Abstract
The association of inadequate dietary sodium intake with bone mineral density (BMD) and the risk of osteoporosis is controversial. To find the association between low sodium diet and the risk of incipient osteoporosis, we performed a population-based cross-sectional analysis using Tanaka method for estimation 24-h urinary sodium excretion (e24hUNaETanaka) as a candidate indicator of sodium intake. We identified 3869 participants without osteoporosis and classified them into quartiles according to their value of e24hUNaETanaka. BMD was measured to find participants at risk of osteoporosis. Lower e24hUNaETanaka was related to decreasing BMD of the distal radius. Multiple Cox-proportional hazard models demonstrated that e24hUNaETanaka had an inverse association with the risk of osteoporosis (adjusted HR = 0.859, 95% CI = 0.751–0.982) and survival analysis revealed that the lowest quartile group had poor osteoporosis-free survival (PLog-rank < 0.0001). Furthermore, our restricted cubic spline analysis revealed that the relationship between e24hUNaETanaka and HR of osteoporosis was negative curvilinear in males and postmenopausal females and positive linear in premenopausal females. Our findings suggest that lower sodium intake was a significant predictor of incipient osteoporosis and there was wide variation in this relationship according to sex and female hormone status.
Similar content being viewed by others
Introduction
Osteoporosis is one of the most common diseases in bone mineral metabolism and it can exert severe negative effects on quality of life and bone health in the aging population1. Furthermore, recent epidemiologic studies had shown that some osteoporotic fractures of the specific site are responsible for increased hospital morbidity and all-cause mortality risk in both inpatient and outpatient subjects2,3. Although osteoporosis has a multi-systemic and polygenic nature, there is very limited data to support the effect of other hidden risk factors, except age, ethnicity, and sex, on bone mineral dysmetabolism and osteoporosis4,5. Thus, identification of new modifiable risk factors of osteoporosis is critical to prevent its irreversible consequences.
Growing evidence showed that not only calcium but also sodium is an important molecule in regulating bone mineral metabolism6. However, there is wide controversy in the relationship between dietary sodium intake and the risk of osteoporosis6. Because previous epidemiologic studies had revealed that increased sodium intake may be related to either sodium-induced calciuria or enhancing bone reabsorption which may contribute to decreased bone mineral density (BMD), current global guidelines recommend a low salt diet to help keep our bone healthy6,7,8. Recently, some authors argued that subjects taking a low sodium diet were likely to have decreased energy intake and other important nutrients, hyponatremia, and/or activation of the renin–angiotensin–aldosterone system, which may contribute to abnormal low BMD9,10. Therefore, there is need for studies to elucidate the exact association between dietary sodium intake and the risk of osteoporosis.
It is well known that sex hormones influence the development of sex-specific traits and they regulate structures and functions of reproductive systems. In addition, accumulating evidence indicates that sex hormones play critical roles in maintaining bone health and that sex hormone insufficiency is strongly associated with the development of osteoporosis and bone fracture11. However, there is very limited data on hormonal influence on the association between a low sodium diet and the risk of incipient osteoporosis. Therefore, we conducted this population-based cross-sectional analysis using the Tanaka method for estimating 24-h urinary sodium excretion (e24hUNaETanaka) as a candidate indicator of sodium intake. After that, we performed subgroup analysis to find out the role of sex hormone on the sodium intake and osteoporosis.
Results
Baseline characteristics
The participants (n = 3869) comprised 1845 male, 1632 premenopausal female, and 392 postmenopausal female participants. When estimating sodium intake with the Tanaka equation, male participants tended to intake more sodium than female participants in this study (men 2.3 ± 0.5 g/day; women 1.9 ± 0.6 g/day; P < 0.001). Participants in the lowest quartile were older and more likely to have increased white blood cell count and platelet count. Participants in the highest quartile were more obese and had increased prevalence of diabetes mellitus, thyroid disease, higher levels of fasting glucose and more urinary excretion of albumin. Moreover, dietary energy intake increased as the 24-h urinary sodium excretion increased. The other demographic data and clinical characteristics are presented in Table 1 and Supplemental Table 1.
Estimated 24-h dietary sodium intake and bone densitometry
We performed linear regression analysis with age, sex, and smoking history as covariates to find the possible relation of e24UNaETanaka with other baseline characteristics related to osteoporosis. As shown in the Table 2, we found that e24UNaETanaka was strongly associated with clinical parameters of systemic inflammation, such as C-reactive protein, hemoglobin, platelet, and albumin, but marginally related with change of areal BMD. Unfortunately, in subgroup analysis according to sex, we did not find a significant relation between e24UNaETanaka and the change of areal BMD during the study period (Fig. 1 and Supplemental Table 2).
Non-linear relation of e24UNaETanaka with the change of (A) ΔT-score of distal radius and (B) ΔT-score of midshaft tibia compared with the chosen reference e24UNaETanaka of 2.0 g/day. Solid line represents the change of bone marrow densitometry indicators and dash lines 95% confidential intervals. *Calculated by restricted cubic spline regression using age, sex, and smoking history as covariates.
Dietary salt intake and osteoporosis
We performed Cox proportional-hazard model, using age, sex, and smoking history as covariates, to find an independent predictor of incipient osteoporosis. We found that decreased dietary salt intake was significantly associated with the development of osteoporosis and this association was not attenuated by further adjustment for BMI, WC, hemoglobin, corrected calcium, eGFR, and TG (e24UNaETanaka, adjusted HR = 0.859, 95% CI = 0.751–0.982, Table 3). To evaluate the linearity between e24UNaETanaka and the risk of osteoporosis, we performed multiple adjusted RCS analyses and found that there was a negative sublinear relationship between them in entire participants (Fig. 2).
Relationship between dietary salt intake and hazard ratio of osteoporosis*. Solid line represents the adjusted HR** of incipient osteoporosis and dash lines 95% CIs**. *Defined as a bone mineral density T-score at either distal radius or tibia shaft below −2.5. **Calculated by restricted cubic spline Cox-proportional hazard regression model using age, sex, and smoking history as covariates and body mass index, waist circumference, systolic BP, diastolic BP, hemoglobin, sodium, corrected calcium, hemoglobin A1c, eGFR, triglyceride, LDL-cholesterol, UACR, and urine potassium/creatinine ratio as predictors. e24hUNaETanaka, Tanaka method for estimating 24-h urinary sodium excretion.
Subsequent Kaplan–Meier analysis with multiple Cox-proportional hazard regression models and log-rank test was performed to compare osteoporosis-free survival among the groups (Fig. 3). We found that participants with the lowest e24UNaETanaka quartile had worse osteoporosis-free survival rate.
The impact of dietary salt intake on osteoporosis-free survival. Q1 had a poor osteoporosis-free survival rate compared with other groups, but there was no significant difference in adjusted HR between Q2–3 and Q4 (adjusted HR* = 0.878, 95% CI* = 0.735–1.050). *Calculated by Cox-proportional hazard model using age, sex, and smoking history as covariates and body mass index, waist circumference, systolic BP, diastolic BP, hemoglobin, sodium, corrected calcium, hemoglobin A1c, eGFR, triglyceride, LDL-cholesterol, UACR, and urine potassium/creatinine ratio as predictors. HR, hazard ratio; CI, confidence interval.
Sex disparity in the association between dietary salt intake and osteoporosis
There was inconsistency between our linear regression and Cox-proportional hazard model results. (Supplemental Table 3) To explore possible confounding factor(s) that could influence the relationship between dietary sodium intake and the risk of incipient osteoporosis, we classified all participants according to their sex and female menopausal status and performed further subgroup analyses. As shown in Fig. 4, our RCS analysis result revealed that there was wide variation in the association between dietary sodium intake and the risk of osteoporosis according to sex hormone status. There was a negative curvilinear relationship in male participants and postmenopausal female participants. In addition, there was a positive linear relationship in premenopausal female participants.
Sex disparity in the relationship between dietary salt intake and hazard ratio of osteoporosis. Solid line represents the adjusted HR* of osteoporosis and dash lines 95% CIs*. *Calculated by restricted cubic spline Cox-proportional hazard regression model using age, sex, and smoking history as covariates and body mass index, waist circumference, hemoglobin, corrected calcium, estimated glomerular filtration rate, triglyceride, and urine potassium/creatinine ratio as predictor.
Discussion
This study provided comprehensive information on the effect of dietary sodium intake on the risk of incipient osteoporosis in the general population. Our results demonstrated that e24UNaETanaka were independent predictors of developing incipient osteoporosis. Tanaka method was developed in Asian participants12. It has the least amount of bias among Kawasaki method and Mage method13. Furthermore, there was wide variation in this association according to sex and female sex hormone state.
Growing evidence showed that osteoporosis and related sequelae have a considerable impact on health-related quality of life including fracture and subsequent morbidity. Particularly, osteoporosis is strongly associated with increased cardiovascular morbidity and mortality in the elderly population1,3,14,15. Although various anti-osteoporosis medications are frequently prescribed to prevent unwanted consequences, it is true that there are potential limitations in the wide use of these medical treatments16. Recently, some authors argued that several nutritional strategies could be another choice to reduce the risk of bone loss and fracture17,18,19,20. Previous epidemiologic studies had demonstrated that excess sodium intake was a potent risk of arterial hypertension and cardiovascular disease and may be associated with decreased urinary calcium reabsorption and increased bone resorption6,7,8. Accordingly, current guidelines recommended that adults at risk of chronic disease should take a low sodium diet in terms of improving body health21,22,23,24. However, the effect of modulation of dietary sodium intake on BMD remains controversial. In our study, we found that there was an inverse association between the estimated sodium intake per day and the risk of osteoporosis in the whole population. It is known that low sodium diet activates the renin-angiotensin-aldostereone system (RAAS) and the components of RAAS are discovered in bone tissue25. When RAAS is activated, it stimulates osteoclast formation and inhibits osteoblast activity to cause osteoporosis26,27. Moreover, insufficient sodium intake leads to a lack of other important nutrients9,10. A low salt diet increases the risk of osteoporosis by consuming fewer calories and increasing bone resorption markers28. Such findings suggested that a balanced nutrition diet is critical to bone health.
The novel finding of our study was that the effect of dietary sodium intake on the risk of incipient osteoporosis varied by sex and menopausal state. Such a result may imply that other confounding factors caused by changes in sex hormones can modulate the effect of sodium intake on bone health6,23. Sex hormones play a critical role in the growth and maintenance of the skeletal system. Manolagas, et. al. demonstrated that estrogen affects several cell types to attenuate bone resorption29.Narla, et. al. revealed that estrogen regulates mainly cortical bone turnover, but testosterone effects on the trabecular bone11. However, there is very limited clinical evidence on the interaction effects of abnormal dietary habits and sex hormonal status on bone homeostasis. In this regard, our results confirmed that sex hormones could regulate the effect of a low sodium diet on the onset of early osteoporosis.
In this study, there was wide inconsistency between results of linear regression analysis showed complex relation between dietary sodium intake and the change of BMD and those of Cox-proportional hazard model revealed apparent negative effects of dietary sodium intake on the risk of osteoporosis. The possible explanations are as following: (i) BMD changes over a long-time scale30; (ii) delayed epidemic of incipient osteoporosis was frequently observed in senile population31,32; and (iii) KoGES was designed to find potential risk factor(s) of chronic disease development in local population group after middle age. It may be another possible explanation that osteoporosis is a polygenic disorder and unknown confounding variables can change the impact of dietary sodium intake on BMD.
Our RSC analysis demonstrated that the osteoporotic effect of a low sodium diet was more prominent in the distal radius rather than the midshaft tibia. It was consistent with a previous prospective cohort study showing that the effect of dietary modulation on the BMD change varied according to the skeletal sites, in which increased intake of dietary sodium reduced the risk of incipient fracture at only femur neck, but not other fracture sites23. However, there is very little experimental evidence to explain these findings. Thus, further studies are needed to evaluate the site-specificity of the osteoporotic effect of decreased sodium intake.
There were several limitations in our study. First, this population-based study did not include data about sex hormone levels in serum, 24-h urine collection of sodium, dual-energy x-ray absorptiometry, and bone markers, such as parathyroid hormone, alkaline phosphatase, osteocalcin or c-telopeptide. Because of these limitations of study design, control of some confounding factors was not possible and a more precise analysis between sex hormone state, dietary sodium intake, BMD change, and the risk of osteoporosis was not performed. In addition, since the prevalence and severity of osteoporosis are influenced by a wide variety of factors, we could not adjust for many other factors other than age, sex, smoking history, body mass index, waist circumference, hemoglobin, corrected calcium, eGFR, TG, and urine potassium-to-creatinine ratio. Second, because of the self-reporting of medical history, medication, and use of tobacco and alcohol, a social-desirability bias cannot be excluded. It may be responsible for results and conclusions that conflicted with previous research. Furthermore, participants may have forgotten relevant details.
In conclusion, this study demonstrated that a lower sodium diet below 2 g per day was an independent predictor for developing incipient osteoporosis and there was a sex disparity in the association between reduced sodium intake and the risk of incipient osteoporosis. Large population-based prospective epidemiologic studies are warranted to confirm these findings.
Methods
Study design and population
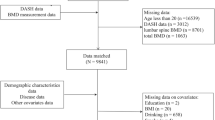
Ansan-Anseong cohort data from the Korean Genome and Epidemiology Study (KoGES) were used in this study. Participants for this study were recruited from the fifth follow-up assessment between 2011 and 2012. Prospective data of Ansan-Anseong study are from a medium-sized city (Ansan) and a rural area (Anseong) near Seoul, South Korea to find the potential effect of candidate genetic variation on various chronic illnesses33. The comprehensive profile and methods concerning the development of KoGES have been represented previously33. Subjects having missing data and those with previously diagnosed osteoporosis, parathyroid-related disease, chronic kidney disease who has eGFR less than 60 or urine albumin-to-creatinine ratio more than 30 or any malignant disease were excluded in this study. All the participants were volunteers and provided written informed consent before enrollment in the study. Their records, except for the date of the survey, were anonymized before analysis. The study protocol was approved by the Institutional Review Board (IRB) of the Hanyang University Seoul Hospital (IRB: HYUH201912015-HE002) and conducted in accordance with the Declaration of Helsinki.
Because our receiver operating characteristic curves analysis demonstrated that e24hUNaETanaka had the best precision in estimating the effect of low sodium intake on incipient osteoporosis as compared with Kawasaki, Mage, and INTERSALT methods, the final 3869 participants were divided into quartiles according to their e24hUNaETanaka results stratified by sex (Supplemental Figs. 1, 2).
Anthropometric and clinical measurements
Anthropometric measurements were made by well-trained examiners and followed by standard methods. Participants wore a lightweight gown or underwear. Height was measured to the nearest 0.1 cm and weight was measured to the nearest 0.01 kg using a portable stadiometer with a weighing scale. Body mass index (BMI) was calculated as participants’ weight in kilograms divided by the square of their height in meters.
Blood pressure (BP) was measured twice, using a mercury sphygmomanometer, once in each arm. Participants were allowed to sit for 5-min rest before measuring blood pressure and between the two measurements34. The average values of the two recorded systolic and diastolic BPs were used in the analysis.
We analyzed diet and nutritional intake using 24-h recall data provided by KoGES. In the case of KoGES, after the face-to-face survey, a phone survey was conducted. A daily energy intake of less than 400 kcal or more than 4000 kcal was excluded35.
Laboratory tests
Venous blood and urine samples were collected after 8 h overnight fasting and sent to the central laboratory (Seoul Clinical Laboratories, Seoul, Republic of Korea) for quantification by biochemical assays. Blood samples are analyzed using a serum separator tubes (SST) and two ethylenediaminetetraacertic acid tubes(EDTA). Urine sample was analyzed by collecting 10 ml of midstream urine33. Fasting plasma concentrations of blood urea nitrogen (BUN), creatinine, albumin, glucose, triglyceride (TG), high-density lipoprotein (HDL)-cholesterol, and low-density lipoprotein (LDL)-cholesterol were determined by an automatic analyzer (ADVIA 1650 and 1680; Siemens, Tarrytown, NY, USA). Glycated hemoglobin (HbA1c) levels were measured by high-performance liquid chromatography (VARIANT II; Bio-Rad Laboratories, Hercules, CA)36. Estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation (CKD-EPI)37. Urine sodium concentrations were measured using ion-selective electrode and 24-h sodium excretion was estimated based on concentrations of sodium and creatinine in spot urine specimen according to 4 different methods (Supplemental Table 438,39,40,41).
Definition of osteoporosis
The speed-of-sound (SOS, m/s) was used to measure areal BMD (Omnisense 7000 s, Sunlight Medical Ltd, Petah Tivka, Israel). According to World Health Organization Guideline, osteoporosis was defined as a bone mineral density T-score at either distal radius or tibia shaft below −2.542.
Statistical analysis
All data, including socio-demographic information, medical conditions, anthropometric and clinical measurements, and laboratory results, were presented as mean ± SD or frequencies (and proportions). The normality of the distribution of parameters was analyzed using the Kolmogorov–Smirnov test. If the original data do not follow a Gaussian distribution, the logarithmic transformation was applied to make the distribution more normal. The Kruskal–Wallis test was used to compare quantitative variables and the chi-square test to compare proportions for categorical variables. Linear regression analysis was used to assess the relationship between potential risk factors associated with e24UNaETanaka and clinical risk factors of osteoporosis. Hazard ratios (HRs) with 95% confidence intervals (CIs) were calculated in multiple Cox-proportional hazards models according to the development of osteoporosis (case vs. control). Kaplan–Meier analysis with the log-rank test was used to compare osteoporosis-free survival between the groups.
Restricted cubic spline (RCS) regression analysis was used to find the possible nonlinear dependency of the association between candidate risk factors and increased risk of the dependent variable43.
A two-tailed P < 0.05 was considered statistically significant. Statistical Analysis Software version 9.4 (SAS Institute Inc, Cary, NC) was used for all analyses.
Data availability
The data that support the findings of this study are available from the corresponding author, J-S Park, upon reasonable request. Data: Data analyzed in this study were obtained from the Korean Genome and Epidemiology Study 2011–2012 (KoGES; 4851–302), National Research Institute of Health, Centers for Disease Control and Prevention, Ministry for Health and Welfare, Republic of Korea.
Abbreviations
- BMI:
-
Body mass index
- BMD:
-
Bone mineral density
- BP:
-
Blood pressure
- BUN:
-
Blood urea nitrogen
- CI:
-
Confidence interval
- CKD:
-
Chronic kidney disease
- eGFR:
-
Estimated glomerular filtration rate
- HbA1c:
-
Glycated hemoglobin A1c
- HDL:
-
High density lipoprotein
- HR:
-
Hazard ratio
- IRB:
-
Institutional Review Board
- KoGES:
-
Korean genome and epidemiology study
- LDL:
-
Low density lipoprotein
- RCS:
-
Restricted cubic spline
- SD:
-
Standard deviation
- TG:
-
Triglyceride
References
Hopman, W. M. et al. Longitudinal assessment of health-related quality of life in osteoporosis: Data from the population-based Canadian Multicentre Osteoporosis Study. Osteoporos Int. 30, 1635–1644 (2019).
Court-Brown, C. M. et al. The spectrum of fractures in the elderly. Bone Jt. J. 96, 366–372 (2014).
Sanders, S. & Geraci, S. A. Outpatient management of the elderly patient following fragility hip fracture. Am. J. Med. 124, 408–410 (2011).
Lacativa, P. G. & Farias, M. L. Osteoporosis and inflammation. ARQ Bras. Endocrinol. Metabol. 54, 123–132 (2010).
NIH consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA. 285, 785–795. (2001)
Teucher, B. & Fairweather-Tait, S. Dietary sodium as a risk factor for osteoporosis: Where is the evidence?. Proc. Nutr. Soc. 62, 859–866 (2003).
Harrington, M. & Cashman, K. D. High salt intake appears to increase bone resorption in postmenopausal women but high potassium intake ameliorates this adverse effect. Nutr. Rev. 61, 179–183 (2003).
Park, S. M. et al. High dietary sodium intake assessed by 24-hour urine specimen increase urinary calcium excretion and bone resorption marker. J. Bone Metab. 21, 189–194 (2014).
Teucher, B. et al. Sodium and bone health: impact of moderately high and low salt intakes on calcium metabolism in postmenopausal women. J. Bone Miner. Res. 23, 1477–1485 (2008).
DiNicolantonio, J. J., Mehta, V., Zaman, S. B. & O’Keefe, J. H. Not salt but sugar as aetiological in osteoporosis: A review. Mo Med. 115, 247–252 (2018).
Narla, R. R. & Ott, S. M. Bones and the sex hormones. Kidney Int. 94, 239–242 (2018).
Tanaka, T. et al. A simple method to estimate populational 24-h urinary sodium and potassium excretion using a casual urine specimen. J. Hum. Hypertens. 16, 97–103 (2002).
Dong, J., Yan, Y., Fan, H., Zhao, X. & Mi, J. Accuracy validation of 8 equations to estimate 24-hour sodium by spot urine in young adolescents. Am. J. Hypertens. 32, 257–264 (2019).
Hallberg, I. et al. Health-related quality of life after osteoporotic fractures. Osteoporos. Int. 15, 834–841 (2004).
Gerber, Y., Melton, L. J. 3rd., Weston, S. A. & Roger, V. L. Osteoporotic fractures and heart failure in the community. Am. J. Med. 124, 418–425 (2011).
Lane, N. E. Epidemiology, etiology, and diagnosis of osteoporosis. Am. J. Obstet. Gynecol. 194, S3-11 (2006).
Ensrud, K. E., & Crandall, C. J. Osteoporosis. Ann Intern Med. 167, ITC17-ITC32. (2017).
Sellmeyer, D. E., Schloetter, M. & Sebastian, A. Potassium citrate prevents increased urine calcium excretion and bone resorption induced by a high sodium chloride diet. J. Clin. Endocrinol. Metab. 87, 2008–2012 (2002).
Dawson-Hughes, B. et al. A controlled trial of the effect of calcium supplementation on bone density in postmenopausal women. N. Engl. J. Med. 323, 878–883 (1990).
Dawson-Hughes, B. et al. Rates of bone loss in postmenopausal women randomly assigned to one of two dosages of vitamin D. Am. J. Clin. Nutr. 61, 1140–1145 (1995).
World Health Organization. Prevention of cardiovascular disease. Guidelines for assessment and management of cardiovascular risk. (World Health Organization, 2007).
McGuire S. U.S. Department of Agriculture and U.S. Department of Health and Human Services, Dietary Guidelines for Americans, 2010. 7th Edition, Washington, DC: U.S. Government Printing Office. January 2011. Adv Nutr. 2, 293–294. (2011).
Carbone, L., et al. Sodium Intake and Osteoporosis. Findings From the Women's Health Initiative. J. Clin. Endocrinol. Metab. 101, 1414–1421. (2016).
Prentice, A. Diet, nutrition and the prevention of osteoporosis. Public Health Nutr. 7, 227–243 (2004).
Okamoto, C. et al. Excessively low salt diet damages the heart through activation of cardiac (pro) renin receptor, renin-angiotensin-aldosterone, and sympatho-adrenal systems in spontaneously hypertensive rats. PLoS ONE 12, 1 (2017).
Mo, C., Ke, J., Zhao, D. & Zhang, B. Role of the renin-angiotensin-aldosterone system in bone metabolism. J. Bone Miner. Metab. 38, 772–779 (2020).
Shimizu, H. et al. Angiotensin II accelerates osteoporosis by activating osteoclasts. FASEB J. 22, 2465–2475 (2008).
Caristia, S. et al. Is caloric restriction associated with better healthy aging outcomes? A systematic review and meta-analysis of randomized controlled trials. Nutrients 12, 2290 (2020).
Manolagas, S. C., O’Brien, C. A. & Almeida, M. The role of estrogen and androgen receptors in bone health and disease. Nat Rev Endocrinol. 9, 699–712 (2013).
Xue, S. et al. Age at attainment of peak bone mineral density and its associated factors: The National Health and Nutrition Examination Survey 2005–2014. Bone 131, 1 (2020).
Kanis, J. A., Melton, L. J. 3rd., Christiansen, C., Johnston, C. C. & Khaltaev, N. The diagnosis of osteoporosis. J. Bone Miner. Res. 9, 1137–1141 (1994).
Tian L, et al. Prevalence of osteoporosis and related lifestyle and metabolic factors of postmenopausal women and elderly men: A cross-sectional study in Gansu province, Northwestern of China. Medicine (Baltimore). 96, (2017).
Kim Y, Han BG; KoGES group. Cohort Profile: The Korean Genome and Epidemiology Study (KoGES) Consortium. Int J Epidemiol. 46 (2017).
Kim, Y. J. et al. Genome-wide interaction study of single-nucleotide polymorphisms and alcohol consumption on blood pressure: The Ansan and Ansung study of the Korean Genome and Epidemiology Study (KoGES). Genet. Epidemiol. 44, 300–310 (2020).
Na, W., Chung, B. & Sohn, C. A relationship between dietary patterns and dyslipidemia in urban-dwelling middle-aged Korean men: Using Korean Genome and Epidemiology Study (KoGES). Clin. Nutr. Res. 8, 219–228 (2019).
Han, S. J., Kim, H. J., Kim, D. J., Lee, K. W. & Cho, N. H. Incidence and predictors of type 2 diabetes among Koreans: A 12-year follow up of the Korean Genome and Epidemiologic Study. Diab. Res. Clin. Pract. 123, 173–180 (2017).
Levey, A. S. et al. A new equation to estimate glomerular filtration rate. Ann. Intern Med. 150, 604–612 (2009).
Peng, Y. et al. Validation and assessment of three methods to estimate 24-h urinary sodium excretion from spot urine samples in Chinese adults. PLoS ONE 11, 1 (2016).
Zhou, L. et al. Validation of spot urine in predicting 24-h sodium excretion at the individual level. Am. J. Clin. Nutr. 105, 1291–1296 (2017).
Cogswell, M. E. et al. Validity of predictive equations for 24-h urinary sodium excretion in adults aged 18–39 y. Am. J. Clin. Nutr. 98, 1502–1513 (2013).
Allen, N. B. et al. The validity of predictive equations to estimate 24-hour sodium excretion: The MESA and CARDIA urinary sodium study. Am. J. Epidemiol. 186, 149–159 (2017).
World Health Organization. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. report of a WHO study group [meeting held in Rome from 22 to 25 June 1992]. (World Health Organization, 1994).
Desquilbet, L. & Mariotti, F. Dose-response analyses using restricted restricted cubic spline functions in public health research. Stat Med. 29, 1037–1057 (2010).
Acknowledgements
The authors declare no conflicts of interest. We had no funding source for this study.
Funding
Data analyzed in this study were obtained from the Korean Genome and Epidemiology Study 2011–2012 (KoGES; 4851–302), National Research Institute of Health, Centers for Disease Control and Prevention, Ministry for Health and Welfare, Republic of Korea.
Author information
Authors and Affiliations
Contributions
J.-S.P. and C.H.L. contributed to the research design; S.H., J.W.C., J.H.L., J.-S.P., and C.H.L. participated in the design and performance of the research and data analysis; and S.H., J.W.C., J.H.L., J.-S.P., and C.H.L. contributed to the writing of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hong, S., Choi, J.W., Park, JS. et al. The association between dietary sodium intake and osteoporosis. Sci Rep 12, 14594 (2022). https://doi.org/10.1038/s41598-022-18830-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-18830-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.