≥ 7) over time.
Dynamic respiratory system compliance, alveolar overdistension, and alveolar collapse for the whole population from admission to beyond a week after. EIT population curves that show mean (solid lines), dynamic compliance (green), overdistension (blue), and collapse (yellow) with 95% confidence intervals (dashed lines) for the whole population from PEEP steps 28 to 8 cmH2O over time for the first 3 days (A), day 4, 5 and 6 (B) and day 7 and above (C).
Figure 4, panels A and D for days 1–3, show ICU non-survivors (upper panel) had a maximal Cdyn of ~ 45 mL/cmH2O, and ICU survivors (lower panel) had a Cdyn of ~ 55 mL/cmH2O, while the intersection of OD and CL curves appeared similar at a PEEP of ~ 12–13 cmH2O. Figure 4, panels B and E show ICU non-survivors had a maximal Cdyn of ~ 38 mL/cmH2O, and ICU survivors had a Cdyn of ~ 50 mL/cmH2O, while the intersection between OD and CL curves shifted to the right for ICU non-survivors to a PEEP of ~ 10 cmH2O and remained at a PEEP of ~ 12–13 cmH20 for ICU survivors for day 4–6. Figure 4, panels C and F show ICU non-survivors had a maximal Cdyn of ~ 25 mL/cmH2O with an intersection between OD and CL curves at a PEEP of ~ 7 cmH2O, and ICU survivors had a Cdyn of ~ 38 mL/cmH2O with an intersection between OD and CL curves at a PEEP of ~ 9 cmH2O for day 7 and thereafter.
Dynamic respiratory system compliance, alveolar overdistension, and alveolar collapse for the whole population from admission to beyond a week after in ICU survivors and ICU non-survivors. EIT population curves that show mean (solid lines), dynamic compliance (green), overdistension (blue), and collapse (yellow) with 95% confidence intervals (dashed lines) from PEEP steps 28 to 8 cmH2O for ICU non-survivors over time for the first 3 days (A), for day 4, 5 and 6 (B) and for day 7 and above (C); and for ICU survivors over time for the first 3 days (D), for day 4, 5 and 6 (E) and for day 7 and above (F).
Additional analyses
We show that in the 283 EIT measurements, OD decreased from ~ 40 to ~ 5%, CL increased from 0 to ~ 8%, with a maximal Cdyn of ~ 40 mL/cmH2O at a PEEP of ~ 13 cmH2O during PEEP steps from 28 to 8 cmH2O (supplementary Figure S2).
Repeating the analyses for PEEP steps above 28 cmH2O showed increasingly less confidence (widening of dashed lines with higher PEEP) than for PEEP < 24 cmH2O (supplementary Figure S3). Repeating the analyses, including EIT data for prone positioning, only showed that OD, CL, and Cdyn curves, on average, including all time points and all PEEP steps, differ from OD, CL, and Cdyn in the supine position (supplementary Figure S4). Stratifying results for men and women showed that the low amount of data for women, on average, led to less precise estimates of OD, CL, and Cdyn curves (supplementary Figure S5).
Discussion
The present study in SARS-CoV-2 patients on mechanical ventilation has four main findings. First, we show that using EIT guided PEEP; OD, CL, and Cdyn change over the course of mechanical ventilation during SARS-CoV-2 infection in a way that suggests that the whole population develops decreased compliance over time. Second, we show that these changes are more unfavourable in non-survivors than survivors. More precisely, over the course of mechanical ventilation, the intersection between OD and CL shifted towards the right which, together with the decreasing Cdyn indicates stiffening of the lungO. Third, after adjustment for age, sex, and APACHE II score, a higher PaO2/FiO2-ratio over time, but not PEEP or driving pressure, was associated with survival. Overall, this suggests that PEEP titration seems to be important both on an individual basis and over time. Fourth, our results show that the unfavourable pattern (i.e., lower Cdyn with higher OD and CL) over time is observed mainly in non-survivors, suggesting that stiffening of the lung over time by COVID-19 related pro-fibrotic processes35 and/or ventilator-induced injury, is associated with unfavourable outcome.
Our prospective cohort study with serial EIT measurements in mechanically ventilated patients with SARS-CoV-2 infection is comprehensive and adds to the previous reports17,19,20,21,22,23,24,33,36,37,38,39,40,41,42. Additional to previous studies, we included much more patients with SARS-CoV-2 infection17,19,20,21,22,23,24,42,43,44,45 or acute respiratory distress syndrome33,36,37,38,39,40, most innovative of this study is that we performed a unique amount of serial EIT measurements (334 EIT observations)17,24,33,36,37,38,39,40,42,43 and mainly included patients with more than 1 PEEP trial per patient (supplementary Table S3)19,20,21,22,24. Moreover, OD, CL, and Cdyn were analysed on the population level using serial measurements to provide more in-depth information on lung pathophysiology over time.
In our centre, optimised EIT-guided PEEP is titrated to a CL ≤ 5%. Other centres titrate PEEP at the intersection of OD and CL. Momentarily, there is no gold standard. However, the best Cdyn in our population is always in the range between CL ≤ 5% and the intersection of OD and CL (supplementary Figure S2).
Our results are similar to van der Zee et al., who showed that high optimum PEEP was required to reach a low OD and low CL by EIT17 and we extend the scarce serial data19,20,21,22. We add that the interrelationship between optimum PEEP, OD, CL, and Cdyn changed over time, showing worse lung dynamics in non-survivors beyond 7 days of mechanical ventilation compared to 1–3 and 4–6 days, respectively. The flattening of the Cdyn curve during the PEEP trial in the non-survivors beyond day 7 indicates the progression of the amount of non-recruitable lung tissue. Furthermore, reducing PEEP in this population does not increase CL. Our results provide evidence for a pathophysiological framework that stiffens the lung during mechanical ventilation over time in a way that leads to an unfavourable outcome. Profibrotic processes, due to increased TGF-β in COVID-19 among other mechanisms35, thus likely play a role in affecting outcome and persistent post-discharge sequelae46. Whether intervention is possible remains unclear. Another explanation might be that lung stiffening results from ventilator-induced injury which in this study is minimized by EIT PEEP optimisation but cannot be totally avoided47. We did not investigated recruitability in this paper. But in our experience, only a small number of patients were responders to recruitment in the early phase after intubation. This effect decreased over time. In general, most patient had a high compliance compared to their PaO2/FiO2-ratio at the start of mechanical ventilation. This may explain the low recruitability. Later in the progress of the disease, compliance decreased and stiffening of the lung occurred. Resulting further in a decreased recruitability which is also illustrated by our finding that reducing PEEP in this population does not increase CL.
For the main analyses, we reported on EIT data determined in patients on mechanical ventilation using PC in a supine position. Additionally, we analysed EIT parameters stratified for prone positioning separately, which is important when investigating Cdyn, OD, and CL over time as prone and supine positioning appeared to affect these parameters differently. Similarly, we additionally stratified the results for men and women. Nevertheless, the amount of data on Cdyn, OD, and CL for women and prone positioning was too small to conclude on in these subgroups based on a population level and over time, respectively.
The strengths of the study include its prospective design and the predefined protocol for serial EIT measurements. Furthermore, to study the course of OD, CL, and Cdyn over time, we used the date of intubation as a starting point. This minimises information bias due to the timing of EIT measurements. The large amount of data increases the precision of the population EIT curves, illustrated by the narrow 95% confidence limits.
Limitations of the study are the inclusion of SARS-CoV-2 infected patients only, which may limit the generalisability of the results to other pulmonary pathology33,36,37,38,39,40. Furthermore, we cannot fully exclude that selection has biased the results as patients were transported between hospitals during the pandemic. However, both the most severe critically ill patients were transported to our institution, for example, when advanced pulmonary support by extracorporeal membrane oxygenation techniques was considered, and the less critically ill patients, because transport was considered most feasible in those patients. Most likely, this averages the severity of pulmonary pathology and critical illness within our population. Patients transported from other centres are likely to be intubated for some time, and thus they may differ in the disease course. Nevertheless, the serial EIT data were aligned from intubation to match the disease courses between patients. Furthermore, although sex-differences likely play a role in COVID-19 pathophysiology28,48, the stratified results for women should be interpreted with caution due to lack of precision. In fact, this result illustrates the importance of a major amount of data when investigating EIT curves on a population level. Finally, we have described observational results, which limit conclusions with regards to causality.
In conclusion, we show that EIT guided PEEP, OD, CL, and Cdyn change over the course of mechanical ventilation during SARS-CoV-2 infection in a way that suggests that all patients develop decreased compliance over time. These changes are more unfavourable in non-survivors as compared to survivors. This suggests that PEEP titration might be valuable not only on an individual basis but also over time; the exact clinical relevance remains to be established. Moreover, we found that a higher PaO2/FiO2-ratio over time, but not PEEP or driving pressure, was associated with survival. Furthermore, these results provide a pathophysiological framework for mechanisms that stiffen the lung contributing to a more unfavourable pattern (i.e., lower Cdyn with higher OD and CL) over time in relation to adverse outcome in COVID-19 patients on mechanical ventilation.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- Cdyn:
-
Dynamic respiratory system compliance
- CL:
-
Alveolar collapse
- CPAP:
-
Continuous positive airway pressure
- EIT:
-
Electrical impedance tomography
- ICU:
-
Intensive Care Unit
- OD:
-
Alveolar overdistension
- PC:
-
Pressure control
- PEEP:
-
Positive end-expiratory pressure
- PS:
-
Pressure support
References
Dergaa, I. et al. Olympic games in COVID-19 times: Lessons learned with special focus on the upcoming FIFA World Cup Qatar 2022. Br. J. Sports Med. 56(12), 654–656 (2022).
Musa, S. et al. BNT162b2 COVID-19 vaccine hesitancy among parents of 4023 young adolescents (12–15 Years) in Qatar. Vaccines (Basel) 9, 9 (2021).
Richardson, S. et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 323(20), 2052–2059 (2020).
King, C. S. et al. Outcomes of mechanically ventilated patients with COVID-19 associated respiratory failure. PLoS ONE 15(11), e0242651 (2020).
Marini, J. J. & Gattinoni, L. Management of COVID-19 respiratory distress. JAMA 323(22), 2329–2330 (2020).
Alhazzani, W. et al. Surviving Sepsis Campaign: Guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19). Intensive Care Med. 46(5), 854–887 (2020).
Gattinoni, L. et al. COVID-19 does not lead to a “typical” acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 201(10), 1299–1300 (2020).
Gattinoni, L. et al. COVID-19 pneumonia: Different respiratory treatments for different phenotypes?. Intensive Care Med. 46(6), 1099–1102 (2020).
Bos, L. D. J., Sinha, P. & Dickson, R. P. The perils of premature phenotyping in COVID-19: A call for caution. Eur. Respir. J. 56, 1 (2020).
Amato, M. B. et al. Driving pressure and survival in the acute respiratory distress syndrome. N. Engl. J. Med. 372(8), 747–755 (2015).
Slutsky, A. S. & Ranieri, V. M. Ventilator-induced lung injury. N. Engl. J. Med. 369(22), 2126–2136 (2013).
Cinnella, G. et al. Physiological effects of the open lung approach in patients with early, mild, diffuse acute respiratory distress syndrome: An electrical impedance tomography study. Anesthesiology 123(5), 1113–1121 (2015).
El-Baradey, G. F. & El-Shamaa, N. S. Compliance versus dead space for optimum positive end expiratory pressure determination in acute respiratory distress syndrome. Indian J. Crit. Care Med. 18(8), 508–512 (2014).
Writing, C. et al. Effect of a lower vs higher positive end-expiratory pressure strategy on ventilator-free days in ICU patients without ARDS: A randomized clinical trial. JAMA 324(24), 2509–2520 (2020).
Manzano, F. et al. Positive-end expiratory pressure reduces incidence of ventilator-associated pneumonia in nonhypoxemic patients. Crit. Care Med. 36(8), 2225–2231 (2008).
Goligher, E. C. et al. Oxygenation response to positive end-expiratory pressure predicts mortality in acute respiratory distress syndrome. A secondary analysis of the LOVS and ExPress trials. Am. J. Respir. Crit. Care Med. 190(1), 70–76 (2014).
van der Zee, P., Somhorst, P., Endeman, H. & Gommers, D. Electrical impedance tomography for positive end-expiratory pressure titration in COVID-19-related acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 202(2), 280–284 (2020).
Pulletz, S. et al. Dynamic relative regional strain visualized by electrical impedance tomography in patients suffering from COVID-19. J. Clin. Monit. Comput. 36, 975–985 (2021).
Tomasino, S. et al. Electrical impedance tomography and prone position during ventilation in COVID-19 Pneumonia: Case reports and a brief literature review. Semin. Cardiothorac. Vasc. Anesth. 24(4), 287–292 (2020).
Safaee Fakhr, B. et al. Bedside monitoring of lung perfusion by electrical impedance tomography in the time of COVID-19. Br. J. Anaesth. 125(5), e434–e436 (2020).
Sella, N. et al. Positive end-expiratory pressure titration in COVID-19 acute respiratory failure: Electrical impedance tomography vs. PEEP/FiO2 tables. Crit. Care 24(1), 540 (2020).
Zarantonello, F., Andreatta, G., Sella, N. & Navalesi, P. Prone position and lung ventilation and perfusion matching in acute respiratory failure due to COVID-19. Am. J. Respir. Crit. Care Med. 202(2), 278–279 (2020).
Mlcek, M. et al. Targeted lateral positioning decreases lung collapse and overdistension in COVID-19-associated ARDS. BMC Pulm. Med. 21(1), 133 (2021).
Taenaka, H. et al. Individualized ventilatory management in patients with COVID-19-associated acute respiratory distress syndrome. Respir. Med. Case Rep. 33, 101433 (2021).
Shono, A., Kotani, T. & Frerichs, I. Personalisation of therapies in COVID-19 associated acute respiratory distress syndrome, using electrical impedance tomography. J. Crit. Care Med. (Targu Mures). 7(1), 62–66 (2021).
Fu, Y. et al. Monitoring bronchoalveolar lavage with electrical impedance tomography: First experience in a patient with COVID-19. Physiol Meas. 41(8), 085008 (2020).
Tas, J. et al. Serial measurements in COVID-19-induced acute respiratory disease to unravel heterogeneity of the disease course: Design of the Maastricht Intensive Care COVID cohort (MaastrICCht). BMJ Open 10(9), e040175 (2020).
Bels, J. L. M. et al. Decreased serial scores of severe organ failure assessments are associated with survival in mechanically ventilated patients; the prospective Maastricht Intensive Care COVID cohort. J. Crit. Care 62, 38–45 (2020).
Prokop, M. et al. CO-RADS: A categorical CT assessment scheme for patients suspected of having COVID-19-definition and evaluation. Radiology 296(2), 97–104 (2020).
Blankman, P., Hasan, D., Erik, G. & Gommers, D. Detection of “best” positive end-expiratory pressure derived from electrical impedance tomography parameters during a decremental positive end-expiratory pressure trial. Crit. Care 18(3), R95 (2014).
Blankman, P. et al. Detection of optimal PEEP for equal distribution of tidal volume by volumetric capnography and electrical impedance tomography during decreasing levels of PEEP in post cardiac-surgery patients. Br. J. Anaesth. 116(6), 862–869 (2016).
Frerichs, I. et al. Chest electrical impedance tomography examination, data analysis, terminology, clinical use and recommendations: Consensus statement of the TRanslational EIT developmeNt stuDy group. Thorax 72(1), 83–93 (2017).
Heines, S. J. H., Strauch, U., van de Poll, M. C. G., Roekaerts, P. & Bergmans, D. Clinical implementation of electric impedance tomography in the treatment of ARDS: A single centre experience. J. Clin. Monit. Comput. 33(2), 291–300 (2019).
Costa, E. L. et al. Bedside estimation of recruitable alveolar collapse and hyperdistension by electrical impedance tomography. Intensive Care Med. 35(6), 1132–1137 (2009).
Osuchowski, M. F. et al. The COVID-19 puzzle: Deciphering pathophysiology and phenotypes of a new disease entity. Lancet Respir. Med. 9(6), 622–642 (2021).
Kunst, P. W., de Vries, P. M., Postmus, P. E. & Bakker, J. Evaluation of electrical impedance tomography in the measurement of PEEP-induced changes in lung volume. Chest 115(4), 1102–1106 (1999).
Pulletz, S. et al. Dynamics of regional lung aeration determined by electrical impedance tomography in patients with acute respiratory distress syndrome. Multidiscip. Respir. Med. 7(1), 44 (2012).
Stahl, C. A. et al. Determination of “recruited volume” following a PEEP step is not a measure of lung recruitability. Acta Anaesthesiol. Scand. 59(1), 35–46 (2015).
Zhao, Z. et al. Positive end-expiratory pressure titration with electrical impedance tomography and pressure-volume curve in severe acute respiratory distress syndrome. Ann. Intensive Care 9(1), 7 (2019).
Guan, W. J. et al. Comorbidity and its impact on 1590 patients with Covid-19 in China: A Nationwide Analysis. Eur. Respir. J. 5, 55 (2020).
Zhao, Z. et al. The use of electrical impedance tomography for individualized ventilation strategy in COVID-19: A case report. BMC Pulm. Med. 21(1), 38 (2021).
Mauri, T. et al. Potential for lung recruitment and ventilation-perfusion mismatch in patients with the acute respiratory distress syndrome from Coronavirus Disease 2019. Crit. Care Med. 48(8), 1129–1134 (2020).
Morais, C. C. A. et al. Bedside electrical impedance tomography unveils respiratory “chimera” in COVID-19. Am. J. Respir. Crit. Care Med. 203(1), 120–121 (2020).
Perier, F. et al. Effect of positive end-expiratory pressure and proning on ventilation and perfusion in COVID-19 acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 202(12), 1713–1717 (2020).
Perier, F. et al. Electrical impedance tomography to titrate positive end-expiratory pressure in COVID-19 acute respiratory distress syndrome. Crit. Care 24(1), 678 (2020).
van Gassel, R. J. J. et al. High prevalence of pulmonary sequelae at 3 months after hospital discharge in mechanically ventilated survivors of COVID-19. Am. J. Respir. Crit. Care Med. 203(3), 371–374 (2021).
Smith, B. J., Grant, K. A. & Bates, J. H. Linking the development of ventilator-induced injury to mechanical function in the lung. Ann. Biomed. Eng. 41(3), 527–536 (2013).
Schiffer, V. et al. The “sex gap” in COVID-19 trials: A scoping review. EClinicalMedicine. 2020, 100652 (2020).
Acknowledgements
The authors thank Kirsten Bos, Sebastiaan de Jongh and the nursing staff of the ICU from Maastricht for assisting during the measurements.
Author information
Authors and Affiliations
Contributions
S.J.H.H., B.C.T.B., R.J.J.G., N.F.L.H., M.C.G.P., R.S., U.S., J.T., I.C.C.H., D.C.J.J.B. contributed to the concept and design of the study. S.J.H.H., M.J.A., R.J.J.G., R.V.M.G., B.J.M.H., R.H., J.K., M.M.G.M., F.R., S.S., J.T. contributed to the collection of data. S.J.H.H., B.C.T.B., F.C.B., J.T., S.M.J.K., D.C.J.J.B. contributed to the data analysis. All authors contributed to the interpretation of the data and the development of the first draft of the article. Subsequent drafts were reviewed and approved by all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Heines, S.J.H., van Bussel, B.C.T., Jong, M.J.Ad. et al. Pulmonary pathophysiology development of COVID-19 assessed by serial Electrical Impedance Tomography in the MaastrICCht cohort. Sci Rep 12, 14517 (2022). https://doi.org/10.1038/s41598-022-18843-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-18843-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.