Abstract
Maximizing food production through integrated management of vegetative and root growth is a major challenge to food security and sustainability in the face of population growth, salinity stress conditions and climatic changes specially in arid and semi-arid regions. This study was conducted to evaluate the effect of foliar application with proline (Pro) at 5 mM, calcium (Ca) at 1.5% or control supplemented with soil application of humic acid (Hc) at 0, 15 g/tree on the nutrition status, osmoregulatory mechanisms and productivity of ‘Wonderful’ pomegranate trees growing under salt stress conditions. Soil and foliar treatments were applied three times: at flowering stage (April), 2 months after fruit set (June) and at fruit maturity (August). Individual application of either Hc or Pro or Ca alleviated the adverse effects of salt stress. Moreover, supplemented soil application of Hc with Pro or Ca as foliar application increased significantly leaf Pro, total carbohydrates, N, P, Ca and K contents, as well as K/Na and Ca/Na ratio. While it significantly decreased leaf Na and Cl concentration. Furthermore, supplemented application of Hc resulted in the highest decrease in leaf Na and Cl concentrations by 94.59%, 44.79% when combined with Pro and by 51.35%, 31.28%, when combined with Ca. In addition, Hc treatment led to the highest mean fruit yield by 139.56% and 90.73%, respectively as mean of both seasons for Pro and Ca treatments, respectively. The results suggest that, exogenous Pro and Ca supplemented with Hc can mitigate salt stress in ‘Wonderful’ pomegranate through enhancing osmoprotectants accumulaton.
Similar content being viewed by others
Introduction
Pomegranate (Punica granatum L.) consider one of the oldest edible fruits, mentioned in ancient Egyptian mythology and in the Holy Quran. It is widely grown in arid and semi-arid regions all over the world1. Pomegranate is more tolerant of dry and semi-arid regions for many reasons, such as adapting to hot, dry summers1, drought tolerance2 and moderate salt tolerance3,4. Consequently, pomegranate production has expanded worldwide, as the quality of available water decreases, such as salt water or recycled water1. In addition to the high nutritional value of pomegranate fruits, which have a wide range of medicinal applications5. ‘Wonderful’ is currently one of the most competitive pomegranate varieties grown in Egypt as it provides the optimum balance between productivity and quality6.
In arid and sem-arid regions, salinity as abiotic stress considers one of the most important environmental factors restricting agricultural productivity. Approximately, twenty percent of the irrigated land and two percent of the dry land agriculture have been affected by salinity worldwide7. Salinity resulted in reduced water use, plant metabolic processes changes, specific ions phytotoxicity (Na, Cl) and nutrition imbalance which reduces growth and productivity8,9.
Pomegranate is moderately salt tolerant species4. Cuttings of cv. ‘Malas Shirin’ pomegranate can tolerate up to 40 mM of NaCl in potted cultures3. Although, El-Khawaga et al.,10 reported that the pomegranate cvs. 'Wonderful’, ‘Manfalouty’ and ‘Nab-Elgamal’ pomegranates under a rate of 6.0 dS m–1 of groundwater had increased leaf Cl concentrations as well as reduced vegetative growth, flowering, and fruiting. On the other hand, pomegranate plants can limit Cl and Na accumulation in leaf tissues tolerating salinity up to EC of 15 dS m−14.
Humic acid (Hc) helps plants mitigate harmful effects of soil salinity by improving soil permeability, aeration, holding capacity, particle aggregation and micronutrient uptake11,12,13. Also, humic acid reduces the uptake of toxic elements8,13,14.
Proline (Pro) accumulation in plants occur naturally under various stress conditions8,15,16,17. In addition, proline is involved in cytoplasmic osmotic adjustment, stabilization of proteins and membranes, buffering cellular redox potential and scavenging reactive oxygen species15,18,19,20. Moreover, the combined application of 10 mM ascorbic acid + 50 mM proline + 100 mM glycin betaine mitigated the negative effects of climatic change stresses in ‘Wonderful’ pomegranate trees21. More recently, exogenous proline reduced the uptake and translocation of Na and Cl9,22.
Calcium ions (Ca) are important for regulating the selective transport of K+ versus Na+ and maintaining cell membrane integrity23,24,25. Sodium ions act by displacing calcium ions from membranes, resulting in increased membrane permeability and elevated intracellular sodium26,27. Ca has very low mobility inside plant cells28.
There are little investigations on the effects of salinity on nutritional status, osmoregulation mechanism and productivity of pomegranate trees4. Also, the individual effects of calcium or Hc on stress tolerance have received much attention, but little information is available on the effects of the applications of Pro and Ca in combination with Hc on the yield and nutritional status of pomegranate trees. This study was conducted to evaluate the integrative effect of Pro or Ca as foliar application in addition to Hc as soil application on the yield and nutritional status of ‘Wonderful’ pomegranate trees under salt stress conditions.
Material and methods
Plant materials
The authors identify that the institutional and/or licensing committee that approved the experiments, including any relevant details, confirm that all experiments were performed in accordance with relevant named guidelines and regulations. This field study was conducted during 2019 and 2020 seasons along with 2021 season for laboratory analysis on 10-year-old pomegranate trees (Punica granatum L. cv. Wonderful) at the experimental station of Cairo University located in Wadi El Natrun, El Behera Governorate, Egypt (30° 41′ 42″ N and 30° 23′ 16″ E, altitude 9 m). Trees were planted 2.5 × 4 m apart in a sandy soil and were irrigated with saline water (Table 1).
Treatments and procedures
Fifty-four trees were subjected to the common horticultural practices and sprayed with either chelated calcium citrate at 1.5%, proline at 0.5 g/L or water as control for foliar treatments using Tween 80 (0.5% v/v) as surfactant. Treated trees in every treatment divided into two groups; first group was treated with 15 g humic acid (Bio Green, Greensboro, GA, USA) as a soil application, and the other trees were not treated with humic acid. All foliar and soil treatments were applied during three critical stages; at flowering stage (April), 2 months after fruit set (June) and at fruit maturity (August). Each treatment consisted of nine trees distributed in three replicates, each one including 3 trees.
Measurements and procedures leaf proline content (µmoles g−1)
The proline content was determined by the following procedure according to Bates et al.29. Leaf samples from each replicate were homogenized in 3% sulphosalicylic acid. After well shake, the samples were treated in a test tube with a mixture of glacial acetic acid and ninhydrin. Then the mixture was heated at 98 °C for 60 min in a water bath and then rapidly cooled at room temperature using crushed ice. Toluene was used to extract proline from the mixture and absorbance was read at 520 nm using spectrophotometer (6300 Visible spectrophotometer, Jenway, Cole-Parmer Ltd., United Kingdom).
Leaf total carbohydrates
Total carbohydrates determination was carried out in the second season and was performed according to Herbert et al.30 as follows; a sample of dry tissue (0.2 g) was added in 10 ml H2SO4 (1 N). Then it was placed in a tube overnight in the oven at 100 °C. The colorimetric method used to determine total sugars31 was as follows: 1 ml of the sugar solution was mixed with 1 ml of 5% phenol followed by 5.0 ml of concentrated H2SO4 (98%). After shaking the tubes well, they were placed in a water bath at a temperature of 23–30 °C for 20 min. Then optical density of the color was measured at 490 nm using spectrophotometer.
Determination of leaf mineral concentrations
Leaf samples were collected in the second season at August from the middle part of the shoots and they were dried at 70 °C for 72 h. Chloride determination was performed according to Mastrogiannidou et al.32 after extracting Cl with distilled water from dried tissues of leaves and titrated with silver nitrate solution (0.02 N). For Na, Ca, and K analyses, 0.2 g of the dried sample was digested into a mixture of H2SO4 and H2O233. The concentrations of Na, Ca, and K were determined using a flame photometer (PFP7, Jenway, Cole-Parmer Ltd United Kingdom) according to Temminghoff and Houba33. Nitrogen concentration was determined using the modified Micro-Kjeldahl method34. Phosphorus concentration was determined sepectrophotometerically by using stannous chloride method35. All mineral concentrations are expressed as percent on a dry weight basis.
Yield and fruit characteristics
The fruits were harvested at the maturity stage during the end of August (180 days after flowering). The fruit yield was calculated by multiplying the weight of the fruits by the number of fruits per tree. Fruit characteristics were determined using nine fruits per each replicate. Fruit weight was determined using a sensitive scale, fruit firmness was measured on the two opposite sides of each fruit (using penetrometer with an 8 mm diameter probe, FT 327). The fruit pulp and peel were weighed separately for the same fruit and then their ratio were calculated. A digital hand refractometer (PR32, ATago, CO. Ltd., Japan) was used to determine the total soluble solids (TSS) in the extracted juice and was expressed as ◦Brix.
Experiment design and statistical analysis
The experiment design was a split plot with soil application in the main plot and the foliar application in the sub plot. The treatments were arranged in a randomized complete block design with two factors (2 soil treatments X 3 foliar treatments) and subjected to variance analysis36. The means of the treatments were compared by least significant difference at 0.05 significance level.
Results and discussion
Proline and carbohydrates as organic osmolytes for osmoregulation
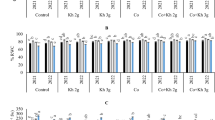
Generally Hc treatment failed to increase proline leaves content compared with other conducted treatments (Fig. 1a). Treatments of Ca alone or with Hc increased leaves proline content by 85.7%, 92.85%, respectively compared to the control. For effects of foliar and soil application of different salt stress ameliorative materials (Fig. 1b), it can be observed that, Hc applied by soil succeeded in increasing carbohydrates percent by 58% compared to the control. Moreover, treatment of Pro alone or with Hc significantly increased the proline content of leaves by 135.71% and 142.85%, respectively compared to the control. Treatments of calcium alone or with Hc increased leaves proline content by 85.71% and 92.85% compared to the control.
Effect of proline (Pro) and calcium (Ca) as foliar application alone or incombination with humic acid (Hc) as soil application on carbohydrates leaves percent (a) and proline (b) leaves content (µmoles g−1) of ‘Wonderful’ pomegranate trees grown under salt stress conditions. The different letters on the bars in each column represent significant difference at p ≤ 0.05; standard error bars represent standerd error of three replicates. Pro: 5 mM proline alone as foliar application; Ca: 1.5% calcium as foliar application alone; Hc: 15 g humic acid as soil application alone; Pro + Hc: 5 mM porline and 15 g humic acid; Ca + Hc: 1.5% calcium and 15 g humic; Ctrl: control (non treated).
Nutritional status
Individual application of Hc succeeded in increasing the plant N, P, K, Ca concentrations significantly by 17.6%, 23.8%, 0.85%, 16.34%, respectively compared to the untreated control (Fig. 2a–d). Moreover, integrated application of Hc with Pro significantly increased leaves N, P, K and Ca by 72.26, 57.14%, 9.78% and 22.17%, respectively. In addition, co-application of humic acid with Ca significantly increased leaves N, P, K and Ca by 74.78%, 47.61%, 5.53% and 28%,and respectively.
Effect of Proline (Pro) and Calcium (Ca) as foliar application alone or incombination with humic acid (Hc) as soil application on (a) nitrogen (N), (b) phosphorous (P), (c) potassium (K) and (d) calcium (Ca) leaves percent based on leaves dry weight of ‘Wonderful’ pomegranate trees grown under salt stress conditions. The different letters on the bars in each column represent significant difference at p ≤ 0.05; errors bars are standerd error of three replicates. Pro: 5 mM proline alone as foliar application; Ca: 1.5% calcium as foliar application alone; Hc: 15 g humic acid as soil application alone; Pro + Hc: 5 mM porline and 15 g humic acid; Ca + Hc: 1.5% calcium and 15 g humic; Ctrl: control (non treated).
Toxic elements and inorganic osmolytes for osmoregulation
Individual application of Hc or Pro or Ca significantly reduced the concentrations of the toxic elements; Na and Cl (Fig. 3a,b) compared to the control. The percent decrease in leaf Na was 94.59%, 64.86%, 51.35%, 40.54% and 29.72% for Pro+Hc, Pro alone, Ca+ Hc, Ca alone and Hc alone, respectively. Also, the decrease in Cl% recorded by 44.79%, 31.28%, 17.76%, 17.61% and 11.53% for Pro+Hc, Ca+Hc, Pro alone, Ca alone and Hc alone, respectively compared to the control. For the inorganic osmoregulation mechanisms (Fig. 3c,d). Hc acid alone failed to achieve a significant increase the concentration of osmoregulated solutes (Ca/Na ratio and K/Na ratio). While the integrated application of Hc plus Pro followed by Ca gave the highest values with significant values.
Effect of Proline (Pro) and Calcium (Ca) as foliar application alone or incombination with humic acid (Hc) as soil application on (a) leaves sodium (Na), (b) chloride (Cl), (c) calcium/sodium ratio (Ca/Na), (d) potassium/sodium ratio (K/Na) based on leaves dry weight of ‘Wonderful’ pomegranate trees grown under salt stress conditions. The different letters on the bars in each column represent significant difference at p ≤ 0.05; errors bars are standerd error of three replicates. Pro: 5 mM proline alone as foliar application; Ca: 1.5% calcium as foliar application alone; Hc: 15 g humic acid as soil application alone; Pro + Hc: 5 mM porline and 15 g humic acid; Ca + Hc: 1.5% calcium and 15 g humic; Ctrl: control (non treated).
Regarding the effect of humic acid on plant nutritional status, our results indicated that, humic acid led to a significant increase in leaf N, P and K concentrations, while it led to a significant decrease in leaf Cl and Na concentration (Fig. 3). In this regard, humic acid has been associated with the preferential accumulation of these nutrients for several fruit trees such as Egyptian lime37, olives38, date palm39, grape8 and Water melon14. The beneficial effect of humic acid in improving the nutritional status of leaves under salt stress conditions may be due to their effect on increasing soil organic matter which subsequently increase soil holding capacity11, improve the exchange capacity, enhance mineral chelation12, increase available mineral, improve root growth11 and maintain nutrients from leaching out37. Most beneficial effect of humic acid under salt stress condition is the uptake limitation of toxic such as Na and Cl. These findings were previously reported in many fruit trees like Mexican lime12 and grape8. The results indicated a non significant effect of humic acid on leaf proline content, the application of humic acid decreased proline concentration in citrus16.
These results indicate that pomegranate plants have a strong capability to restrict Na and Cl accumulation in leaf tissues. In this regard, Sun et al.4 concluded that pomegranate plant is very tolerant to saline water irrigation up to an EC of 15 dS m−1 with little foliar salt damage and a slight growth reduction.
As for the effects of foliar Pro treatments under salt stress conditions, our results indicate that exogenous Pro significantly increased leaf K, Ca, proline contents, while it significantly decreased leaf Na and Cl concentrations. Most studies have been tested foliar spraying of proline on field crops and vegetable crops such as common beans9,40 mustard41, barley42, Aloe vera43, rice44, onion20 and pea plant45. Lima-Costa46 found that, exogenous proline at 5 mM improved vegetative growth of citrus plants under 100 mM NaCl. Proline application for Simmondsia chinensis at 20 mM significantly increased N, K and significantly decreased Cl and Na under salt stress conditions47. The results showed the great effect of Pro on improving plant nutritional status. Also, the integrative spraying of 6 mM Proline in addition to potassium silicate increased leaf N, P, K concentrations9 and decreased leaf Na concentration of common bean under NaCl at 150 mM9,22. Moreover, application of 10 mM proline increased leaf N, P, K and yield of sugar beet under drought stress48. In addition, inculated barley plants with two strains produced proline and IAA, enhancing plant water and nutrient uptake42. Exogenously application of proline of drought stressed onion improved photosynthetic efficiency, up-regulating osmoprotectants and water use efficiency20.
The results indicated that Ca led to a significant increase in leaf Ca, K, proline contents as well as significantly decreased leaf Cl and Na concentrations. These results were agreed with Ahmad et al.49 who treated tomato plants with Ca after salt exposure that reduced Na uptake. Also, Jasim et al.50 treated Berhi date palm with Ca which led to a significant increase in K+ and K/Na ratio in leaves, whereas Cl concentration was decreased. Moreover, Jasim et al.50 found that, Ca significantly increased leaf K+ and K+/Na+ ratios in date leaves, whereas Cl− concentration were decreased. Ca is nesseccary for uptake K+ versus Na+23,24,25. Ca regulate some stress adaption mechanism such as cell polarity51, stomatal closure52, membrane stability and prevent the leakage of the solute from plant cell cytoplasm53. Ca plays an important role in cell elongation, cell division, membrane permeability, nitrogen metabolism and carbohydrate translocation54. In general, Ca is mostly applied to field crops and vegetable crops such as Festuca ovina55, endives56, tomato49, indica rice57, Legume58, wheat59 and pepper60. In ‘Elstar’ apple, leaf Ca concentration increased after Ca spraying at 6 to 9 kg ha−161. On the other hand, Hagagg et al.62 found there was no clear effect of Ca treatments (3%, 5% and 7% CaCo3) on the mineral content of ‘Kalamata’ and ‘Manzanillo’ olive trees.
Under salt stress conditions, management of both root system (Hc treatment) and vegetative system (Ca, Pro treatments) increased K/Na ratio, Ca/Na ratio, carbohydrates and nutrients as well as decreased toxic elements (Na, Cl) suggests the mechanism of using pomegranate K, Ca and proline as osmoprotectants. Moreover, Pro treatments have achieved the most promising effects to alleviate salt stress may be come from providing plant energy, carbohydrates and nutrients from producing more endogenous proline content and becoming more nutritional balanced with lower toxic nutrients. Thus providing nitrogen to various roots, leaves and fruits building plant tissues. Furthermore, professional treatments have achieved the most promising effects of salt stress relief that come from providing plant energy to produce more endogenous proline content and become more balanced with lower toxic nutrients.
Yield
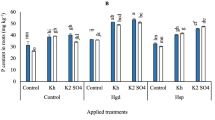
Single use of Hc resulted in a significant increase in fruit number, weight and yield compared to the untreated control (Fig. 4a,b,c). The percentage increase in fruit number due to Hc application was 45% and 21%, for fruit weight was 4.6% and 7.2% whereas for fruit yield were 29.39 and 51.84% for the first and second seasons, respectively. Also, the integrated application of Hc plus Pro increased fruit number by 121%, 87.93%, fruit weight by 15.2%, 18.7%, fruit yield by 162.6%, 116.4% for the first and second seasons, respectively. Furthermore, integrated application of Hc plus Ca increased fruit number by 54.96% and 77.77%, fruit weight by 14.98%, 14.4%, fruit yield by 77.17% and 104.28%, for the first and second season, respectively.
Effect of Proline (Pro) and Calcium (Ca) as foliar application alone or incombination with humic acid (Hc) as soil application on (a) fruit number, (b) fruit weight (g) and (c) yield (kg/tree) of ‘Wonderful’ pomegranate trees grown under salt stress conditions. The different letters on the bars in each column represent significant difference at p ≤ 0.05; errors bars are standerd error of three replicates. Pro: 5 mM proline alone as foliar application; Ca: 1.5% calcium as foliar application alone; Hc: 15 g humic acid as soil application alone; Pro + Hc: 5 mM porline and 15 g humic acid; Ca + Hc: 1.5% calcium and 15 g humic; Ctrl: control (non treated).
Fruit quality
The combined use of Hc and Ca significantly increased fruit pulp/peel ratio followed by the application of Hc plus Pro (Fig. 4a,b,c). Both Hc acid or Pro alone failed to increase fruit TSS concentration, while during the first season the integrated application of Hc plus Ca increased fruit TSS significantly by 12.38% compared to the control. The integrated application of Hc plus Ca gave the highest fruit firmness in the first season with a significant value by 18.99% compared to the control.
Pro, Ca and Hc treatments increased nutrionnal status (N, P, K, Ca) as well as increasing carbohydates and proline as osmoprotectants mechanism which increased the supply of these growth-stimulated nutrients to different plant organ resulting in increased fruit yield.
With regard to fruit yield, the results indicated that humic acid has a great effect on increasing ‘Wonderful’ fruit weight and number subsequently fruit yield besides the pulp/peel ratio (Fig. 5). These results of the application of humic acid on increasing fruit yield were previously observed in many plants such as Egyptian lime trees37 and mango63. Recently, Masoud et al.64 found that, foliar application of ‘manfalouty’ pomegranate trees with 1% humic acid during the fruit growth improved fruit yield and quality. Increasing ‘Wonderful’ pomegranate yield under salt stress condition may be due to enhanced soil and plant mineral availablility and content. The main components of soil organic matter are humic substances (65–70%), which improve plant growth due to increased cell membrane permeability, phosphorus and oxygen uptake, physiological processes (photosynthesis, respiration) and root cell growth65. Finally, humic acid has direct effects on plant growth and nutritional status beside their great indirect effects on the soil66.
Effect of Proline (Pro) and Calcium (Ca) as foliar application alone or incombination with humic acid (Hc) as soil application on (a) fruit pulp/peel ratio, (b) fruit TSS percent, (c) fruit firmness (kg/cm3) (kg/tree) of ‘Wonderful’ pomegranate trees grown under salt stress conditions. The different letters on the bars in each column represent significant difference at p ≤ 0.05; errors bars are standerd error of three replicates. Pro: 5 mM proline alone as foliar application; Ca: 1.5% Calcium as foliar application alone; Hc: 15 g humic acid as soil application alone; Pro + Hc: 5 mM porline and 15 g humic acid; Ca + Hc: 1.5% calcium and 15 g humic; Ctrl: control (non treated).
The results showed a role of Pro in improving yield and fruit quality. These results were in agreement with El Sayed et al.67 as they found that spraying ‘Manfalouty’ proline at 100 ppm improved fruit weight, TSS and yield. Also, application of 10 mM proline increased sugar beet yield under drought stress48. Increasing fruit number via proline application has been mentioned by Mattioli et al.68 as they reported that under salinity stress proline accumulated in pollen grains protected pollen fertility and improved yield stability.
The results showed that, Ca treatment significantly increased fruit weight, fruit number and pulp/peel ratio. These reults are in harmony with Masoud et al.64 who mentioned that, spraying ‘Manfalouty’ pomegranate trees with 2% calcium chloride significantly increased fruit yield. Increasing leaf Ca content of ‘Elstar’ apple after Ca spraying enhanced yield at 6–9 kg ha−161. Calcium has been shown to mitigate the harmful effects of salinity on various plant species49,55,56,57,58,59,60.
The presence of Ca ions alleviated the toxic effects of salinity by promoting tissue growth. These effects may be resulting from the role of Ca in plant cell elongation and division, permeability of cell membrane, nitrogen metabolism and carbohydrate transport54. In fruit trees, spraying ‘Samany’ and ‘Zaghloul’ date palm with 5% calcium carbonate three times increased bunch weight and consequently total yield, fruit weight, thickness and TSS content69. Similar results were found in pomegranate64, olive62 and date palm69.
Conclusion
Individual application of humic acid (15 g/tree) as soil application or proline (5 mM) and Ca (1.5%) as foliar application three times a season was successful in alleviating the adverse effect of salt stress in ‘Wonderful’ pomegranate trees. The integrated applications of humic acid in addition to foliar application of proline or calcium counteract the negative effect of salinity via increasing organic and inorganic organic osmolytes, improving plant nutritional status and reducing the uptake of toxic elements which resulted in increasing fruit yield.
Data availability
The data generated and/or analysed during the current study are available per request to the corresponding author.
References
Holland, D., Hatib, K. & Bar-Ya’akov, I. Pomegranate: Botany, horticulture and breeding. In Horticultural Reviews Vol. 35 (ed. Janick, J.) 127–191 (Wiley, 2009).
Fayek, M. A., Mohamed, A. E. & Rashedy, A. A. Responses of five pomegranate (Punica granatum L.) cultivars to contrasting water availability: Leaf morphophysiological and anatomical adaptation. Appl. Ecol. Environ. Res. 20, 967–978 (2022).
Naeini, M. R., Khoshgoftarmanesh, A. H., Lessani, H. & Fallahi, E. Effects of sodium chloride-induced salinity on mineral nutrients and soluble sugars in three commercial cultivars of pomegranate. J. Plant Nutr. 27(8), 1319–1326 (2005).
Sun, Y., Niu, G., Masabni, J. G. & Ganjegunte, G. Relative salt tolerance of 22 pomegranate (Punica granatum) cultivars. HortScience 53(10), 1513–1519 (2018).
Lansky, E. P. & Newman, R. A. Review: Punica granatum (pomegranate) and its potential for prevention and treatment of inflammation and cancer. J. Ethnopharmacol. 109(2), 177–206 (2007).
Khedr, E. H. Application of different coating treatments to enhance storability and fruit quality of pomegranate (Punica granatum L., cv. Wonderful) during prolonged storage. Rev. Braz. Fruitc. 44(2), 1–13 (2022).
FAO (Food and Agriculture organization). Extent and causes of salt-affected soils in participating countries. Global network on integrated soil management for sustainable use of salt-affected soils. FAO-AGL website. Available in https://xueshu.baidu.com/usercenter/paper/show?paperid=9e5044cfc974c52d785834bbd8438017 (2000).
Mehanna, H. T., Fayed, T. A. & Rashedy, A. A. Response of two grape rootstocks to some salt tolerance treatments under saline water conditions. J. Hortic. Sci. Ornam. Plants 2(2), 93–106 (2010).
Rady, M. M., Elrys, A. S., Abo El-Maati, M. F. & Desoky, E. M. Interplaying roles of silicon and proline effectively improve salt and cadmium stress tolerance in Phaseolus vulgaris plant. Plant Physiol. Biochem. 139, 558–568 (2019).
El-Khawaga, A. S., Zaeneldeen, E. M. A. & Yossef, M. A. Response of three pomegranate cultivars (Punica granatum L.) to salinity stress. Middle East J. Agric. Res. 1(1), 64–75 (2013).
Khaled, H. & Fawy, H. A. Effect of different levels of humic acids on the nutrient content, plant growth, and soil properties under conditions of salinity. Soil Water Res. 6(1), 21–29 (2011).
Jahromi, A. A. & Khankahdani, H. H. Effect of humic acid on some vegetative traits and ion concentrations of Mexican Lime (Citrus aurantifolia Swingle) seedlings under salt stress. Int. J. Hortic. Sci. Technol. 3(2), 255–264 (2016).
Hatami, E., Shokouhian, A. A., Ghanbari, A. R. & Naseri, L. A. Alleviating salt stress in almond rootstocks using of humic acid. Sci. Hortic. 237, 296–302 (2018).
Shalaby, O. A. E. & El-Messairy, M. M. Humic acid and boron treatment to mitigate salt stress on the melon plant. Acta Agric. Slov. 111(2), 349–356 (2018).
Kavi Kishor, P. B. et al. Regulation of proline biosynthesis, degradation, uptake and transport in higher plants: Its implications in plant growth and abiotic stress tolerance. Curr. Sci. 88(3), 424–438 (2005).
Mahmoudi, M. & Aryaee, P. Study the effects of fulvic acid on physiological traits of citrus unshu under salt stress. Int. J. Chem. Environ. Biol. Sci. 3(3), 198–200 (2015).
Kaya, C., AKram, N. A., Ashraf, M. & Sonmez, O. Exogenous application of humic acid mitigates salinity stress in maize (Zea mays L.) plants by improving some key physico-biochemical attributes. Curr. Sci. 46, 67–78 (2018).
Hayat, S. et al. Role of proline under changing environments. Plant Signal. Behav. 7(11), 1456–1466 (2012).
Meena, M. et al. Regulation of L-proline biosynthesis, signal transduction, transport, accumulation and its vital role in plants during variable environmental conditions. Heliyon 5, e02952 (2019).
Semida, W. M., Abdelkhalik, A., Rady, M. O. A., Marey, R. A. & Abd El-Mageed, T. A. Exogenously applied proline enhances growth and productivity of drought stressed onion by improving photosynthetic efficiency, water use efficiency and up-regulating osmoprotectants. Sci. Hortic. 272, 109580 (2020).
Abo-ogiala, A. Crop production of pomegranate cv. wonderful via foliar application of ascorbic acid, proline and glycinbetaine under environmental stresses. Int. J. Environ. 7(3), 95–103 (2018).
El Moukhtari, A., Cabassa-Hourton, C., Farissi, M. & Savoure, A. How does proline treatment promote salt stress tolerance during crop plant development?. Front. Plant Sci. 11, 1127 (2020).
Orlov, S. N., Aksentsev, S. L. & Kotelevtsev, S. V. Extracellular calcium is required for the maintenance of plasma membrane integrity in nucleated cells. Cell Calcium 38(1), 53–57 (2005).
Wu, G. Q. & Wang, S. M. Calcium regulates K+/Na+ homeostasis in rice (Oryza sativa L.) under saline conditions. Plant Soil Environ. 58(3), 121–127 (2012).
Cheng, X., Zhang, X., Yu, L. & Xu, H. Calcium signaling in membrane repair. Semin. Cell Dev. Biol. 45, 24–31 (2015).
Wang, P. et al. J. Na+/Ca2+ exchanger-like protein (AtNCL) involved in salt stress in Arabidopsis. J. Biol. Chem. 287, 44062–44070 (2012).
Li, P. H., Zhang, G. Y., Gonzales, N., Guo, Y. Q., Hu, H. H., Park, S. & Zhao, J. Ca2+-regulated and diurnal rhythm-regulated Na+/Ca2+ exchanger AtNCL affects flowering time and auxin signalling in Arabidopsis. Plant Cell Environ. 39, 377–392 (2016).
Paiva, E. A. S. Are calcium oxalate crystals a dynamic calcium store in plants?. New Phytol. 223, 1707–1711 (2019).
Bates, L. S., Waldren, R. P. & Teare, I. Rapid determination of free proline for water-stress studies. Plant Soil 39, 205–207 (1973).
Herbert, D., Phipps, P. J. & Strange, R. E. Chemical analysis of microbial cells. J. Microbiol. Methods 5, 209–344 (1971).
Smith, G. S., Johnston, C. M. & Cornforth, I. S. Comparison of nutrient solutions for growth of plants in sand culture. New Phytol. 94(4), 537–548 (1983).
Mastrogiannidou, E., Chatzissavvidis, C., Antonopoulou, C., Tsabardoukas, V., Giannakoula, A. & Therios, I. Response of pomegranate cv. wonderful plants to salinity. J. Soil Sci. Plant Nutr. 16(3), 621–636 (2016).
Temminghoff, E. E. J. M. & Houba, V. J. G. Plant Analysis Procedures. Second Edition Analysis 94–96 (Kluwer Academic Publishers, 2004). https://doi.org/10.1007/978-1-4020-2976-9.
Jones, J. B. Jr. Kjeldahl Method for Nitrogen Determination (Micro-Macro Publishing, 1991).
Association of Official Analytical Chemists—A. O. A. C. Official Methods of Analysis of the Association of the Analytical Chemists 17th edn, 2200 (AOAC International, 2000).
Snedecor, W. & Cochran, W. G. Statistical Methods 8th edn, 503 (Iowa State University Press, 1989).
Ennab, H. A. Effect of humic acid on growth and productivity of egyptian lime trees (Citrus aurantifolia swingle) under salt stress conditions. J. Agric. Res. (Kafr El-Shaikh Univ.) 42(4), 494–505 (2016).
Genaidy, E. A. E., Merwad, M. A. & Haggag, L. F. Effect of algae, humic acid and waste organic material in culture media on growth performance of “Picual” olive seedlings. Int. J. Chemtech Res. 8(11), 43–50 (2015).
Fekry, W. M. E., Rashad, M. A. & Alalaf, A. H. Attempts to improve the growth and fruiting of barhi date palms under salinity stress. Asian J. Plant Sci. 19, 146–151 (2020).
Abdelhamid, M. T., Rady, M. M., Osman, A. S. H. & Abdalla, M. A. Exogenous application of proline alleviates saltinduced oxidative stress in Phaseolus vulgaris L. plants. J. Hortic. Sci. Biotechnol. 88(4), 439–446 (2013).
Wani, A. S., Ahmad, A., Hayat, S. & Tahir, I. Epibrassinolide and proline alleviate the photosynthetic and yield inhibition under salt stress by acting on antioxidant system in mustard. Plant Physiol. Biochem. 135, 385–394 (2019).
Ben Mahmoud, O. M. et al. Auxin and proline producing rhizobacteria mitigate salt-induced growth inhibition of barley plants by enhancing water and nutrient status. S. Afr. J. Bot. 128, 209–217 (2020).
Nakhaie, A., Habibi, G. & Vaziri, A. Exogenous proline enhances salt tolerance in acclimated Aloe vera by modulating photosystem II efficiency and antioxidant defense. S. Afr. J. Bot. 147, 1–10 (2020).
Hasanuzzaman, M. et al. Exogenous proline and glycine betaine mediated upregulation of antioxidant defense and glyoxalase systems provides better protection against salt-induced oxidative stress in two rice (Oryza sativa L.) varieties. BioMed Res. Int. 2014, 1–17 (2014).
Shahid, M. A. et al. Exogenous proline and proline-enriched Lolium perenne leaf extract protects against phytotoxic effects of nickel and salinity in Pisum sativum by altering polyamine metabolism in leaves. Turk. J. Bot. 38, 914–926 (2014).
Lima-Costa, M.E., Ferreira, S., Duarte, A. & Ferreira, A. L. Alleviation of salt stress using exogenous proline on a citrus cell line. Acta Hortic. 868, 109–112 (2010).
Alotaibi, S., Ali, E., Darwesh, H., Ahmed, A. & Al-Thubaiti, E. Effect of proline on growth and nutrient uptake of Simmondsia chinensis (link) schneider under salinity stress. Pak. J. Biol. Sci. 22(9), 412–418 (2019).
AlKahtani, M. D. F. et al. Evaluation of silicon and proline application on the oxidative machinery in drought-stressed sugar beet. Antioxidants 10(3), 398 (2021).
Ahmad, P. et al. Exogenous application of calcium to 24-epibrassinosteroid pretreated tomato seedlings mitigates NaCl toxicity by modifying ascorbate–glutathione cycle and secondary metabolites. Sci. Rep. 8, 13515 (2018).
Jasim, A. M., Abbas, M. F. & Shareef, H. J. Calcium application mitigates salt stress in Date Palm (Phoenix dactylifera L.) offshoots cultivars of Berhi and Sayer. Acta Agric. Slov. 107(1), 103–112 (2016).
Zhou, L., Lan, W., Jiang, Y., Fang, W. & Luan, S. Calcium-dependent protein kinase interacts with and activates a calcium channel to regulate pollen tube growth. Mol. Plant 7(2), 369–376 (2014).
Zou, J. J. et al. Arabidopsis calcium-dependent protein kinse8 and catalase3 function in abscisic acid-mediated signaling and H2O2 homeostasis in stomatal guard cells under drought stress. Plant Cell 27(5), 1445–1460 (2015).
El-Beltagi, H. S. & Mohamed, H. I. Alleviation of cadmium toxicity in Pisum sativum L. seedlings by calcium chloride. Not. Bot. Horti. Agrobot. Cluj Napoca 41, 157–168 (2013).
White, P. J. Calcium channels in higher plants. Biochim. Biophys. Acta (BBA) Biomembr. 1465(1–2), 171–189 (2000).
Salahshoor, F. & Kazemi, F. Effect of calcium on reducing salt stress in seed germination and early growth stage of Festuca ovina. Plant Soil Environ. 62, 460–467 (2016).
Tzortzakis, N. G. Potassium and calcium enrichment alleviate salinity-induced stress in hydroponically grown endives. Sci. Rep. 8, 13515 (2010).
Cha-um, S., Singh, H. P., Samphumphuang, T. & Kirdmanee, C. Calcium-alleviated salt tolerance in indica rice (Oryza sativa L. spp. indica): Physiological and morphological changes. Aust. J. Crop Sci. 6(1), 176–182 (2012).
Murillo-Amador, B. et al. Influence of calcium silicate on growth, physiological parameters and mineral nutrition in two legume species under salt stress. J. Agron. Crop Sci. 193(6), 413–421 (2007).
Zaman, B., Niazi, B.H., Athar, M. & Ahmad, M. Response of wheat plants to sodium and calcium ion interaction under saline environment. Int. J. Environ. Sci. Technol. 2, 7–12 (2005).
Akladious, S. A. & Mohamed, H. I. Ameliorative effects of calcium nitrate and humic acid on the growth, yield component and biochemical attribute of pepper (Capsicum annuum) plants grown under salt stress. Sci. Hortic. 236, 244–250 (2018).
Wójcik, P., Filipczak, J. & Wójcik, M. Effects of prebloom sprays of tryptophan and zinc on calcium nutrition, yielding and fruit quality of ‘Elstar’ apple trees. Sci. Hortic. 246, 212–216 (2019).
Hagagg, L. F., Abd-Alhamid, N. & Maklad, M. F. Effect of kaolin and calcium carbonate on vegetative growth, leaf pigments and mineral content of kalamata and manzanillo olive trees. Middle East J. Agric. Res. 8(1), 298–310 (2019).
El-Hoseiny, H. M., Helaly, M. N., Elsheery, N. I. & Alam-Eldein, S. M. Quality of mango trees humic acid and boron to minimize the incidence of alternate bearing and improve the productivity and fruit quality of mango trees. HortScience 55, 1026–1037 (2020).
Masoud, A. A. B., Khodair, O. A. & Gouda, F. E. M. Effect of gibberellic acid, naphthalenacetic acid, calcium and zinc spraying on fruiting of manfalouty pomegranate trees. Assiut J. Agric. Sci. 50(2), 219–228 (2019).
Russo, R. O. & Berlyn, G. P. The use of organic biostimulants to help low input sustainable agriculture. J. Sustain. Agric. 1(2), 19–42 (1990).
Chen, Y. & Aviad, T. Effects of humic substances on plant growth. In Humic Substances in Soil and Crop Science: Selected Readings (ed. Maccarthy, P.) 161–186 (CSSA and ASA, 1990).
El Sayed, O. M., El Gammal, O. H. M. & Salama, A. S. M. Effect of proline and tryptophan amino acids on yield and fruit quality of Manfalouty pomegranate variety. Sci. Hortic. 69, 1–5 (2014).
Mattioli, R., Palombi, N., Funck, D. & Trovato, M. Proline accumulation in pollen grains as potential target for improved yield stability under salt stress. Front. Plant Sci. 11, 582877 (2020).
Badran, M. A. Benefits of calcium carbonate sprays on yield and fruit quality of samany and zaghloul date palm under new reclaimed soils. Assiut J. Agric. Sci. 46(5), 48–57 (2015).
Acknowledgements
The authors extend their thanks for the funding and facilities at Cairo University to conduct this study. Also, the authors thank a lot Dr. H.M. Abdel-Lattif for statistical advice.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by A.A.R., E.H.K. and M.H.A. The first draft of the manuscript was written by A.A.R. and E.H.K. and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rashedy, A.A., Abd-ElNafea, M.H. & Khedr, E.H. Co-application of proline or calcium and humic acid enhances productivity of salt stressed pomegranate by improving nutritional status and osmoregulation mechanisms. Sci Rep 12, 14285 (2022). https://doi.org/10.1038/s41598-022-17824-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-17824-6
This article is cited by
-
Protective role of manganese, proline and rice straw extract in wheat against drought driven oxidative stress
Acta Physiologiae Plantarum (2024)
-
Effects of Salinity and Proline On Growth and Physiological Characteristics of Three Olive Cultivars
Gesunde Pflanzen (2023)
-
Novel Sustainable Adsorbents Prepared by Banana/Pomegranate Peels via EDTA Grafting for Effective Removal of Cd(II), Co(II), Mn(II), and Ni(II) from Sewage System
Water, Air, & Soil Pollution (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.