Abstract
Cordyceps militaris (CM) is a popular medicinal fungus; however, few studies have focused on its impact on the male reproductive system. We evaluated the effects of CM fermentation products on the reproductive development of juvenile male (JM) mice. Mice were divided into four experimental groups, each fed 5% CM products (weight per weight (w/w) in normal diet): extracellular polysaccharides (EPS), fermentation broth (FB), mycelia (MY), and whole fermentation products (FB plus MY, FBMY) for 28 days, while mice in the control group (CT) were fed a normal diet. Basic body parameters, testicular structure, sperm parameters, and sex hormones concentrations were analyzed. Compared to the CT group, mice in the EPS, MY, and FBMY groups showed a significantly increased mean seminiferous tubule area (p < 0.05), mice in the FB and MY groups had significantly higher sperm concentrations (p < 0.05), and mice in the EPS, FB, and FBMY groups showed significantly increased ratios of motile sperm (p < 0.05). Meanwhile, EPS significantly promoted the ability of JM mice to synthesize testosterone (p < 0.05). Furthermore, all CM products significantly increased the food intake of JM mice (p < 0.05) but did not significantly change their water intake and body weight gain (p > 0.05). In conclusion, CM products, especially EPS, exhibit strong androgen-like activities that can promote male reproductive development.
Similar content being viewed by others
Introduction
Testis development during juvenile stages is essential for male reproductive function1. Obesity2, environmental pollution3, psychological stress4, and even COVID-195 impact testicular function and spermatogenesis. Exposure to these risk factors early in life may have long-term effects on male fertility6. A meta-analysis reported that sperm concentration has decreased globally by approximately 50% over the past 50 years, and male infertility has become a major problem for many couples7. The World Health Organization (WHO) predicts that infertility will become the third major health threat after cancer and cardiovascular diseases in the twenty-first century8. Currently, some drugs have been developed for treating male infertility9. For example, sildenafil citrate (Viagra) is used clinically to treat erectile dysfunction. However, many adverse events have been observed, including flushing, headaches, and dyspepsia10. Therefore, it is necessary to identify natural products that enhance male reproductive development and function without significant side effects.
Ophiocordyceps sinensis (O. sinensis), referred to as the “Himalayan Viagra,” is a scarce and expensive medicinal fungus11. Cordyceps militaris (C. militaris), also known as Northern O. sinensis, is a species of the genus Cordyceps12. Studies have shown that there are the levels of some bioactive ingredients, such as amino acids, unsaturated fatty acids, and adenosine, are higher in C. militaris than in O. sinensis13. Recently, C. militaris has been widely used as a substitute for O. sinensis in traditional Chinese medicine and health supplement12.
Specifically, the culture of C. militaris using a liquid fermentation process can shorten the production cycle and increase yield14. This process yields large amounts of extracellular polysaccharides (EPS) and mycelia (MY) in a short period. EPS and MY have been proven to possess antioxidant, antimicrobial, and anti-inflammatory activities15,16. However, due to the large volume of fermentation broth (FB) and low content of active ingredients, it is often discarded as waste. In this study, we investigated the effects of C. militaris fermentation products on the reproductive development of juvenile male (JM) mice. Basic body parameters, testicular structure, sperm parameters, and serum concentrations of sex hormones were analyzed.
Results and discussion
Main components of the four C. militaris fermentation products
Table 1 showed that the content of polysaccharides in the EPS group was the highest, and was significantly higher than those in the FB, MY and FBMY groups (p < 0.05). There were significant differences in the contents of cordycepin and adenosine in the four C. militaris fermentation products (p < 0.05), and all had the highest content in the FB. Except between the FB and EPS, protein concentrations in different fermentation products were all significant differences (p < 0.05). The protein concentrations in the MY was the highest, followed by in the FBMY, in the FB, and in the EPS was the lowest. In this study, EPS were obtained from FB by ethanol precipitation. When the EPS in FB were precipitated with ethanol, cordycepin could not be simultaneously precipitated because of its good solubility in water and ethanol. Thus, the cordycepin content in the EPS was below detectable levels.
Effects of C. militaris fermentation products on food and water intake and body weight gain
Food and water intake and body weight gain in mice of all groups are presented in Fig. 1. The food intake of JM mice in the experimental groups fed with C. militaris fermentation products (EPS, FB, MY, and FBMY) was significantly higher than that of the CT group that were fed a normal diet (all p < 0.05; Fig. 1A). JM mice in the EPS group had the highest food intake (p < 0.001 vs. CT, p = 0.007 vs. FB, p = 0.034 vs. MY, p = 0.343 vs. FBMY). A possible explanation for this phenomenon is that the sweetness of EPS increases the palatability of the pellets. Moreover, there were no significant differences in water intake (Fig. 1B) or body weight gain for 28 days (Fig. 1C) between the experimental groups and the CT group (all p > 0.05). Although mice in the EPS group ate more than mice in the other groups, their body weight gains were only significantly increased when compared with the FB group (p < 0.05). These results imply that body weight gain in JM mice is controlled or regulated by EPS addition, thereby preventing obesity.
Effects of C. militaris fermentation products on food and water intake and body weight gain of JM mice during the 28-day experiment. Food intake (A), water intake (B), and body weight gain (C) were shown in different indicated groups. Values are presented as means ± SD (n = 5 for all test groups). Panels marked with different lowercase letters are significantly different (p < 0.05).
Recently, the regulatory role of some medicinal fungi on obesity has been gradually clarified through 16S rRNA gene sequencing and metabolomic studies17,18,19,20,21,22. Since intestinal bacteria can not only increase the absorption of intestinal monosaccharides and short-chain fatty acids and promote the fat accumulation in the liver tissue23, but the lipopolysaccharide of gram-negative bacteria can also bind to receptors on the surface of immune cells, causing an inflammatory response thereby aggravating obesity24. Previous studies have demonstrated that O. sinensis can reduce the amounts of Enterococcus cecorum and modulate a bile acid receptor, thereby attenuating lipid metabolism disorders and their associated inflammation in high-fat diet (HFD) mice25. C. militaris polysaccharides could increase gut bacteria negatively associated with obesity traits, decrease the population of gram-negative bacteria, effectively improve intestinal flora dysbiosis in HFD-induced mice, and regulate the levels of metabolites, thereby reducing body weight, fat accumulation, and pro-inflammatory cytokine levels in mice26,27. Selenium-enriched C. militaris could reduce serum triglyceride and low-density lipoprotein cholesterol levels, inhibit serum lipopolysaccharide-binding proteins, adiponectin levels, and pro-inflammatory gene expression in HFD mice, and improve the expression of related anti-inflammatory genes28. Although the mechanisms remain to be elucidated, C. militaris fermentation products, especially EPS, may effectively prevent obesity during the development of JM mice by modulating the intestinal flora.
Effects of C. militaris fermentation products on apparent testicular parameters
Testicular volumes and weights of JM mice in each group are shown in Fig. 2. There were no significant differences in testicular volume between the experimental and CT groups (all p > 0.05; Fig. 2A). Mice in the MY group had the largest testicular volumes. Furthermore, mice in all groups, except the EPS group, showed similar results (all p > 0.05; Fig. 2B). Mice in the EPS group had the lowest testicular weight and showed significant differences compared with mice in the other groups (p = 0.004 vs. CT; p = 0.013 vs. FB; p = 0.002 vs. MY; p = 0.005 vs. FBMY).
Effects of C. militaris fermentation products on apparent testicular parameters of JM mice. Testicular volumes (A) and testicular weights (B) were shown in different indicated groups. Values are presented as means ± SD (n = 5 for all test groups). Panels marked with different lowercase letters are significantly different (p < 0.05).
It has been reported that cordycepin reduces fat accumulation around the epididymis of HFD rats29 and significantly reduces epididymal adipocyte sizes in a dose-dependent manner30. In addition, C. militaris polysaccharides can decrease epididymal fat index by modulating the expression of key genes and proteins in epididymal fat31, including reducing sterol regulatory element binding protein-1c level by approximately 49%, inhibiting the expression of peroxisome proliferator-activated receptor γ protein by approximately 67%32, thereby significantly reducing epididymal adipogenesis. This suggests that the decreased testicular weight of JM mice in the EPS group may be related to the fact that EPS can reduce lipid and adipocyte differentiation in testis and surrounding tissue. Since the testes play crucial roles in producing sperms and synthesizing androgens, the testicular structure, sperm parameters, and sex hormone levels in sera were further evaluated.
Effects of C. militaris fermentation products on testicular morphology and structure
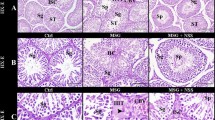
The hematoxylin and eosin (H&E) staining images of testicular sections of JM mice from each group are shown in Fig. 3. The morphology and structure of the seminiferous tubule (STs) were normal, and germ cells in the STs were in a regular arrangement in all groups. In the same field of view, STs were larger, and sperm cell densities were higher in all experimental groups than in the CT group. These results indicate that C. militaris fermentation products promote ST development in the testes of JM mice. Therefore, we evaluated the effect of C. militaris fermentation products on the morphological indicators of STs.
Effects of C. militaris fermentation products on testicular morphology and structure. Testicular sections of the CT group (A), EPS group (B), FB group (C), MY group (D), and FBMY group (E) were stained with H&E. Scale bars: 200 μm. Panels marked with different lowercase letters are significantly different (p < 0.05).
The number and mean area of the STs of JM mice in each group are presented in Fig. 4. The number of STs in all experimental groups was significantly lower than that in the CT group (p < 0.05; Fig. 4A). Mice in the EPS group had the lowest number of STs (p < 0.001 vs. CT, p < 0.001 vs. FB, p = 0.761 vs. MY, p = 0.052 vs. FBMY). Moreover, the mean area of STs in all experimental groups, except for the FB group, was significantly larger than that in the CT group (all p < 0.05; Fig. 4B). The mean area of STs was the largest in the MY group (p < 0.001 vs. CT, p = 0.221 vs. EPS, p < 0.001 vs. FB, p = 0.025 vs. FBMY). The larger the mean area of the STs, the more mature the STs.
The STs of the testis are the site of spermatogenesis and are composed of spermatogenic cells and Sertoli cells, the former producing sperm and the latter supporting and nourishing spermatogenic cells33. In this study, except that EPS significantly reduced testicular weight, C. militaris fermentation products did not significantly affect testicular volumes or weights of JM mice, but significantly increased the size and maturation of STs. These results suggest that C. militaris enhances testicular development, thereby promoting spermatogenesis in JM mice.
Effects of C. militaris fermentation products on sperm parameters
To further assess male reproductive development, sperm concentrations and ratios of motile sperm in the epididymis were analyzed (Table 2). Sperm concentrations in JM mice in all experimental groups were higher than in the CT group. Mice in both the FB and MY groups showed significant increases in sperm concentrations compared to the CT group (p < 0.05). Mice in the MY group had the highest sperm concentrations. Furthermore, the ratio of motile sperm was also higher in all experimental groups than in the CT group. Except for the MY group, the motile sperm ratios in all experimental groups were significantly higher than in the CT group (all p < 0.05). Ratios of motile sperm were the highest in the FBMY group.
Based on the results shown in Figs. 3, 4 and Table 2, C. militaris fermentation products promoted the development and maturation of STs, enhanced spermatogenesis in the testes, and increased mature sperm concentration and motile sperm ratio in the epididymis. These results are consistent with reports showing increased sperm concentrations and motilities in boars34 and Sprague–Dawley rats35 following MY supplementation.
Oxidative stress is an important cause of testicular damage, which can lead to testicular dysplasia, spermatogenic cell necrosis and apoptosis, resulting in male reproductive dysfunction36. C. militaris is a natural antioxidant with excellent ability to scavenge reactive oxygen radicals37. Among them, cordycepin can regulate the activities of downstream antioxidant enzymes through the SIRT1/Foxo3a signaling pathway38, as well as regulate NF-κB activation and MAPKs signaling39, effectively improving sexual dysfunction in aged rats. Moreover, another study showed that the EPS extracted from the C. militaris FB could promote the proliferation of spleen T and B lymphocytes through MAPK signaling40; thus, EPS may also be able to resist testicular oxidation through this pathway. Therefore, the antioxidant activities of C. militaris may explain why these fermented products can promote male reproductive development in JM mice.
Effects of C. militaris fermentation products on serum sex hormone levels
To investigate the effects of supplementation with C. militaris fermentation products on male reproductive development, the serum sex hormone levels of JM mice were analyzed (Fig. 5). Although mice in both the EPS and FB groups showed higher testosterone (T) concentrations than those in the CT group, there were no significant differences in T concentrations between each experimental group and the CT group (all p > 0.05; Fig. 5A). Mice in the EPS group had the highest T levels. However, T levels in mice in the FBMY group were significantly lower than those in the other experimental groups (all p < 0.05), but not in the CT group.
Effects of C. militaris fermentation products on serum sex hormone levels in different indicated groups. T concentration (A), E2 concentration (B), LH concentration (C), and calculated T/T ratio (D) were shown. Values are presented as means ± SD (n = 5 for all test groups). Panels marked with different lowercase letters are significantly different (p < 0.05).
Since estradiol (E2) levels may antagonize the effects of T, serum E2 levels were also analyzed. Although mice in both the FB and MY groups showed higher E2 concentrations in sera than those in the CT group, there were no significant differences in E2 concentrations between each experimental group and the CT group (all p > 0.05; Fig. 5B). However, E2 levels were the lowest in the FBMY group but only significantly different when compared to the FB and MY groups (both p < 0.05). In males, E2 is mostly converted through the aromatization of T41. The results shown in Figs. 5A,B support the positive correlations between T and E2 levels, and the lower E2 concentration, resulting in lower testicular volumes, number of STs, and sperm concentrations in epididymis of mice in the FBMY group. However, the reason for the lower T and E2 levels in the FBMY group than in the other groups remains to be elucidated.
Since luteinizing hormone (LH) is secreted by the anterior pituitary gland, which stimulates ovulation in females and androgen synthesis in males, serum LH levels were analyzed. Except for the FB group, there were no significant differences in LH concentrations among the other groups (all p > 0.05; Fig. 5C). However, the LH levels were significantly lower in the FB group than in the CT, EPS, and MY groups (all p < 0.05). The synthesis and secretion of sex hormones are regulated mainly by the hypothalamus-pituitary-gonadal axis. Thus, increases in both T and E2 levels in the FB group may have a negative effect on the LH levels secreted by the pituitary gland. This explains why mice in the FB group had the lowest LH levels.
Since T concentrations in testes are at least 25–100 times higher than those in sera42, the ratios of serum T levels to testicular weights (T/T) were calculated to directly assess the ability of testes to synthesize T. Results showed that T/T ratios were higher in all experimental groups, except the FBMY group, than those in the CT group (Fig. 5D). The T/T ratios were the highest in the EPS group and showed significant differences when compared with the CT, MY, and FBMY groups (all p < 0.05). Moreover, the T/T ratios of the FBMY group were the lowest and showed a significant decreases when compared with those of the EPS and FB groups ( p < 0.05), but there was no significant difference with the CT group.
The results shown in Fig. 5 indicated that C. militaris fermentation products, especially EPS, promote the development of testes and elevate serum levels of T and T/T ratios in JM mice. Our findings agree with one previous study that demonstrated a C. militaris-induced increase in T productions in Sprague-Dawley rats 43. Another study showed that O. sinensis stimulates T productions in vivo and in vitro 44. Polysaccharides and glycoproteins of O. sinensis share structural similarities with LH and can be recognized by LH receptors on Leydig cells to stimulate T productions. Although the specific mechanism remains unknown, C. militaris fermentation products, especially EPS, showed androgen-like activity, which can promote male reproductive development in JM mice.
The results shown in Figs. 2, 3, 4 and 5 and Table 2 indicate that C. militaris fermentation products, especially EPS, promote the development of testes, elevate serum levels of T and T/T ratios, and then increase sperm concentrations and ratios of motile sperms in the epididymis of JM mice. Since all mice in this study were fed ad libitum, it is possible that the EPS-containing feed increased the mice's appetite due to its sweetness. From our results, increased EPS intake may better promote reproductive development in male JM mice. In addition, C. militaris extract has been reported to prevent obesity and fat accumulation in ovariectomized rats, lower triglyceride levels, and enhance estrogen receptor agonistic activity and phosphorylation, effectively regulating menopause-induced obesity45. Therefore, these findings suggest that C. militaris fermentation products can promote the reproductive system health and prevent metabolic syndromes including obesity.
Conclusion
In this study, we evaluated the effects of four different C. militaris fermentation products on male reproductive development in JM mice. EPS showed the strongest androgen-like activity, which promoted male reproductive development. EPS supplementation can increase the mean area and maturation of STs in the testes, sperm concentrations and ratios of motile sperms in the epididymis, and serum T levels and T/T ratios. Therefore, EPS supplementation can effectively improve male reproductive development in JM mice, and may prevent or assist in treating male reproductive dysfunction or infertility. Furthermore, it is important to note that C. militaris fermentation products can effectively control or regulate body weight gain in JM mice during the experimental period of 28 days, thus it has the potential to assist in obesity treatment. However, it must be noted that clinical studies are required to determine if these beneficial effects of C. militaris can apply to humans.
Materials and methods
Materials
C. militaris fermentation products including EPS, FB, and MY, were gifts from Dalong Biotechnology Co. Ltd. (Taichung, Taiwan). EPS were obtained from ethanol precipitation of the FB, centrifugation, and freeze-drying. The yields of polysaccharides, cordycepin, adenosine, and proteins of the EPS, FB, MY and whole fermentation product (FBMY) were detected by the phenol-sulfuric acid method 46 high-performance liquid chromatography (HPLC) method 47, and Bio-Rad Protein Assay (Bio-Rad, Hercules, CA, USA) according to the manufacturer's instructions, respectively (Table 1). The feed pellets of different experimental groups were produced by mixing 5% (w/w) dry weight of EPS, FB, MY, and FBMY with 95% Laboratory Rodent Diet 5001.
Animals
Three-week-old male mice (C57BL/6JNarl), 25 in number, were purchased from the National Laboratory Animal Center (Taipei, Taiwan). During the experimental period, they were kept in an air-conditioned room at 24 ± 2 °C and 50–60% humidity with a 12 h light/dark cycle. They were randomly divided into five groups (n = 5 each). Mice in the control (CT) group were fed Laboratory Rodent Diet 5001 only, while mice in the experimental groups were fed pellets containing 5% (w/w) EPS, FB, MY, and FBMY for 28 days. Mice were sacrificed using the cervical dislocation method after anesthesia with isoflurane (Baxter, CA, USA) at the end of the experiment. Blood samples were obtained by cardiac puncture. In addition, testes and epididymides were removed for measurement and analysis.
H&E staining of testis sections
Each right testis was fixed in a 10% neutral formalin solution (Leica Surgipath Inc., Buffalo Grove, IL, USA) for more than 16 h. After fixation, testes were cut in half at the widest point and embedded in paraffin. Five-micrometer histological sections were deparaffinized, rehydrated, and stained with H&E according to the manufacturer’s instructions (Sigma-Aldrich Corp., St. Louis, USA). Morphological images were obtained using a TissueFAXS system (Tissue Gnostics, Vienna, Austria) at the Instrument Center of Chung Shan Medical University. Seminiferous tubule (ST) numbers were recorded using Scope Photo 3.0 (Micro Imaging Ltd., Auckland, New Zealand).
Sperm concentrations and motilities
The right epididymis was cut into three pieces and incubated in 1 mL of Ren’s solution at 37 °C for 30 min. After centrifugation at 100 g for 5 min, sperm concentrations were determined using a hemocytometer and Motic BA300 Binocular Compound Microscope (Motic Asia, Hong Kong, China). For each mouse, sperm numbers were obtained for five grids and then divided by five. Sperm concentrations were expressed in millions per milliliter (106 cells/mL), and sperm images were captured at 5 s intervals using a Dino-Lite digital microscope (AnMo Electronics Corp., Taipei, Taiwan) and DinoCapture 2.0. Active sperms were defined as sperm in different positions on the two images. Ratios of motile sperm (%) = (number of total sperm–number of inactive sperm)/number of total sperm × 100%.
Serum hormone assays
The collected blood samples were centrifuged at 10,000 g for 10 min, and serum was collected. Serum testosterone (T), estradiol (E2), and luteinizing hormone (LH) levels were determined according to the manufacturer’s protocols (Elabscience Biotechnology Co., Ltd., Wuhan, Hubei, China).
Statistics
Data are expressed as mean ± standard deviation (SD). The differences between multiple groups were evaluated using one-way analysis of variance (ANOVA) and post-hoc least significant difference (LSD) tests. Statistical significance was set at p < 0.05. All figures were created using Graph Pad Prism version 6.0.0 for Windows (Graph Pad Software, www.graphpad.com).
Ethical approval
Experimental protocols were approved by the Animal Care and Use Committee of Da-Yeh University (approval number: 107025), all animals were treated according to the guidelines for animal experimentation of Da-Yeh University in Changhua, Taiwan. The animal experiments were also performed in accordance with the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Zhang, N. et al. Single-cell transcriptome analysis of bisphenol A exposure reveals the key roles of the testicular microenvironment in male reproduction. Biomed. Pharmacother. 145, 112449. https://doi.org/10.1016/j.biopha.2021.112449 (2022).
Liu, Y. & Ding, Z. Obesity, a serious etiologic factor for male subfertility in modern society. Reproduction (Cambridge, England) 154, R123-131. https://doi.org/10.1530/rep-17-0161 (2017).
Sun, S. et al. Identifying critical exposure windows for ambient air pollution and semen quality in Chinese men. Environ. Res. 189, 109894. https://doi.org/10.1016/j.envres.2020.109894 (2020).
Ilacqua, A., Izzo, G., Emerenziani, G. P., Baldari, C. & Aversa, A. Lifestyle and fertility: The influence of stress and quality of life on male fertility. Reprod. Biol. Endocrinol. RB&E 16, 115. https://doi.org/10.1186/s12958-018-0436-9 (2018).
Li, C. et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections by intranasal or testicular inoculation induces testicular damage preventable by vaccination in golden Syrian hamsters. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. https://doi.org/10.1093/cid/ciac142 (2022).
Delbes, G. et al. Effects of endocrine disrupting chemicals on gonad development: Mechanistic insights from fish and mammals. Environ. Res. 204, 112040. https://doi.org/10.1016/j.envres.2021.112040 (2022).
Branch, F. M., Perry, M. J., Chen, Z. & Louis, G. M. B. Metal(loid)s and human semen quality: The life study. Reproductive toxicology (Elmsford, N,Y,) 106, 94–102. https://doi.org/10.1016/j.reprotox.2021.10.006 (2021).
He, C. et al. Roles of noncoding RNA in reproduction. Front. Genet. 12, 777510. https://doi.org/10.3389/fgene.2021.777510 (2021).
Zhou, Z. et al. Epidemiology of infertility in China: a population-based study. BJOG Int. J. Obstet. Gynaecol. 125, 432–441. https://doi.org/10.1111/1471-0528.14966 (2018).
Graziano, S. et al. Sildenafil-associated hepatoxicity: a review of the literature. Eur. Rev. Med. Pharmacol. Sci. 21, 17–22 (2017).
Liu, G., Han, R. & Cao, L. Artificial cultivation of the Chinese Cordyceps from injected ghost moth larvae. Environ. Entomol. 48, 1088–1094. https://doi.org/10.1093/ee/nvz099 (2019).
Dong, C. H., Yang, T. & Lian, T. A comparative study of the antimicrobial, antioxidant, and cytotoxic activities of methanol extracts from fruit bodies and fermented mycelia of caterpillar medicinal mushroom Cordyceps militaris (Ascomycetes). Int. J. Med. Mushrooms 16, 485–495. https://doi.org/10.1615/intjmedmushrooms.v16.i5.70 (2014).
Chen, L., Liu, Y., Guo, Q., Zheng, Q. & Zhang, W. Metabolomic comparison between wild Ophiocordyceps sinensis and artificial cultured Cordyceps militaris. Biomed. Chromatogr. BMC. https://doi.org/10.1002/bmc.4279 (2018).
Kang, C. et al. Optimization of large-scale culture conditions for the production of cordycepin with Cordyceps militaris by liquid static culture. Sci. World J. 2014, 510627. https://doi.org/10.1155/2014/510627 (2014).
Zhang, J., Wen, C., Duan, Y., Zhang, H. & Ma, H. Advance in Cordyceps militaris (Linn) Link polysaccharides: Isolation, structure, and bioactivities: A review. Int. J. Biol. Macromol. 132, 906–914. https://doi.org/10.1016/j.ijbiomac.2019.04.020 (2019).
Jung, S. J. et al. Immunomodulatory effects of a mycelium extract of Cordyceps (Paecilomyces hepiali; CBG-CS-2): a randomized and double-blind clinical trial. BMC Complement. Altern. Med. 19, 77. https://doi.org/10.1186/s12906-019-2483-y (2019).
Yang, J. et al. Cordyceps cicadae polysaccharides ameliorated renal interstitial fibrosis in diabetic nephropathy rats by repressing inflammation and modulating gut microbiota dysbiosis. Int. J. Biol. Macromol. 163, 442–456. https://doi.org/10.1016/j.ijbiomac.2020.06.153 (2020).
Zhang, D. et al. The protective effect of polysaccharide SAFP from Sarcodon aspratus on water immersion and restraint stress-induced gastric ulcer and modulatory effects on gut microbiota dysbiosis. Foods (Basel, Switzerland) https://doi.org/10.3390/foods11111567 (2022).
Chen, J. et al. Sarcodon aspratus polysaccharides ameliorated obesity-induced metabolic disorders and modulated gut microbiota dysbiosis in mice fed a high-fat diet. Food Funct. 11, 2588–2602. https://doi.org/10.1039/c9fo00963a (2020).
Si, Y. C. et al. Integrative analysis of the gut microbiota and metabolome in obese mice with electroacupuncture by 16S rRNA gene sequencing and HPLC-MS-based metabolic profiling. Am. J. Chin. Med. 50, 673–690. https://doi.org/10.1142/s0192415x22500276 (2022).
Chen, X. et al. Characterization of the gut microbiota in Chinese children with overweight and obesity using 16S rRNA gene sequencing. PeerJ 9, e11439. https://doi.org/10.7717/peerj.11439 (2021).
Rangel-Huerta, O. D., Pastor-Villaescusa, B. & Gil, A. Are we close to defining a metabolomic signature of human obesity? A systematic review of metabolomics studies. Metab. Off. J. Metab. Soc. 15, 93. https://doi.org/10.1007/s11306-019-1553-y (2019).
Verhaar, B. J. H., Prodan, A., Nieuwdorp, M. & Muller, M. Gut microbiota in hypertension and atherosclerosis: A review. Nutrients. 12, 2982. https://doi.org/10.3390/nu12102982 (2020).
Hersoug, L. G., Møller, P. & Loft, S. Role of microbiota-derived lipopolysaccharide in adipose tissue inflammation, adipocyte size and pyroptosis during obesity. Nutr. Res. Rev. 31, 153–163. https://doi.org/10.1017/s0954422417000269 (2018).
Wu, G. D. et al. Cordyceps improves obesity and its related inflammation via modulation of enterococcus cecorum abundance and bile acid metabolism. Am. J. Chin. Med. 50, 817–838. https://doi.org/10.1142/s0192415x22500343 (2022).
Lee, B. H. et al. Polysaccharides obtained from Cordyceps militaris alleviate hyperglycemia by regulating gut microbiota in mice fed a high-fat/sucrose diet. Foods (Basel, Switzerland) https://doi.org/10.3390/foods10081870 (2021).
Huang, R. et al. Polysaccharides from Cordyceps militaris prevent obesity in association with modulating gut microbiota and metabolites in high-fat diet-fed mice. Food Res. Int. (Ottawa, Ont.) 157, 111197. https://doi.org/10.1016/j.foodres.2022.111197 (2022).
Yu, M. Anti-hyperlipidemia and gut microbiota community regulation effects of selenium-rich Cordyceps militaris polysaccharides on the high-fat diet-fed mice model, Foods (Basel, Switzerland), 10, 2252, https://doi.org/10.3390/foods10102252 (2021).
An, Y. et al. Cordycepin reduces weight through regulating gut microbiota in high-fat diet-induced obese rats. Lipids Health Dis. 17, 276. https://doi.org/10.1186/s12944-018-0910-6 (2018).
Tran, N. K. S. et al. Fermented Cordyceps militaris extract prevents hepatosteatosis and adipocyte hypertrophy in high fat diet-fed mice. Nutrients.10, 1015. https://doi.org/10.3390/nu11051015 (2019).
Yu, W. Q. et al. Polysaccharide CM1 from Cordyceps militaris hinders adipocyte differentiation and alleviates hyperlipidemia in LDLR((+/-)) hamsters. Lipids Health Dis. 20, 178. https://doi.org/10.1186/s12944-021-01606-6 (2021).
Yin, F. et al. The Cordyceps militaris-derived polysaccharide CM1 alleviates atherosclerosis in LDLR((-/-)) mice by improving hyperlipidemia. Front. Mol. Biosci. 8, 783807. https://doi.org/10.3389/fmolb.2021.783807 (2021).
Staub, C. & Johnson, L. Review: spermatogenesis in the bull. Anim. Int. J. Anim. Biosci. 12, s27–s35. https://doi.org/10.1017/s1751731118000435 (2018).
Lin, W. H. et al. Improvement of sperm production in subfertile boars by Cordyceps militaris supplement. Am. J. Chin. Med. 35, 631–641. https://doi.org/10.1142/s0192415x07005120 (2007).
Chang, Y. et al. Effect of Cordyceps militaris supplementation on sperm production, sperm motility and hormones in Sprague-Dawley rats. Am. J. Chin. Med. 36, 849–859. https://doi.org/10.1142/s0192415x08006296 (2008).
Dutta, S., Sengupta, P., Slama, P. & Roychoudhury, S. Oxidative stress, testicular inflammatory pathways, and male reproduction. Int. J. Mol. Sci. 22, 10043. https://doi.org/10.3390/ijms221810043 (2021).
He, M. T., Lee, A. Y., Park, C. H. & Cho, E. J. Protective effect of Cordyceps militaris against hydrogen peroxide-induced oxidative stress in vitro. Nurs. Res. Pract. 13, 279–285. https://doi.org/10.4162/nrp.2019.13.4.279 (2019).
Huang, T. et al. Cordycepin, a major bioactive component of Cordyceps militaris, ameliorates diabetes-induced testicular damage through the Sirt1/Foxo3a pathway. Andrologia 54, e14294. https://doi.org/10.1111/and.14294 (2022).
Kopalli, S. R., Cha, K. M., Cho, J. Y., Kim, S. K. & Koppula, S. Cordycepin from medicinal fungi Cordyceps militaris mitigates inflammaging-associated testicular damage via regulating NF-κB/MAPKs signaling in naturally aged rats. Mycobiology 50, 89–98. https://doi.org/10.1080/12298093.2022.2035515 (2022).
Yu, Y. et al. Isolation and immune activity of a new acidic Cordyceps militaris exopolysaccharide. Int. J. Biol. Macromol. 194, 706–714. https://doi.org/10.1016/j.ijbiomac.2021.11.115 (2022).
Cooke, P. S., Nanjappa, M. K., Ko, C., Prins, G. S. & Hess, R. A. Estrogens in male physiology. Physiol. Rev. 97, 995–1043. https://doi.org/10.1152/physrev.00018.2016 (2017).
Lombardo, F. et al. Androgens and fertility. J. Endocrinol. Invest. 28, 51–55 (2005).
Hong, I.-P. et al. Effect of Cordyceps militaris on testosterone production in Sprague-Dawley rats. Int. J. Ind. Entomol. 23, 143–146. https://doi.org/10.7852/ijie.2011.23.1.143 (2011).
Hsu, C. C., Huang, Y. L., Tsai, S. J., Sheu, C. C. & Huang, B. M. In vivo and in vitro stimulatory effects of Cordyceps sinensis on testosterone production in mouse Leydig cells. Life Sci. 73, 2127–2136. https://doi.org/10.1016/s0024-3205(03)00595-2 (2003).
Jang, D. et al. System-level investigation of anti-obesity effects and the potential pathways of Cordyceps militaris in ovariectomized rats. BMC Complement. Med. Ther. 22, 132. https://doi.org/10.1186/s12906-022-03608-y (2022).
Dubois, M., Gilles, K. A., Hamilton, J. K., Rebers, P. T. & Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 28, 350–356 (1956).
Iamtham, S., Kaewkam, A., Chanprame, S. & Pan-utai, W. Effect of spirulina biomass residue on yield and cordycepin and adenosine production of Cordyceps militaris culture. Bioresour. Technol. Rep. 17, 100893. https://doi.org/10.1016/j.biteb.2021.100893 (2022).
De Percie Sert, N. et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 18, e3000410. https://doi.org/10.1371/journal.pbio.3000410 (2020).
Acknowledgements
Authors thank the Dalong Biotechnology Co., Ltd. (Taichung, Taiwan) for kindly providing different fermentation products of C. militaris for this study. We would like to thank Ms. Cheryl Robbins for manuscript editing and Editage (www.editage.cn) for English language editing.
Funding
The funding was provided by The Ministry of Science and Technology of Taiwan (Grant No. MOST 107-2622-E-212-008-CC20, MOST 110-2320-B-040-008), National Chung Hsing University and Chung Shan Medical University inter-school project (Grant No. NCHU-CSMU 10802), The Key Projects of Natural Science Foundation of Zhejiang Province (Grant No. LXZ22H300001), Basic Scientific Research Project of Hangzhou Medical College, (Grant No. KYZD202005).
Author information
Authors and Affiliations
Contributions
Conceptualization, T.-H.H. and M.-S.T.; data curation, D.-Z.J. and S.L.; formal analysis, S.-H.W. and M.-S.T.; investigation, S.L, W.-K.H. and T.-C.L.; methodology, S.L, S.-H.W. and W.-K.H.; re-sources, T.-H.H. and D.-Z.J.; supervision, D.-Z.J. and T.-C.L.; validation, M.-S.T. and H.-L.S.; visualization, S.L. and M.-S.T.; writing—original draft, S.L.; writing—review and editing, D.-Z.J., S.-H.W. and M.-S.T. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lin, S., Hsu, WK., Tsai, MS. et al. Effects of Cordyceps militaris fermentation products on reproductive development in juvenile male mice. Sci Rep 12, 13720 (2022). https://doi.org/10.1038/s41598-022-18066-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-18066-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.