Abstract
Systemic mastocytosis (SM) is characterized by multifocal accumulation of neoplastic mast cells (MCs), predominately affecting the bone marrow (BM). Imaging with computed tomography (CT) is used for assessment of bone mineral density and structure. However, the value of functional imaging with dual-energy CT (DECT) and the assessment of virtual-non-calcium attenuation values (VNCa-AV) for visualization of BM disease burden in SM has not yet been assessed. DECT of the axial skeleton was performed in 18 patients with SM (indolent SM [ISM], n = 6; smoldering SM [SSM]/advanced SM [AdvSM], n = 12) and 18 control subjects. VNCa-AV were obtained in 5 representative vertebraes per patient and correlated with laboratory, morphologic and molecular parameters. VNCa-AV strongly correlated with quantitative BM MC infiltration (r = 0.7, R2 = 0.49, P = 0.001) and serum tryptase levels (r = 0.7, R2 = 0.54, P < 0.001). Mean VNCa-AV were significantly higher in SSM/AdvSM as compared to ISM (− 9HU vs. − 54HU, P < 0.005) and controls (− 38HU, P < 0.005). Nine of 10 (90%) patients with a VNCa-AV > − 30HU and 7/7 (100%) patients with a VNCa-AV > − 10HU had SSM or AdVSM. BM VNCa-AV provide information about the MC burden of SM patients and correlate with SM subtypes. DECT may therefore serve as a supplementary tool for SM diagnosis, subclassification and monitoring in a one-stop-shop session.
Similar content being viewed by others
Introduction
Systemic mastocytosis (SM) is a rare hematologic neoplasm characterized by neoplastic expansion and accumulation of clonal mast cells (MCs), predominantly affecting bone marrow (BM), skin and visceral organs1,2,3,4,5. SM is subcategorized in indolent SM (ISM), smoldering SM (SSM) and advanced SM (AdvSM), the latter comprising aggressive SM (ASM), SM with an associated hematologic neoplasm (SM‐AHN) and mast cell leukemia (MCL)6. While ISM patients benefit from an almost normal life expectancy, AdvSM is associated with poor prognosis and a median overall survival of less than 3–4 years1,7,8,9,10,11,12,13,14.
BM histology with qualitative and quantitative assessment of MC and AHN is of fundamental importance for diagnosis and subclassification of SM. In addition to blood counts and serum chemistry, radiological imaging including X-ray, computed tomography (CT) and magnetic resonance imaging (MRI) are pivotal supplementary tools for visualizing anatomical disease extent and specifically bone and BM involvement9,15,16,17,18. In a recent study on measurement of bone mineral density (BMD) through CT, an increased BMD/osteosclerosis was associated with a more aggressive phenotype and inferior survival19. Moreover, an activated BM, reflected as BM edema in MRI, is indicative for a high MC burden, organ damage and adverse survival20. However, BM edema can only be visualized but not quantified by MRI and decreased/increased BMD can only be quantified by CT.
To overcome those individual limitations of CT and MRI, functional imaging through dual-energy CT (DECT) and analysis of virtual-non-calcium attenuation values (VNCa-AV) has recently been established for detection of BM edema in trauma patients21,22 and discrimination between various infiltration patterns in multiple myeloma23. We therefore sought to investigate whether functional imaging with DECT may also be useful in patients with SM for visualization of BM disease burden.
Methods
Patients and control group
This retrospective analysis included 18 patients with SM (female, n = 8 [44; median age 63 years, range 45–86). Detailed demographic and SM-associated disease characteristics are presented in Tables 1 and 2. Eighteen control patients (female [n = 7, 39%]; median age 61 years, range 41–83) were included using the same DECT protocol. All control patients were diagnosed with non-metastatic malignant melanoma; other hematologic neoplasms were not present. The analysis adhered to the tenets of the Declaration of Helsinki and was approved by the relevant institutional review board (Medical Faculty Mannheim, University of Heidelberg). All patients gave written informed consent.
Diagnosis and subclassification
Diagnosis and subclassification were performed according to the revised world health organization (WHO) 2017 classification: ISM in 6/18 (33%) and SSM/AdvSM in 12/18 (67%) patients (Tables 1, 2)1,2,24. Diagnosis of SSM was established through presence of at least two of three B-findings (MC infiltration > 30% and serum tryptase level > 200 ng/mL; signs of dysplasia or myeloproliferation in non-MC lineage compartments of the BM, but no AHN; hepatomegaly without impairment of liver function and/or splenomegaly and/or lymphadenopathy). Diagnosis of ASM was based on the presence of one or more C-findings (cytopenia with neutropenia < 1 × 109/L, anemia <10 g/dL or thrombocytopenia < 100 × 109/L, palpable hepatomegaly with impaired liver function, palpable splenomegaly with signs of hypersplenism, malabsorption with weight loss due to gastrointestinal MC infiltrates or skeletal involvement with large osteolytic lesions and/or pathological fractures). SM-AHN met the criteria for SM and for an AHN (e.g. chronic myelomonocytic leukemia, myelodysplastic/myeloproliferative neoplasm unclassified [MDS/MPNu] or chronic eosinophilic leukemia). MCL was diagnosed based on the presence of at least ≥ 20% MCs in a BM smear.
Treatment
Patients with ISM received a symptom-directed conventional therapy including H1- and H2-antagonists, cromolyn acid, proton pump inhibitors and corticosteroids. One patient with SSM was treated with hydroxurea. All patients with AdvSM were treated with the multikinase inhibitor midostaurin; 3 patients further received the purine analogue cladribine as second-line treatment. The use of bisphosphonates was not documented in any patient at time of DECT.
DECT scan protocol, image reconstruction and postprocessing
All examinations were performed on a dual-source CT system in dual-energy mode (SOMATOM Force; Siemens Healthineers, Forchheim, Germany). Median time between diagnosis and DECT was 1.0 years (range 0–11.0). For evaluation of osteosclerosis, weighted-average coronal and sagittal multiplanar reformations were calculated. For DECT postprocessing, axial slices with a thickness of 1.0 mm were reconstructed. Postprocessing was performed on a dedicated dual-energy software (Syngo.via; version VB30A; Siemens Healthineers) with a three-material decomposition algorithm for bone mineral, yellow marrow and red marrow25. For further assessment, DECT images were viewed as weighted-average CT merged with a colour-coded VNCa overlay using the BM setting (Siemens Healthineers).
CT image evaluation
Qualitative image analysis of osteosclerosis
CT images were reviewed in consensus by two attending radiologists with 10 years of experience each in body imaging on a commercially available MacPro workstation (Apple, Cupertino, CA) running OsiriX DICOM Viewer 64-bit Version 5.5.2 (OsiriX Foundation, Geneva, Switzerland) without knowledge of the clinical findings or classification. Based on our prior experience, three distinct patterns were defined: normal bone structure, diffuse osteosclerosis and multiple focal sclerotic bone lesions.
Quantitative image analysis
All images were separately analyzed in a randomized order by two readers (J.R. and P.R.) with 10 years of experience each in oncologic imaging. The readers were blinded to clinical data and VNCa-AV measurements were performed in consensus. In patients with SM and patients from the control group, five circular region of interest (ROI) measurements of at least 100 mm2 were obtained between Th11–12 and L1–3. ROI borders were maintained 2 mm away from adjacent cortical bone in order to only include BM in the evaluation. Non-myelomatous lesions, such as Schmorl nodes and haemangiomas were not included in the ROIs. For further analysis, mean values of the five ROIs were used.
Statistics
All statistical analyses considered clinical and laboratory parameters obtained at time of imaging. The Mann–Whitney U test was used to compare continuous variables and medians of distributions. Pearson’s correlation coefficient was used to compare VNCa-AV with various disease-specific parameters (e.g. BM MC infiltration, serum tryptase level). P-values of < 0.05 (two-sided) were considered as significant. SPSS version 22 (IBM Corporation, Armonk, NY, USA) was used for statistical analysis.
Results
Qualitative image analysis of osteosclerosis
Conventional CT revealed normal bone structure in all patients of the control group (18/18) and in 5/6 (83%) patients with ISM. ISM patient #18 showed multiple focal sclerotic bone lesions. A normal bone structure was identified in only 2/12 (17%) SSM/AdvSM patients, while 6/12 (50%) patients showed diffuse osteosclerosis (Fig. 1) and 4/12 (33%) patients revealed multiple focal sclerotic bone lesions.
Quantitative image analysis of VNCa-AV
In total, 180 ROIs were evaluated: 90 in control subjects, 30 in ISM patients and 60 in AdvSM patients. VNCa-VA were strongly correlated with quantitative bone marrow mast infiltration (r = 0.7, R2 = 0.49, P = 0.001) and serum tryptase levels (r = 0.7, R2 = 0.54, P < 0.001). Further significant correlations were identified upon the KIT D816V allele burden in peripheral blood (r = 0.6, R2 = 0.4, P = 0.016) and hypoalbuminemia (r = − 0.5, R2 = 0.3, P = 0.041) (Table 3, Fig. 2). The overall results indicated a strong association between a pathologically elevated VNCa-AV and an advanced phenotype.
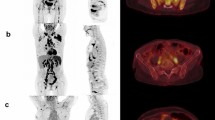
Mean VNCa-AV were significantly higher in patients with SSM/AdvSM compared to ISM (− 9HU vs. − 54HU, P<0.005) and controls (− 38HU, P < 0.005). VNCa-AV were not different between ISM and control subjects (Fig. 3). Nine of 10 (90%) patients with a VNCa-AV > − 30HU and 7/7 (100%) patients with a VNCa-AV > − 10HU had SSM or AdVSM. Pathologically elevated VNCa-AV indicating diffuse BM edema were not observed in ISM patients (0/6) but in 8/12 (67%) SSM/AdvSM patients. Of note, two of these patients (#6 and #8) with an aggressive phenotype (BM MC infiltration: 35% and 70%, serum tryptase: 377 µg/L and 885 µg/L, genetics: KIT D816V+/SRSF2+ and KIT D816V negative) had normal bone structures on conventional CT (Fig. 4).
(A) Patient #8 with MCL showing normal bone structures on computer tomography (CT). (B) Dual-energy CT revealed elevated virtual-non-calcium values with diffuse edema of the bone marrow. The high bone marrow mast cell infiltration (70%) and high serum tryptase levels (885 µg/L) indicates a high mast cell disease burden.
Comparative analysis of increased and normal VNCa-AV throughout all SM subgroups
Within the cohort of SSM/AdvSM, patients with increased VNCa-AV (group 1) presented with a significantly stronger BM MC infiltration (mean 45% vs. 25%, P = 0.088) and higher levels of serum tryptase (mean 437 µg/L vs. 143 µg/L, P = 0.008), alkaline phosphatase (mean 339 U/L vs. 121U/L, P = 0.160) and bilirubin (mean 0.83 mg/dL vs. 0.58 mg/dL, P = 0.344) as compared to patients with normal VNCa-AV (group 2) (Table 4). In comparison to group 1, mean values for ISM patients (group 3) were: BM MC infiltration 16% vs. 45% (P = 0.004), serum tryptase 87 µg/L vs. 437 µg/L (P = 0.003) and alkaline phosphatase 88 U/L vs. 339 U/L (P = 0.109).
Discussion
Non-invasive imaging techniques such as CT and MRI are important supplementary tools in the diagnostic work-up of SM. Beside the visualization of potential involvement of visceral organs through organomegaly, this particularly includes qualitative and quantitative assessment of bone lesions such as osteopenia, osteoporosis, osteosclerosis and osteoporotic/osteolytic lesions with pathologic fractures19. The correlation between HU values and BM MC infiltration has been established in non-DECT examinations18. While osteopenia and osteoporosis are typically present in ISM, osteosclerosis is more frequently identified in AdvSM26,27. We recently showed that an increased BMD is strongly associated with advanced disease and inferior outcome19. However, conventional CT technology does not allow visualization and quantification of BM edema, which is frequently identified in patients with SSM/AdvSM by MRI20.
A recent study successfully applied VNCa from DECT in patients with multiple myeloma. Kosmala et al. showed, that the VNCa-AV significantly differed according to the BM infiltration pattern on MRI and that a diffuse infiltration pattern could be confidently determined using DECT23.
In patients with SSM/AdvSM diffuse BM infiltration patterns have also been described using MRI technique20, however no data is available about the applicability of DECT in these patients. We therefore evaluated DECT-generated VNCa-AV in clinically and morphologically well characterized patients with ISM and SSM/AdvSM. VNCa-VA were strongly correlated with quantitative bone marrow MC infiltration and serum tryptase levels but also other characteristics, e.g. levels of alkaline phosphatase, albumin or KIT D816V variant allele frequency, indicating a strong correlation with an advanced phenotype. Mean VNCa-AV were significantly higher in patients with SSM/AdvSM with values > − 30HU almost exclusively and values > − 10HU exlusively found in SSM/AdvSM. Within the SSM/AdvSM patient group, patients with an elevated VNCa-AV revealed a more aggressive phenotype. Of note, two AdvSM patients with regular bone structure by conventional CT showed markedly elevated VNCa-AV. Both patients were associated with a poor clinico-genetic risk profile, e.g. KIT D816V negative MCL and multimutated SM-MDS/MPNu progressing to secondary acute myeloid leukemia even more indicating DECT as a supplementary tool for identification of high-risk disease.
The increased VNCa-AV may be best explained by variable displacement of healthy adipose marrow by tumor cells or rather MCs. Because an AHN is present in 70–80% of AdvSM patients, it could be argued that the AHN also contributes to the pathologically elevated VNCa-AV, e.g. through hypercellularity. However, the strong association between VNCa-AV and several SM-specific factors outside of the bone marrow such as serum tryptase levels and to a lesser extent alkaline phosphatase, albumin and KIT D816V are clearly in favor of SM.
Several limitations of this study have to be addressed. First, the control group consisted of patients with non-metastatic malignant melanoma and not healthy probands. This patient group was chosen, because (i) the retrospective design of this study did not allow to enroll healthy probands, (ii) scans for metastatic melanomatous deposits in patients with malignant melanoma were mandatory and (iii) the absence of metastases in patients with malignant melanoma and otherwiese “healthy” BM comprised a control group similar to what we expect in a general sex- and age-matched population. Further, the small sample size of 18 patients derived from a single center limits statistical power, especially for subgroup analysis. However, SM is a very rare disease and the application of DECT is not yet part of the routine clinical work-up. Larger patient populations and a prospective study design would be desirable.
Last, to underline our results, the correlation of VNCa-AV with the BM morphology on MRI might be of interest. Unfortunately, due to the retrospective study design timely spine MRI was not available for the patients included in this study. This aspect should also be highlighted in future studies.
Imaging techniques such as DECT are appreciated for its remarkable value in biological characterization of tissue involvement through generating functional information upon the tissue microstructure. Radiological biomarkers, which allow a quantitative and objective analysis of these images, are of increasing importance in this context. We conclude, that DECT represents an excellent supplementary tool for one-stop-shop imaging of organomegaly, osteopenia, osteoporosis, osteosclerosis and bone marrow edema in patients with SM. The technique allows non-invasive assessment of the mast cell burden and may therefore serve as supplementary tool for diagnosis, subclassification and monitoring of the SM disease course.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AdvSM:
-
Advanced systemic mastocytosis
- AHN:
-
Associated hematologic neoplasm
- ASM:
-
Aggressive systemic mastocytosis
- BM:
-
Bone marrow
- BMD:
-
Bone mineral density
- CT:
-
Computed tomography
- DECT:
-
Dual-energy computed tomography
- ISM:
-
Indolent systemic mastocytosis
- MC:
-
Mast cell
- MCL:
-
Mast cell leukemia
- MDS/MPNu:
-
Myelodysplastic/myeloproliferative neoplasm unclassified
- MRI:
-
Magnetic resonance imaging
- ROI:
-
Region of interest
- SM:
-
Systemic mastocytosis
- SM-AHN:
-
Systemic mastocytosis with an associated hematologic neoplasm
- SSM:
-
Smoldering systemic mastocytosis
- VNCa-AV:
-
Virtual-non-calcium attenuation values
References
Valent, P., Akin, C. & Metcalfe, D. D. Mastocytosis: 2016 updated WHO classification and novel emerging treatment concepts. Blood 129, 1420–1427. https://doi.org/10.1182/blood-2016-09-731893 (2017).
Valent, P. et al. Diagnosis and treatment of systemic mastocytosis: State of the art. Br. J. Haematol. 122, 695–717 (2003).
Metcalfe, D. D. Mast cells and mastocytosis. Blood 112, 946–956. https://doi.org/10.1182/blood-2007-11-078097 (2008).
Jawhar, M. et al. Molecular profiling of myeloid progenitor cells in multi-mutated advanced systemic mastocytosis identifies KIT D816V as a distinct and late event. Leukemia 29, 1115–1122. https://doi.org/10.1038/leu.2015.4 (2015).
Horny, H. P., Parwaresch, M. R. & Lennert, K. Bone marrow findings in systemic mastocytosis. Hum. Pathol. 16, 808–814. https://doi.org/10.1016/s0046-8177(85)80252-5 (1985).
Arber, D. A. et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 127, 2391–2405 (2016).
Jawhar, M. et al. Additional mutations in SRSF2, ASXL1 and/or RUNX1 identify a high-risk group of patients with KIT D816V(+) advanced systemic mastocytosis. Leukemia 30, 136–143. https://doi.org/10.1038/leu.2015.284 (2016).
Sperr, W. R. et al. International prognostic scoring system for mastocytosis (IPSM): A retrospective cohort study. Lancet Haematol. 6, e638–e649. https://doi.org/10.1016/s2352-3026(19)30166-8 (2019).
Jawhar, M. et al. The clinical and molecular diversity of mast cell leukemia with or without associated hematologic neoplasm. Haematologica 102, 1035–1043. https://doi.org/10.3324/haematol.2017.163964 (2017).
Lübke, J. et al. Superior efficacy of midostaurin over cladribine in advanced systemic mastocytosis: A registry-based analysis. J. Clin. Oncol. https://doi.org/10.1200/jco.21.01849 (2022).
Lübke, J. et al. Inhibitory effects of midostaurin and avapritinib on myeloid progenitors derived from patients with KIT D816V positive advanced systemic mastocytosis. Leukemia 33, 1195–1205. https://doi.org/10.1038/s41375-019-0450-8 (2019).
Jawhar, M. et al. MARS: Mutation-adjusted risk score for advanced systemic mastocytosis. J. Clin. Oncol. https://doi.org/10.1200/jco.19.00640 (2019).
Schwaab, J. et al. Importance of adequate diagnostic work-up for correct diagnosis of advanced systemic mastocytosis. J. Allergy Clin. Immunol. Pract. https://doi.org/10.1016/j.jaip.2020.05.005 (2020).
Schwaab, J. et al. Comprehensive mutational profiling in advanced systemic mastocytosis. Blood 122, 2460–2466. https://doi.org/10.1182/blood-2013-04-496448 (2013).
Huang, T. Y., Yam, L. T. & Li, C. Y. Radiological features of systemic mast-cell disease. Br. J. Radiol. 60, 765–770. https://doi.org/10.1259/0007-1285-60-716-765 (1987).
Johansson, C., Roupe, G., Lindstedt, G. & Mellstrom, D. Bone density, bone markers and bone radiological features in mastocytosis. Age Ageing 25, 1–7 (1996).
Manara, M. et al. Osteoporosis with vertebral fractures in young males, due to bone marrow mastocytosis: A report of two cases. Clin. Exp. Rheumatol. 28, 97–100 (2010).
Meyer, H. J., Pönisch, W., Monecke, A., Gundermann, P. & Surov, A. Can diagnostic low-dose whole-body CT reflect bone marrow findings in systemic mastocytosis? Anticancer Res. 40, 1015–1022. https://doi.org/10.21873/anticanres.14036 (2020).
Riffel, P. et al. An increased bone mineral density is an adverse prognostic factor in patients with systemic mastocytosis. J. Cancer Res. Clin. Oncol. 146, 945–951. https://doi.org/10.1007/s00432-019-03119-3 (2020).
Riffel, P. et al. Magnetic resonance imaging reveals distinct bone marrow patterns in indolent and advanced systemic mastocytosis. Ann. Hematol. 98, 2693–2701. https://doi.org/10.1007/s00277-019-03826-4 (2019).
Suh, C. H. et al. Diagnostic performance of dual-energy CT for the detection of bone marrow oedema: A systematic review and meta-analysis. Eur. Radiol. 28, 4182–4194. https://doi.org/10.1007/s00330-018-5411-5 (2018).
Bierry, G., Venkatasamy, A., Kremer, S., Dosch, J. C. & Dietemann, J. L. Dual-energy CT in vertebral compression fractures: Performance of visual and quantitative analysis for bone marrow edema demonstration with comparison to MRI. Skelet. Radiol. 43, 485–492. https://doi.org/10.1007/s00256-013-1812-3 (2014).
Kosmala, A. et al. Dual-energy CT of the bone marrow in multiple myeloma: Diagnostic accuracy for quantitative differentiation of infiltration patterns. Eur. Radiol. 28, 5083–5090. https://doi.org/10.1007/s00330-018-5537-5 (2018).
Sperr, W. R. & Valent, P. Diagnosis, progression patterns and prognostication in mastocytosis. Expert Rev. Hematol. 5, 261–274. https://doi.org/10.1586/ehm.12.12 (2012).
Liu, X., Yu, L., Primak, A. N. & McCollough, C. H. Quantitative imaging of element composition and mass fraction using dual-energy CT: Three-material decomposition. Med. Phys. 36, 1602–1609. https://doi.org/10.1118/1.3097632 (2009).
Kushnir-Sukhov, N. M., Brittain, E., Reynolds, J. C., Akin, C. & Metcalfe, D. D. Elevated tryptase levels are associated with greater bone density in a cohort of patients with mastocytosis. Int. Arch. Allergy Immunol. 139, 265–270. https://doi.org/10.1159/000091172 (2006).
Barete, S. et al. Systemic mastocytosis and bone involvement in a cohort of 75 patients. Ann. Rheum. Dis. 69, 1838–1841. https://doi.org/10.1136/ard.2009.124511 (2010).
Acknowledgements
Deutsche José Carreras Leukämie-Stiftung’ (Grant No. DJCLS 08R/2020).
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
R.J., L.J., R.A. and R.P. have assessed and verified the data. R.J., L.J., R.A. and R.P. contributed to concept and design. R.J., L.J., S.J., N.N., J.M., O.D., S.S.O., H.W.K., R.A. and R.P. were involved in acquisition of data. R.J., L.J., R.A. and R.P. contributed to statistical analysis. R.J., L.J., R.A. and R.P. were involved in interpretation of data. R.J., L.J., R.A. and R.P. were involved in manuscript writing. All authors contributed to critical revision of the manuscript for important intellectual content. All authors read and approved the final manuscript. All authors are accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Riffel, J., Lübke, J., Naumann, N. et al. Functional imaging with dual-energy computed tomography for supplementary non-invasive assessment of mast cell burden in systemic mastocytosis. Sci Rep 12, 14228 (2022). https://doi.org/10.1038/s41598-022-18537-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-18537-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.