Abstract
There is growing evidence that surgery can drive an inflammatory response in the brain. However, the mechanisms behind this response are incompletely understood. Here, we investigate the hypotheses that 1. Cerebrospinal fluid (CSF) cytokines increase after vascular surgery and 2. That these changes in CSF cytokines are interrelated. Patients undergoing either open or endovascular elective surgery of the thoracic aorta were invited to participate in this study. Cerebrospinal fluid samples were taken before surgery and on the first post-operative day. These were analysed for the presence of ten cytokines by immunoassay to examine for post-operative changes in cytokine levels. After surgery, there were significant increases in six out of the ten measured CSF cytokines (IL-1β, 2, 6, 8, 10 and 13). This included changes in both putative pro-inflammatory (IL-1β, 6 and 8) and putative anti-inflammatory (IL-2, 10 and 13) cytokines. The greatest increases occurred in IL-6 and IL-8, which showed a 63-fold and a 31-fold increase respectively. There was strong intercorrelation between CSF cytokines after the operation. Following surgery on the thoracic aorta, there was a marked increase in CSF cytokines, consistent with a potential role in neuroinflammation. The ten measured cytokines showed intercorrelation after the operation, indicating that a balance between multiple pro- and anti-inflammatory cytokines may be present.
Similar content being viewed by others
Introduction
Neuroinflammation is a complex and incompletely understood process which is partly mediated through cytokines1. It has been argued that the cytokine mediated neuroinflammatory process could drive peri-operative neurocognitive disorders (PND), such as post-operative delirium (POD), however this is a putative idea which has yet to be proven2. PND is the commonest post-operative complication in older patients undergoing surgery and is associated with worse clinical outcomes3; understanding its pathophysiology is essential for developing prevention and cure.
One hypothesis for the aetiology of PND is that a physiological insult from surgery induces the release of local inflammatory mediators (such as cytokines) from macrophages at the surgical site4. An intact blood–brain-barrier (BBB) prevents these inflammatory mediators from affecting the closely regulated environment of the CNS. However if the BBB is weakened by aging, neurodegeneration5, or the response to a surgical insult6, inflammatory mediators can enter the CNS. The processes by which the BBB may become weakened are complex, but in Alzheimer’s disease (AD), this includes brain capillary leakage, cellular infiltration, degeneration of pericytes and loss of endothelial integrity5. Once inflammatory mediators enter the CNS, microglial activation occurs, which drives further inflammation, partly through cytokine release7,8. Cytokine release and the inflammatory response can then trigger neuronal injury and dysfunction 5, which theoretically could manifest as PND. There is currently no treatment for PND9. If the process of neuroinflammation can be better understood, this may open the door to cytokine manipulation, potentially reducing levels of consequent PND.
Previous investigations on peri-operative cytokine changes has shown greater increases in CSF cytokines than serum or plasma cytokines after an operation10,11,12. This suggests a key role for CSF cytokines in post-operative neuroinflammation. However, the number of patients involved in these studies was small. There is a further suggestion that the CSF cytokines, IL-6 and IL-8, are increased in patients with worse neurocognitive outcomes, but again the sample sizes of these studies are inadequately powered to provide definitive evidence11,13.
To date, only one study has investigated peri-operative changes in both CSF and serum following vascular surgery on the thoracic aorta14. In this study of 23 patients, the cytokine IL-6 was increased in both serum and CSF after the operation. In patients with spinal cord injury, the CSF IL-6 increase was more marked. While this study examined a wide variety of proteins relevant to neurology, the number of cytokines studied was small.
Spinal cord injury leading to paraplegia is the most devastating consequence of morbidity following surgery on the thoracic aorta15. Draining CSF intraoperatively with a spinal catheter has been shown to reduce the chances of this complication16, by increasing blood flow to the spinal cord17. As such patients already have a spinal catheter in place. This allows for easy access to CSF peri-operatively, making patients undergoing thoracic-vascular surgery a valuable cohort for studying neuroinflammation.
We investigated cytokines with both a pro-inflammatory role (IL-1β, IL-6, IL-8, IL-12p70, IFN-γ and TNF-α) and those with an anti-inflammatory role (IL-2, IL-4, IL-10 and IL-13)18,19 in a cohort of patients undergoing vascular surgery. The aim of this study was to evaluate the neuroinflammatory response to vascular surgery in order to:
-
1.
Determine changes in pro- and anti-inflammatory CSF cytokines before and after vascular surgery.
-
2.
Investigate potential intercorrelations between cytokines in the CSF, to establish whether multiple cytokines are involved in driving neuroinflammation.
-
3.
Establish if the changes in CSF cytokines are reflected in serum cytokine levels. If so, this would enable serum cytokines to be used as biomarkers of CSF changes.
Methods
This was a prospective observational study looking at peri-operative changes in cytokine levels in the CSF and serum of patients undergoing elective vascular surgery for any pathology of the thoracic aorta.
Study population
Patients were eligible for recruitment into the study if they were undergoing either open or endovascular repair for any thoracic aortic pathology in which a spinal catheter was to be used as part of standard peri-operative care. The decision about whether a spinal catheter would be used during an operation was a joint decision made pre-operatively in the vascular multidisciplinary team meeting involving the operating surgeon and anaesthetist. Patients did not have neuroimaging performed before or after the operation.
Recruitment took place at St Mary’s Hospital, London, over a one-year period. Patients unable to give informed written consent were excluded from the study. The study received approval from the London Westminster Research Ethic Committee (13/LO/0210). The study conformed to the precepts set out in the Declaration of Helsinki of 1975. All patients gave informed, written consent prior to surgery.
Specimen collection and storage
Study patients had a spinal catheter inserted prior to surgery by an anaesthetist, following administration of a general anaesthetic. The spinal catheter was used to maintain the CSF pressure at 10 mmHg. If the pressure rose above 10 mmHg, CSF was slowly drained until the target was met. CSF was collected by the same operator (author AP) prior to surgery and on the first post-operative day. Approximately 1-3 mL of CSF was collected each time. The spinal catheter was removed when it was no longer clinically indicated. Samples were centrifuged at 4000RPM for 5 min to remove any red blood cells present in the sample. Samples were then pipetted into cryo-tubes in 500 μL aliquots and stored in a −80 °C freezer.
Immunoassays
The V-PLEX Proinflammatory Panel 1 Human Kit (Meso Scale Discovery, Maryland, USA) was chosen as it measures both pro-inflammatory (IL-1β, IL-6, IL-8, IL-12p70, IFN-γ and TNF-α) and anti-inflammatory (IL-2, IL-4, IL-10 and IL-13) cytokines19. Analysis followed the manufacturer’s instructions (www.mesoscale.com). This method of cytokine measurement in peri-operative CSF and blood samples has been used previously10. It uses electrochemiluminescence to quantify the levels of ten cytokines in 25 μL of peri-operative CSF or blood samples.
The range for the lowest level of detection (LLD) was between 0.00861 pg/mL for IL-4 and 0.605 pg/mL for IFN-γ. If samples were above the upper limit of detection (ULD) for the assay, they were diluted in Diluent 2 which was supplied with the kit assay and re-analysed, according to the manufacturer’s instructions. This was necessary for a small number of IL-8 measurements. For cytokine levels below the LLD or detected, but below the fit curve, the LLD value for the cytokine assay was used, as previously described20. All cytokine analysis was completed in the Infectious Diseases Laboratory at Imperial College London, London.
Statistical analysis
Analyses were carried out using Python, version 3.7 (available from www.python.org). A Wilcoxon Signed-Rank test with Bonferroni correction was used to examine changes in cytokines before and after surgery. Spearman’s rank correlation coefficient, again with a Bonferroni correction, assessed cytokine intercorrelation. An adjusted-p value of < 0.05 was considered statistically significant. Patient demographics (age and sex) were assessed for correlation with CSF cytokine changes using Spearman’s rank correlation coefficient with a Bonferroni correction and logistic regression respectively. A power calculation to determine the necessary sample size was not undertaken before starting the study, as the number of patients who would be able to consent and complete the study was anticipated to be small.
Ethics approval and consent to participate
The study received approval from the London Westminster Research Ethic Committee (13/LO/0210). The study conformed to the precepts set out in the Declaration of Helsinki of 1975. All patients gave informed, written consent prior to surgery.
Results
During a 1-year period, ten patients were recruited into the study. Table 1 shows the demographic and pre-operative information for the patients. Four of the patients were female, with a mean age of 65 yrs (SD 12 years). Seven patients had undergone previous vascular surgery, often with serious post-operative morbidity. Two patients died in critical care within 30-days of the operation. No patient developed post-operative paraplegia or a clinically apparent stroke.
Timetable of sample collection
All patients had pre-operative (timepoint 1: T1) and post-operative (timepoint 2: T2) CSF samples taken. Five patients also had simultaneous paired serum samples. Patient 5 had their post-operative samples taken on the day of surgery. Supplementary table 1 summarises the aetiology of the thoracic aorta pathology, the type of vascular surgery and the post-operative complications.
Cytokine changes following surgery
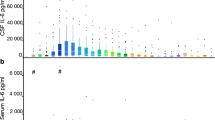
Table 2 and Fig. 1 show the cytokine changes in CSF following surgery. The greatest increases occurred in IL-6 and IL-8, which showed a 63-fold and a 31-fold increase respectively. Six cytokines showed statistically significant increases between T1 and T2 (IL-1β, 2, 6, 8, 10 and 13), as shown in Fig. 1. Patient demographics (age and sex) were not significantly correlated with any CSF cytokine changes. Of the cytokines which showed statistically significant increases, three are traditionally classified as pro-inflammatory (IL-1β, 6 and 8) and three (IL-2, 10 and 13) as anti-inflammatory18.
Post-operative changes in CSF cytokines. Levels of cytokines in cerebrospinal fluid (CSF) before surgery (T1) and the day after surgery (T2). Each patient is represented by a colour that is consistent across the box-plots. Only cytokines that showed statistically significant changes following surgery are shown.
The levels of most CSF cytokines tended to rise following surgery. However, levels of IFN-γ and IL-12p70 were generally below the LLD in both the pre-and post-operative CSF samples, so no change could be detected. For the cytokines IL-1β, IL-2 and IL-4, pre-operative levels were often below the LLD, but post-operative levels rose into the detection range.
Intercorrelation between CSF cytokines
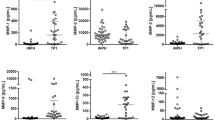
Figure 2 shows the intercorrelation between CSF cytokines before and after surgery. At T1, the significant correlations observed in the CSF were between IL-1β and IL-2 (r = 0.95, adjusted-p < 0.001). At T2, there were a number of strong positive correlations between 20 pairs of different cytokines, as shown in Supplementary table 2.
Serum cytokine changes
Only five of the ten patients had serum samples drawn at the same time as the CSF samples. The greatest increase was in IL-6 which showed a 71-fold increase in serum levels after the operation. There was limited intercorrelation between CSF and serum cytokines. At T1, four combinations of CSF and serum cytokines showed significant correlation (r = 1, adjusted-p < 0.05). These included IL-2 and IL-1β, IL-4 and IL-12, IFN-γ and Il-2 and IL-1β and IL-1β. At T2 there were no significant correlations between the CSF and serum cytokine levels (data not shown).
Discussion
CSF cytokine findings
We demonstrated increases in both pro- and anti-inflammatory CSF cytokines after vascular surgery on the thoracic aorta. The most marked increases occurred in the pro-inflammatory cytokines, IL-6 and IL-8. The increases in CSF IL-6 confirm previous findings in a similar cohort14, whereas, to our knowledge, we are the first group to show similar changes in CSF IL-8. Interleukin-8 is a member of the CXC chemokine family, implicated in a wide variety of inflammatory diseases21. CSF IL-8 has been shown to be increased in AD, Parkinson’s disease22 and following traumatic brain injury23. In patients undergoing other forms of surgery, findings of marked increases in CSF IL-8 have also been demonstrated10,12.
The increase in CSF cytokines in this study could have resulted from a dysregulated inflammatory response to the peripheral stimulus of surgery driving neuroinflammation within the brain. Alternatively, these findings may have been secondary to silent cerebral infarcts, which have been shown to be increased following thoracic aortic endovascular procedures24. Ischaemic strokes have been shown to drive an increase in proinflammatory cytokines25. It is also possible that the CSF cytokine changes may have occurred due to other post-operative complications such as endoleaks or infection.
Intercorrelation findings
Levels of cytokines in the CSF, while not exhibiting an association with each other prior to surgery, strongly correlate on day one after surgery. Figure 2 highlights the complex balance between pro- and anti-inflammatory cytokines which may drive neuroinflammation. The lack of an association between post-operative CSF and serum samples is consistent with other studies in this area10,12, and suggests that changes in cytokine levels in blood cannot be used reliably as surrogate markers of CSF cytokine changes.
Proposed mechanisms
The results of this study need to be interpreted alongside the current postulated mechanisms of brain dysfunction. These include excessive neuroinflammation26, the production of reactive oxygen species (ROS)27, and dysregulated neurotransmission28.
These mechanisms are all mediated through activation of microglia, which when stimulated can release ROS27 and cytokines29. Microglia are also key drivers of the kynurenine inflammatory pathway, which, in turn, can drive glutamatergic neurotransmission30. In the healthy brain, microglia are fundamental in maintaining tissue homeostasis by removing accumulated debris27. However, their overactivity may be harmful in disease states31.
The postulated mechanisms of brain dysfunction are not mutually exclusive, with multiple mechanisms likely to be acting together28. Indeed, pro-inflammatory cytokines have been shown to activate the kynurenine pathway, which in turn leads to the generation of ROS through quinolinic acid production32. The cytokine changes demonstrated in this study may therefore have implications for several mechanisms of brain dysfunction.
Therapeutic targets
In contrast to neurodegenerative processes, peri-operative brain dysfunction occurs at a predictable time point, giving a potential opportunity for prevention33. Currently, no effective treatment for PND exists34. The suggestion that CSF IL-6 and IL-8 hold a key role in the post-operative neuroinflammatory pathway, raises the question of whether direct cytokine inhibition could attenuate these effects. However, as we have demonstrated in this paper, multiple cytokines, both pro- and anti-inflammatory, increase after an operation and thus blocking the action of only one of these cytokines may not necessarily inhibit neuroinflammation. Furthermore, as has been suggested in Alzheimer’s disease, a degree of cytokine-driven neuroinflammation may be neuroprotective35,36.
Limitations
Similar to many studies in this area, this study had a small patient cohort, which may have limited our ability to demonstrate the true magnitude of peri-operative cytokine changes. Future studies should involve larger sample sizes across multiple settings to address this problem. Samples were only taken at two time points, with one patient’s CSF sample taken after surgery on day 0 rather than day 1. This patient was not excluded due to an already small cohort. Within the cohort, the surgical approach was not homogenous, with some patients undergoing open surgery and others undergoing endovascular surgery, which could represent a further complicating factor. The small numbers of patients in different surgical groups limits meaningful comparisons. A further confounder was that seven out of ten patients had undergone previous vascular surgery, often with serious post-operative morbidity. Ideally, this study would have corrected for underlying co-morbidity and baseline inflammatory status. In future studies, neuroimaging, to look for radiological evidence of stroke, should be included to investigate how much of the neuroinflammatory burden may be driven by ischaemic strokes. Finally, we cannot exclude the possibility that the inflammatory response was driven by the insertion of the spinal catheter, rather than surgery or anaesthesia, but this is felt to be unlikely due to the magnitude of cytokine changes12.
This study looked solely at cytokine changes, which is only part of the neuroinflammatory process after surgery33. Measurement of the Q-albumin to determine the integrity of the BBB would also have been useful5. Future studies would ideally also examine the CSF cell count and immunoglobulin subtypes37, and other markers of neuronal injury6 to more fully understand pathophysiological processes. This was not possible within the scope of this study.
A final key limitation of this study was that patients did not undergo peri-operative cognitive testing. Formal cognitive testing for delirium, using screening tools such as the 4AT38 and neuropsychological testing, would allow for the more direct investigation of correlations between observed CSF cytokine changes and the magnitude of cognitive dysfunction in PND.
Conclusions
After vascular surgery there is a large increase in cytokines in the CSF, particularly in the pro-inflammatory cytokines IL-6 and IL-8. This may be secondary to peripheral changes in the circulation crossing the BBB and driving neuroinflammation. A strong correlation was found between CSF cytokines on day one after the operation, suggesting that it may be the balance between multiple pro- and anti-inflammatory cytokines which drives neuroinflammation.
Data availability
The dataset supporting the conclusions of this article is available from the corresponding author upon reasonable request.
Abbreviations
- AD:
-
Alzheimer’s disease
- BBB:
-
Blood–brain barrier
- CNS:
-
Central nervous system
- CSF:
-
Cerebrospinal fluid
- IFN-γ:
-
Interferon gamma
- IL:
-
Interleukin
- LLD:
-
Lower limit of detection
- POD:
-
Post-operative delirium
- PND:
-
Peri-operative neurocognitive disorders
- ROS:
-
Reactive oxygen species
- T1:
-
Time-point 1 (pre-operation)
- T2:
-
Time-point 2 (day 1 post-operation)
- TEVAR:
-
Thoracic endovascular aortic repair
- TNF-α:
-
Tumour necrosis factor alpha
- ULD:
-
Upper limit of detection
References
DiSabato, D. J., Quan, N. & Godbout, J. P. Neuroinflammation: The devil is in the details. J. Neurochem. 139, 136–153 (2016).
Pereira, C., Dani, M., Taylor-Robinson, S. D. & Fertleman, M. Putative involvement of Cytokine modulation in the development of perioperative neurocognitive disorders. Int. J. Gen. Med. 15, 5349–5360 (2022).
Evered, L., Atkins, K., Silbert, B. & Scott, D. A. Acute peri-operative neurocognitive disorders: A narrative review. Anaesthesia 77, 34–42 (2022).
Marcantonio, E. R. Postoperative delirium: A 76-year-old woman with delirium following surgery. JAMA J. Am. Med. Assoc. 308, 73–81 (2012).
Sweeney, M. D., Sagare, A. P. & Zlokovic, B. V. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 14, 133–150 (2018).
Reinsfelt, B. et al. Cerebrospinal fluid markers of brain injury, inflammation, and blood-brain barrier dysfunction in cardiac surgery. Ann. Thorac. Surg. 94, 549–555 (2012).
Garden, G. A. & Möller, T. Microglia biology in health and disease. J. Neuroimmune Pharmacol. 1, 127–137 (2006).
Bohlen, C. J., Bennett, F. C. & Bennett, M. L. Isolation and Culture of Microglia. Curr. Protoc. Immunol. 125, e70 (2019).
Safavynia, S. A. & Goldstein, P. A. The role of neuroinflammation in postoperative cognitive dysfunction: Moving from hypothesis to treatment. Front. Psych. 9, 752 (2019).
Fertleman, M. et al. Cytokine changes in cerebrospinal fluid and plasma after emergency orthopaedic surgery. Sci. Rep. 12, 2221 (2022).
Hirsch, J. et al. Perioperative cerebrospinal fluid and plasma inflammatory markers after orthopedic surgery. J. Neuroinflammation 13, 211 (2016).
Bromander, S. et al. Changes in serum and cerebrospinal fluid cytokines in response to non-neurological surgery: An observational study. J. Neuroinflammation 9, 242 (2012).
Danielson, M. et al. Neuroinflammatory markers associate with cognitive decline after major surgery: Findings of an explorative study. Ann. Neurol. 87, 370–382 (2020).
Lindblom, R. P. F. et al. Protein Profiling in Serum and Cerebrospinal Fluid Following Complex Surgery on the Thoracic Aorta Identifies Biological Markers of Neurologic Injury. J. Cardiovasc. Transl. Res. 11, 503–516 (2018).
Coselli, J. S. & LeMaire, S. A. Tips for Successful Outcomes for Descending Thoracic and Thoracoabdominal Aortic Aneurysm Procedures. Semin. Vasc. Surg. 21, 13–20 (2008).
Miyamoto, K., Ueno, A., Wada, T. & Kimoto, S. A new and simple method of preventing spinal cord damage following temporary occlusion of the thoracic aorta by draining the cerebrospinal fluid. J. Cardiovasc. Surg. (Torino) 1, 188–197 (1960).
Epstein, N. Cerebrospinal fluid drains reduce risk of spinal cord injury for thoracic/thoracoabdominal aneurysm surgery: A review. Surg. Neurol. Int. 9 (2018).
Dinarello, C. A. Historical insights into cytokines. Eur. J. Immunol. 37, S34–S45 (2007).
Meso Scale Diagnostics. Proinflammatory Panel 1 (human) Kits. https://www.mesoscale.com/~/media/files/productinserts/proinflammatorypanel1humaninsert.pdf (2020).
Thwaites, R. S. et al. Inflammatory profiles across the spectrum of disease reveal a distinct role for GM-CSF in severe COVID-19. Sci. Immunol. 6, (2021).
Roebuck, K. A. Regulation of interleukin-8 gene expression. J. Interferon Cytokine Res. 19, 429–438 (1999).
Zhang, J. et al. CSF Multianalyte Profile Distinguishes Alzheimer and Parkinson Diseases. Am. J. Clin. Pathol. 129, 526–529 (2008).
Kossmann, T. et al. Interleukin-8 released into the cerebrospinal fluid after brain injury is associated with blood-brain barrier dysfunction and nerve growth factor production. J. Cereb. Blood Flow Metab. 17, 280–289 (1997).
Perera, A. H. et al. Cerebral embolization, silent cerebral infarction and neurocognitive decline after thoracic endovascular aortic repair. Br. J. Surg. 105, 366–378 (2018).
Tuttolomondo, A., Di Raimondo, D., di Sciacca, R., Pinto, A. & Licata, G. Inflammatory Cytokines in Acute Ischemic Stroke. Curr. Pharm. Des. 14, 3574–3589 (2008).
Dokalis, N. & Prinz, M. Resolution of neuroinflammation: mechanisms and potential therapeutic option. Semin. Immunopathol. 2019 416 41, 699–709 (2019).
Heneka, M. T., Kummer, M. P. & Latz, E. Innate immune activation in neurodegenerative disease. Nat. Rev. Immunol. 14, 463–477 (2014).
Maldonado, J. R. Neuropathogenesis of delirium: Review of current etiologic theories and common pathways. Am. J. Geriatr. Psychiatry 21, 1190–1222 (2013).
Song, W. M. & Colonna, M. The identity and function of microglia in neurodegeneration. Nature Immunol. 19, 1048–1058 (Nature Publishing Group, 2018).
Hughes, T. D., Güner, O. F., Iradukunda, E. C., Phillips, R. S. & Bowen, J. P. The Kynurenine Pathway and Kynurenine 3-Monooxygenase Inhibitors. Molecules 27, 273 (2022).
Saxena, S., Kruys, V., Vamecq, J. & Maze, M. The Role of Microglia in Perioperative Neuroinflammation and Neurocognitive Disorders. Front. Aging Neurosci. 13 (2021).
Stone, T. W., Forrest, C. M. & Darlington, L. G. Kynurenine pathway inhibition as a therapeutic strategy for neuroprotection. FEBS J. 279, 1386–1397 (2012).
Yang, T., Velagapudi, R. & Terrando, N. Neuroinflammation after surgery: from mechanisms to therapeutic targets. Nat. Immunol. 21, 1319–1326 (2020).
Granger, K. T. & Barnett, J. H. Postoperative cognitive dysfunction: an acute approach for the development of novel treatments for neuroinflammation. Drug Discovery Today 26, 1111–1114 (2021).
Chakrabarty, P. et al. Massive gliosis induced by interleukin-6 suppresses Aβ deposition in vivo: Evidence against inflammation as a driving force for amyloid deposition. FASEB J. 24, 548–559 (2010).
Chakrabarty, P., Herring, A., Ceballos-Diaz, C., Das, P. & Golde, T. E. Hippocampal expression of murine TNF results in attenuation of amyloid deposition in vivo. Mol. Neurodegener. 6, (2011).
Reiber, H. & Peter, J. B. Cerebrospinal fluid analysis: Disease-related data patterns and evaluation programs. J. Neurol. Sci. 184, 101–122 (2001).
Bellelli, G. et al. Validation of the 4AT, a new instrument for rapid delirium screening: A study in 234 hospitalised older people. Age Ageing 43, 496–502 (2014).
Acknowledgements
The authors acknowledge the United Kingdom National Institute for Health Research (NIHR) Biomedical Facility at Imperial College London for infrastructure support. SDT-R was funded by a Wellcome Trust ISSF grant at Imperial College London.
Funding
The study was funded by a grant awarded to Miss Rudarakanchana from the National Institute of Health Research (NIHR) Imperial Biomedical Research Centre (BRC).
Author information
Authors and Affiliations
Contributions
C.P.—undertook all cytokine experiments and writing of the manuscript. A.H.P. was responsible for patient recruitment and biofluid collection. N.R. offered supervision with the project M.D.—undertook manuscript review. B.H.L.H.—undertook statistical analysis. M.D.G.—undertook statistical analysis. S.D.T.R.—offered guidance and supervision with the project and manuscript review. M.F.—was responsible for supervision of the project and manuscript review. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pereira, C., Perera, A.H., Rudarakanchana, N. et al. Cytokine changes in cerebrospinal fluid following vascular surgery on the thoracic aorta. Sci Rep 12, 12839 (2022). https://doi.org/10.1038/s41598-022-16882-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-16882-0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.