Abstract
Detection of heavy transition metals is of importance protecting humans and the environment from their toxicity. Amongst them, copper(II) and cobalt(II) need more attention due to their wide applications in industry, in which exposures with excess amounts of them cause heathy concerns. Hence, fast and efficient distinguishing copper(II) and cobalt(II) could be worthy. While electrochemical approaches could determine these cations, expensive instrumentation and time-consuming analysis are significant drawbacks, leading to concentrations on spectroscopic and colorimetric pathways. Herein, graphene quantum dot was modified with Eriochrome Black T (EBT) to generate a novel sensor with the ability of sensing mentioned cations. This new nanocompound demonstrated new optical properties when complexed with cobalt(II) in natural pH, and copper(II) in acidic media. Both cations successfully were detected by the new nanosensor through revealing distinct spectroscopic signals. Moreover, cobalt(II) was distinguished by this sensor colorimetrical, appearing green solution. Linear ranges of cobalt(II) and copper(II) were obtained as 0.02–0.08 M and 0.015–0.2 M, with the limit of detections 0.014 and 0.018 M, respectively.
Similar content being viewed by others
Introduction
According to the classical definition, heavy metals are classified as elements with molecular weights ranging from 63.5 to 200.6, which most of them like Cu, Co, Pd, Hg, and Cd have potential to threat the environment and mankind1. Some of these elements such as Co and Cu are widely employing in the industry, which their toxicity could be significant concerns for the human2. Alarms and asthma, cardiac and thyroid damage, heart failure and heart disease, and elevated red blood cells are some disorders caused by exposure with an excess amounts of Co(II). Hereditary copper metabolism disorders and neurodegenerative ailments are the issues ascribed to Cu(II) pollutions3. These concerns persuade researchers to continue seeking for efficient sensors to swift detect of the trace amounts of toxic metals especially in industrial wastes as the primary source of the pollution4. Developing well-functioning, cost-effective, rapid, and recoverable sensors could utterly refute traditional detection methods like Atomic Absorption Spectroscopy (AAS), Induced Coupled Plasma, Ion Chromatography, and electrochemistry5. Optical sensors have created fantastic progress in the metal distinguishing, in which all the positives are accessible. A wide variety of synthetic materials have deserved optical metal determination by either spectroscopic or colorimetric approaches6. It was demonstrated that graphene-based sensors productively detect heavy metals due to the superior optical activity, arising from a large conjugated π-network7,8. CoS/reduced porous graphene oxide for colorimetric detection of Hg(II)9, CuS and NiS nanoparticles-decorated on porous-reduced graphene oxide for the colorimetric detection of Hg(II)10, and AuNPs@MoS2/reduced graphene oxide immunosensor of the electrochemical neuron-specific enolase (NSE)11 are some examples of an unaccountable number of reports on graphene sensors for the detection of organic and mineral compounds. Moreover, graphene quantum dot (GQD) applications are boasting due to its high electrical conductivity, chemical stability, and surface area12. A remarkable number of GQD composites were employed in the synthesis of sensors such as serine and histidine-functionalized GQD for detecting carbendazim13, gold-histidine functionalized GQD-graphene hybrid for sensing chlorpyrifos14, GQD/multi-walled carbon nanotubes for ascertaining Interleukin-615, gold nanoparticles functionalized sulfur-doped GQD and h-ZnS-CdS NC for determining Interleukin-616, and nitrogen and boron-doped GQD for distinguishing cardiac troponin I17.
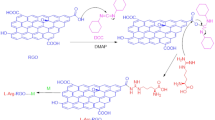
Eriochrome Black T (EBT) is an azo dye with an appropriate chemical structure to be employed as a complexometric indicator of Ca(II). While the indicator plays a crucial role in determining water hardness, pollution created by this azo dye causes severe issues for plants and animals. This concern has led to a vast number of reports on the EBT removal from water wastes, and encouraged scientists to come up with the idea of binding EBT on a support18. Surprisingly, EBT deposited on various supports demonstrated novel application in detecting various organic and inorganic materials. For example, poly(benzopyrene) films doped with EBT as a Pb2+-sensitive sensor19, poly(EBT)-modified carbon paste an electrode for Hg2+ distinguishing20, EBT/graphite composite for Theophylline determination21, and poly(EBT) on glassy carbon electrode for recognition of Isoniazid are some successful reports in this area22. Most of these detections were focused on electrochemical pathways, while we believe that EBT has significant optical potential on account of its extensive π-system. We recently demonstrated that the perching of Alizarine Red S on GQD generates a powerful sensor for detecting metal cations23. Easy, rapid, and cost-effective synthesis of GQD in combination with sustainability, and facile modification are some characteristics of this material that encouraged us to its modification with EBT24,25,26. Therefore, GQD was modified with EBT (GQD-EBT) to obtain a novel sensor of metal cations via esterification reaction (Scheme 1). This reaction efficiently was progressed by the N,N′-dicyclohexylcarbodiimide (DCC) and 4,4′-dimethylaminopyridine (DMAP) as the esterification agents27,28. The sensor successfully detected two significant metal cations, including Co2+ and Cu2+, which their fast detections are not well-stablished till now29,30,31.
Experimental section
Materials and methods
All reagents were purchased from Sigma-Aldrich and used without further purification. TEM, and SEM micrographs were obtained by FEI Tecnai G2 F20 SuperTwin TEM, and FESEM TESCAN MIRA3, respectively. FT-IR of AVATAR FTIR instrument of Thermo Company in the range of 600–4000 cm−1 was used to determine functional groups of compounds. XRD of XRD Philips PW1730 was employed with the Cu kα (λ = 1.540598 nm), a voltage of 140 kV, and scanning from 10° to 80°. UV–Vis spectra were obtained by WPA Biowave LifeScience UV–Vis spectrometer using quartz cuvettes with a path length of 10 mm in H2O as the solvent.
Synthesis of GQD-EBT
GQD (2.0 g), DCC (0.4 g), and DMAP (0.03 g) were added to a round bottom flask containing 9 ml of dimethyl sulfoxide, and 1 ml of H2O. The resulted mixture was stirred magnetically at 90 °C for 10 min. Then, EBT (0.5 g) was added to the reaction mixture, and stirring was continued for 2 h. Next, the precipitate was filtered off, and the residue was washed with H2O (3 × 10 ml) to give GQD-EBT as a dark solid after drying at 60 °C.
General procedure for detecting Mx+
To 5 ml of an adjusted Mx+ solution (pH = 3 with HCl and pH = 10 with NaOH) in the desired concentration, 0.05 g of GQD-EBT was added. Color changes were recorded as the addition of EBT-GQD. UV–Vis spectroscopies were carried out after calibration of the instrument with the standard solution of media.
Interfere study of cations on Co2+
GQD-EBT (0.05 g) was added to 10 ml of 0.02 M solution of Ca2+, Cu2+, Hg2+, Pb2+, Fe3+, Cd2+, Al3+, and Ni2+. Color changes were recorded as the addition of EBT-GQD. UV–Vis spectroscopy was carried out after calibration of the instrument with the standard media solution.
Results and discussion
Characterization of GQD-EBT
Organic transformations could be quickly approved by FT-IR spectroscopy, which highlights the addition, elimination, and transformation of functional groups with active vibrational modes. FT-IR spectra of GQD before and after modification indicated remarkable changes. Three prominent peaks of GQD appeared in both spectra, including stretching modes of OH, C=O, and C=C. The peak of C=O indicated a blueshift when GQD was modified with EBT due to the esterification reaction (Fig. 1)24.
Raman spectrum of GQD-EBT was also employed to characterize the structure with the ability to provide worthy information about the graphene-based structures. The spectrum of GQD-EBT demonstrated peaks of D-band at 1313 cm−1 attributed to the bending vibrations of C-H at the defects/edges and G-band at 1598 cm−1 ascribed to in-plane C=C vibrations, respectively. The high intensity of the D-band compared to G-band is a reliable sign for the presence of abundant functional groups on GQD (Fig. 2). Also, a strong peak appeared at 1313 cm−1 for EBT relating to C=C bonds32.
1H NMR spectroscopy was also employed to affirm the modification reaction, where H-aromatic of EBT in the GQD-EBT spectrum could confirm loading of the indicator on GQD. Spectrum of GQD-EBT indicated peaks for H-aromatic at about 7.0 and 8.2 ppm (Fig. 3), which did not observe in the GQD spectrum (Figure S1). These peaks clearly confirm the loading of EBT on GQD.
Elemental analysis was conducted by Energy Dispersive X-ray Spectroscopy (EDX) on GQD-EBT to determine various elements in the structure (Fig. 4). X-ray detector of SEM instrument revealed C and O atoms of GQD with high intensities. The analysis also indicated atoms ascribed to EBT, including S, Na, and N. To disclose the EBT distribution on GQD surface, elemental mapping was carried out, in which homogeneous distribution of S, N, and Na atoms observed (Fig. 3). This homogeneity in the elements’ dispersal implies success of the homogeneous sensor synthesis.
TEM micrographs of GQD were prepared before and after modification to see the structure and changes made during EBT loading on GQD (Fig. 5). TEM image of GQD showed the formation of nanoparticles in the range of 4–8 nm. The reaction time of citric acid transformation to graphene derivatives determines the dimensions of the synthesized particles with the fine particles in a shorter time33. TEM of GQD-EBT demonstrated that the thermal treatment of GQD during the modification reaction led to the nanoparticles growing, although they are still GQD with a size under 100 nm.
Detecting heavy metals by GQD-EBT
The optical behavior of GQD-EBT was studied by a spectrophotometer in the presence and absence of metal cations. GQD-EBT showed an absorption at 550 nm without any cation in a natural pH (Fig. 6). While the addition of GQD-EBT to most of the cations solutions (0.02 M) such as Ca2+, Cu2+, Hg2+, Pb2+, Fe3+, Cd2+, Al3+, and Ni2+ did not create impressive changes in the absorption wavelength in the natural pH, charging to Co2+ solution (0.02 M) led to the redshift of absorption to 514 nm (Fig. 6). Surprisingly, GQD-EBT could not spectroscopically or colorimetrical detect Ca2+ opposite to EBT. Intriguingly, the new sensor distinguished Co2+ colorimetrical by changing the brown GQD-EBT solution to green. The amount of GQD-EBT was optimized as 0.05 g, in which the best color detection of Co2+ was obtained at the lowest amounts of the sensor. A standard addition technique for Co2+ detection by GQD-EBT revealed a calibration curve of y = 1.80x + 0.12 with a linear range lying between 0.02–0.08 M. Limit of detection (LOD) was calculated as 0.014 M.
To elucidate the effect of solution pH on the detection ability of GQD-EBT, recognitions of the mentioned cations were carried out in two pHs of 10 and 3. As the first result, the detection of Co2+ was lost in both acidic and basic conditions. However, the result of this experiment was fascinating for Cu2+ in pH = 3, in which a new peak appeared at about 800 nm in addition to a peak of 550 nm (Fig. 7). A standard addition technique for Cu2+ detection by GQD-EBT demonstrated a calibration curve of y = 3.0653x + 0.1314 with a linear range lying between 0.015–0.2 M and an LOD of 0.018 M. The colorimetric distinguishing of Cu2+ was impossible due to the no detectable color change with the addition of the cation to the sensor solution.
Selectivity of the sensor was examined in a solution containing various cations at pH = 7, leading to the successful determination of Co2+ by spectroscopic and colorimetric methods. The peak of Co2+/GQD-EBT was observed in the spectrum of the solution which gained deep green color, indicating noninterference of other cations. Investigation of the selectivity at pH = 3 was also showed the possibility of the Cu2+ detection spectroscopically by observing the related peak at about 800 nm. Real samples were studied by GQD-EBT, with the various concentrations of Co2+ and Cu2+ in the tape and river water, in which both cations were successfully distinguished by the sensor after adjusting pH. Table 1 describes the results of detecting various concentrations of Co2+ and Cu2+.
A remarkable number of sensors has been reported for the Co2+ and Cu2+ distinguishing, in which some of them indicated superior sensitivity and selectivity than GQD-EBT23,30,31. However, introducing new approach to the functionalizing GQD with an indicator such as EBT could be a promising pathway to afford a lot of new sensors via this protocol. Easy synthesis of GQD from inexpensive citric acid, high optical activity of GQD, facile GQD modification through carboxylic acid groups, useful changing in the GQD’s optical properties due to modification with an indicator are some of significant features highlighted in this study. Therefore, synthesis of an economical sensor for the fast detection of Co2+ and Cu2+ are most valuable positives of this pathway compared to the most of reported sensors.
Conclusion
In summary, a novel sensor was synthesized from GQD and EBT by the facile and efficient pathway of esterification reaction. GQD modified EBT demonstrated different performance in the detection of cations compared to EBT, such as inability in Ca2+ detection. The new sensor successfully detected Co2+, and Cu2+ in natural, and acidic solutions, respectively. Moreover, colorimetric detection of Co2+ was also affordable with a green color induced by the sensor. This study offers that modifying GQD with optically active compounds could generate new nanomaterials with characteristic optical activities. Therefore, a considerable number of researches could be conducted focusing on the synthesis of new sensors for the detection of heavy metal cations.
References
Malik, L. A., Bashir, A., Qureashi, A. & Pandith, A. H. Detection and removal of heavy metal ions: A review. Environ. Chem. Lett. 17, 1495–1521. https://doi.org/10.1007/s10311-019-00891-z (2019).
Okamoto, S. & Eltis, L. D. The biological occurrence and trafficking of cobalt. Metallomics 3, 963–970. https://doi.org/10.1039/c1mt00056j (2011).
Abdulazeez, I., Basheer, C. & Al-Saadi, A. A. A selective detection approach for copper(ii) ions using a hydrazone-based colorimetric sensor: Spectroscopic and DFT study. RSC Adv. 8, 39983–39991. https://doi.org/10.1039/C8RA08807A (2018).
Lu, Y. et al. A review of the identification and detection of heavy metal ions in the environment by voltammetry. Talanta 178, 324–338. https://doi.org/10.1016/j.talanta.2017.08.033 (2018).
Jin, M. et al. Review of the distribution and detection methods of heavy metals in the environment. Anal. Methods 12, 5747–5766. https://doi.org/10.1039/D0AY01577F (2020).
Oehme, I. & Wolfbeis, O. S. Optical sensors for determination of heavy metal ions. Microchim. Acta 126, 177–192. https://doi.org/10.1007/BF01242319 (1997).
Gao, X.-G., Cheng, L.-X., Jiang, W.-S., Li, X.-K. & Xing, F. Graphene and its derivatives-based optical sensors. Front. Chem. 9, 615164. https://doi.org/10.3389/fchem.2021.615164 (2021).
Zhao, Y., Li, X.-G., Zhou, X. & Zhang, Y.-N. Review on the graphene based optical fiber chemical and biological sensors. Sens. Actuators B Chem. 231, 324–340. https://doi.org/10.1016/j.snb.2016.03.026 (2016).
Borthakur, P., Darabdhara, G., Das, M. R., Boukherroub, R. & Szunerits, S. Solvothermal synthesis of CoS/reduced porous graphene oxide nanocomposite for selective colorimetric detection of Hg(II) ion in aqueous medium. Sens. Actuators B Chem. 244, 684–692. https://doi.org/10.1016/j.snb.2016.12.148 (2017).
Borthakur, P., Boruah, P. K. & Das, M. R. CuS and NiS nanoparticle-decorated porous-reduced graphene oxide sheets as efficient peroxidase nanozymes for easy colorimetric detection of Hg(II) ions in a water medium and using a paper strip. ACS Sustain. Chem. Eng. 9, 13245–13255. https://doi.org/10.1021/acssuschemeng.1c04203 (2021).
Karaman, C. et al. Electrochemical neuron-specific enolase (NSE) immunosensor based on CoFe2O4@Ag nanocomposite and AuNPs@MoS2/rGO. Anal. Chim. Acta 1200, 339609. https://doi.org/10.1016/j.aca.2022.339609 (2022).
Keshipour, S. & Khezerloo, M. Nanocomposite of hydrophobic cellulose aerogel/graphene quantum dot/Pd: Synthesis, characterization, and catalytic application. RSC Adv. 9, 17129–17136. https://doi.org/10.1039/C9RA01799B (2019).
Ruiyi, L. et al. Serine and histidine-functionalized graphene quantum dot with unique double fluorescence emission as a fluorescent probe for highly sensitive detection of carbendazim. Sens. Actuators B Chem. 343, 130099. https://doi.org/10.1016/j.snb.2021.130099 (2021).
Ruiyi, L., Hui, J., Nana, L., Dan, X. & Zaijun, L. Electrochemical detection of chlorpyrifos in fruits with gold-histidine functionalized graphene quantum dot-graphene hybrid and target-induced DNA cycle amplification. Sens. Actuators B Chem. 355, 131314. https://doi.org/10.1016/j.snb.2021.131314 (2022).
Özcan, N., Karaman, C., Atar, N., Karaman, O. & Yola, M. L. A novel molecularly imprinting biosensor including graphene quantum dots/multi-walled carbon nanotubes composite for interleukin-6 detection and electrochemical biosensor validation. ECS J. Solid State Sci. Technol. 9, 121010. https://doi.org/10.1149/2162-8777/abd149 (2020).
Atar, N. & Yola, M. L. A novel QCM immunosensor development based on gold nanoparticles functionalized sulfur-doped graphene quantum dot and h-ZnS-CdS NC for Interleukin-6 detection. Anal. Chim. Acta 1148, 338202. https://doi.org/10.1016/j.aca.2021.338202 (2021).
Karaman, O. et al. Electrochemical cardiac troponin I immunosensor based on nitrogen and boron-doped graphene quantum dots electrode platform and Ce-doped SnO2/SnS2 signal amplification. Mater. Today Chem. 23, 100666. https://doi.org/10.1016/j.mtchem.2021.100666 (2022).
Cittan, M. & Çelik, A. An electrochemical sensing platform for trace analysis of Eriochrome Black T using multi-walled carbon nanotube modified glassy carbon electrode by adsorptive stripping linear sweep voltammetry. Int. J. Environ. Anal. Chem. 99, 1540–1552. https://doi.org/10.1080/03067319.2019.1625342 (2019).
Lisak, G. et al. Electrochemical behaviour of poly(benzopyrene) films doped with Eriochrome Black T as a Pb2+-sensitive sensors. Electroanalysis 22, 2794–2800. https://doi.org/10.1002/elan.201000459 (2010).
Guha, K., Mascarenhas, R., Thomas, T. & D’Souza, O. Differential pulse anodic stripping voltammetric determination of Hg2+ at poly(Eriochrome Black T)-modified carbon paste electrode. Ionics 20, 849–856. https://doi.org/10.1007/s11581-013-1040-9 (2013).
Devarushi, U. S., Shetti, N. P., Bukkitgar, S. D. & Tuwar, S. M. in 3rd International Conference on Emerging Technologies - Micro to Nano (ETMN). (Amer Inst Physics, 2018).
Asadpour-Zeynali, K. & Baghalabadi, V. Electrocatalytic determination of isoniazid by a glassy carbon electrode modified with poly (Eriochrome Black T). Anal. Bioanal. Chem. Res. 4, 31–40 (2017).
Ahmadi, H., Keshipour, S. & Ahour, F. New water-soluble colorimetric pH and metal ione sensor based on graphene quantum dot modified with alizarine red S. Sci. Rep. 10, 14185. https://doi.org/10.1038/s41598-020-70821-5 (2020).
Hadidi, M., Ahour, F. & Keshipour, S. Electrochemical determination of trace amounts of lead ions using D-penicillamine-functionalized graphene quantum dot-modified glassy carbon electrode. J. Iran. Chem. Soc. 19, 1179–1189. https://doi.org/10.1007/s13738-021-02367-w (2022).
Esmaeili, M., Ahour, F. & Keshipour, S. Sensitive and selective determination of trace amounts of mercury ions using a dimercaprol functionalized graphene quantum dot modified glassy carbon electrode. Nanoscale 13, 11403–11413. https://doi.org/10.1039/D1NR00076D (2021).
Babazadeh, S., Moghaddam, P. A., Keshipour, S. & Mollazade, K. Colorimetric sensing of imidacloprid in cucumber fruits using a graphene quantum dot/Au (III) chemosensor. Sci. Rep. 10, 14327. https://doi.org/10.1038/s41598-020-71349-4 (2020).
Al-Azmi, A. & Keshipour, S. Dimaval as an efficient ligand for binding Ru(III) on cross-linked chitosan aerogel: Synthesis, characterisation and catalytic investigation. Cellulose 27, 895–904. https://doi.org/10.1007/s10570-019-02838-7 (2020).
Keshipour, S. & Adak, K. Magnetic d-penicillamine-functionalized cellulose as a new heterogeneous support for cobalt(II) in green oxidation of ethylbenzene to acetophenone. Appl. Organomet. Chem. 31, e3774. https://doi.org/10.1002/aoc.3774 (2017).
Pramanik, A., Amer, S., Grynszpan, F. & Levine, M. Highly sensitive detection of cobalt through fluorescence changes in β-cyclodextrin-bimane complexes. Chem. Commun. 56, 12126–12129. https://doi.org/10.1039/D0CC05812B (2020).
Alamgir, S., Mhahabubur Rhaman, M., Basaran, I., Powell, D. R. & Alamgir Hossain, M. Colorimetric and spectroscopic cobalt(II) sensing by a simple Schiff base. Polyhedron 187, 114681. https://doi.org/10.1016/j.poly.2020.114681 (2020).
Elkhatat, A. M. et al. Recent trends of copper detection in water samples. Bull. Natl. Res. Centre 45, 218. https://doi.org/10.1186/s42269-021-00677-w (2021).
Szabó, L., Herman, K., Leopold, N., Buzumurgă, C. & Chiş, V. Surface-enhanced Raman scattering and DFT investigation of Eriochrome Black T metal chelating compound. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 79, 226–231. https://doi.org/10.1016/j.saa.2011.02.041 (2011).
Qu, D. et al. Formation mechanism and optimization of highly luminescent N-doped graphene quantum dots. Sci. Rep. 4, 5294. https://doi.org/10.1038/srep05294 (2014).
Acknowledgements
We gratefully acknowledge the facilities provided by Urmia University.
Author information
Authors and Affiliations
Contributions
S.K.: Conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, resources, supervision, validation, visualization, writing-original draft, and writing-review & editing. L.V.: Data curation, formal analysis, investigation, methodology, resources, validation, visualization, and writing-review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vahab, L., Keshipour, S. Novel nanosensor of cobalt(II) and copper(II) constructed from graphene quantum dots modified with Eriochrome Black T. Sci Rep 12, 13179 (2022). https://doi.org/10.1038/s41598-022-17616-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-17616-y
This article is cited by
-
Symmetrical Ligand’s Fabricated Porous Silicon Surface Based Photoluminescence Sensor for Metal Detection and Entrapment
Journal of Fluorescence (2024)
-
Ratiometric Fluorescence and Chromogenic Probe for Trace Detection of Selected Transition Metals
Journal of Fluorescence (2024)
-
Quantum mechanisms for selective detection in complex gas mixtures using conductive sensors
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.