Abstract
To date, significant efforts have been put into searching for materials with advanced magnetocaloric properties which show promise as refrigerants and permit realization of efficient cooling. The present study, by an example of Ho1−xErxNi2, develops the concept of magnetocaloric efficiency in the rare-earth Laves-phase compounds. Based on the magneto-thermodynamic properties, their potentiality as components of magnetocaloric composites is illustrated. The determined regularities in the behaviour of the heat capacity, magnetic entropy change, and adiabatic temperature change of the system substantiate reaching high magnetocaloric potentials in a desired temperature range. For the Ho1−xErxNi2 solid solutions, we simulate optimal molar ratios and construct the composites used in magnetic refrigerators performing an Ericsson cycle at low temperatures. The tailored magnetocaloric characteristics are designed and efficient procedures for their manufacturing are developed. Our calculations based on the real empirical data are very promising and open avenue to further experimental studies. Systems showing large magnetocaloric effect (MCE) at low temperatures are of importance due to their potential utilization in refrigeration for gas liquefaction.
Similar content being viewed by others
Introduction
The magnetocaloric effect discovered by Weiss and Piccard1 in 1917, consists of heating or cooling of a magnetic material under the magnetic field variation. The nature of MCE was explained, and its practical use to reach ultralow temperatures via adiabatic demagnetization was suggested independently by Debye2 and by Giauque3. To date, it is still one of the most used techniques to reach very low temperatures. Magnetic refrigeration based on the magnetocaloric effect has become an attractive alternative to conventional cooling methods owing to its energy efficiency and ecological safety. Up to now, an intensive search for materials suitable for the use as the working body of magnetocaloric refrigerators is under way4,5,6,7,8,9. All magnetic materials intrinsically show MCE, although the intensity of the effect depends on the properties of each material. The phenomenon is due to the coupling of magnetic sublattice of a solid with an applied magnetic field, which changes the magnetic contribution to the entropy of the solid. In the case of conventional MCE, isothermal magnetization reduces the entropy of a magnetic material, which subsequently can be cooled by adiabatic demagnetization, like the gas compression. In a reversible process, demagnetizing restores zero-field magnetic entropy of a system. Generally, MCE is defined by the isothermal entropy change, ΔS, in an isothermal process and by the temperature change, ΔTad, in an adiabatic process. The values of the above magnetocaloric potentials usually are highest in the vicinity of a magnetic ordering temperature and decrease smoothly to zero beyond the magnetic phase transition region. The other correlated parameters that allow one to determine the magnetocaloric performance of magnetic material are the refrigerant capacity (RC) and relative cooling power (RCP) or temperature averaged entropy change (TEC)10.
The search for new materials for cryogenics among RNi2 (R—is a rare-earth metal) compositions, has its base in their structural, magnetic, and thermodynamic properties. Magnetocaloric cooling with the rare-earth-based Laves-phase materials offers higher efficiency for liquifying gases compared to conventional methods, e.g., hydrogen which is of great interest as an energy carrier in the decarbonization of the economy.
The RNi2 compounds belong to the Laves phases and crystallize with the formation of a simple regular MgCu2-type structure (C15). However, the majority of the RNi2 compounds crystallize in a cubic structure characterized by regular arrangement of vacancies at the rare earth sites, which stabilize these compounds in a structure derived from the ideal C15 cubic structure11,12,13. The ordering of the R vacancies on special lattice sites leads either to a tetragonal14 or cubic superstructure12,13,15. The superstructure derived from C15 can be described within the space group F-43 m and is characterized by the doubled lattice parameter a compared to the C15 structure16. The R atoms occupy five different crystallographic sites, whereas the ordered vacancies are located only at one of these 5 sites, namely, the 4a sites15. However, the 4a sites are not completely empty, and the occupancy varies among the investigated RNi2 compounds. This variation is due to different sizes of different R atoms occupancy and can also depend on other factors, such as, e.g., the starting stoichiometry of samples.
The RNi2 compounds are characterized by high localized magnetic moments originating from the incompletely filled 4f-electron shell of lanthanides. The non-magnetic state of Ni atoms in these compounds is the cause of low magnetic ordering temperatures because the range of wave functions derived from lanthanides is lower than the interatomic distances, and 4f-4f interactions are weak. The majority of RNi2 compounds are found to be ferromagnetically ordered at low-temperatures and, upon ordering, exhibit the second-order magnetic phase transition17,18. These features of RNi2 compounds determine the marked MCE and, hence, their promise as cryogenic refrigeration materials.
To provide the effective operation in the ideal Ericsson magnetic regenerator cycle, a magnetic working material should have a magnetic entropy change − ΔSmag that is constant in the cycled temperature span19. The above considerations determine the possibility to make a “table-like” temperature dependence − ΔSmag (T), namely, to reach the almost unchanged significant value of − ΔSmag over a desired temperature range. In particular, it can be done with the Ho1−xErxNi2 compositions, for which the Curie temperatures (TC) range between 13.5 and 6.5 K for HoNi2 and ErNi220, respectively. It should be noted that the “table-like” behavior of − ΔSmag(T) is the essential requisite for an ideal Ericsson-like refrigeration cycle21.
The purpose of this work is to characterize the structure and magneto-thermodynamic properties of Ho1−xErxNi2 and to analyze their evolution in accordance with the substitutions in the rare-earth sublattice. We focus on the practical magnetocaloric aspect of the Ho1−xErxNi2 solid solutions. Direct and indirect measurements of magnetocaloric potentials in a wide magnetic-field range allow us to extend the knowledge on the magnetocaloric nature of considered compositions with the magnetic dilution determining their properties as composite refrigerant components.
Results
Structural analysis
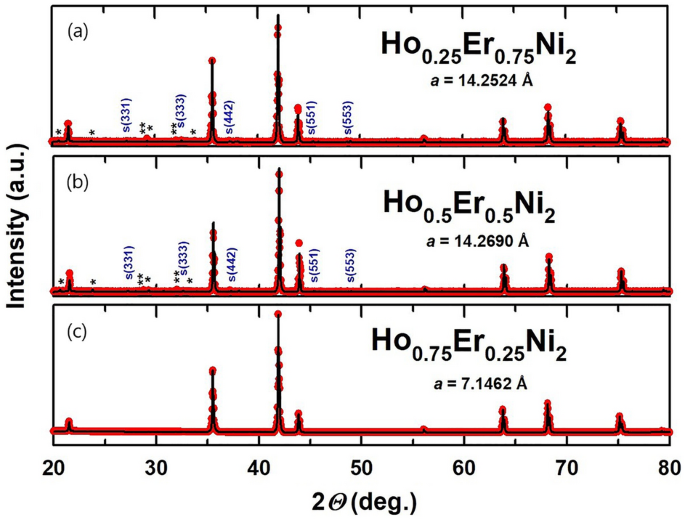
The XRD patterns recorded for the Ho1-xErxNi2 solid solutions at room temperature were analyzed by the Rietveld method and are depicted in Fig. 1. Through the substitution of erbium for holmium in Ho0.5Er0.5Ni2 and Ho0.25Er0.75Ni2, the ordering of R vacancies preserved in the structure of HoNi2 phase takes place, and the 2a cubic superstructure (space group F-43 m) forms, indicated by indexed peaks marked with S in Fig. 1 a, b. For the Ho0.75Er0.25Ni2 stoichiometric composition, in contrast to Ho0.5Er0.5Ni2 and Ho0.25Er0.75Ni2, this effect is not so evident, reflections of the superstructure do not appear in the X-ray diffraction pattern, and the structure can be described by the space group Fd-3 m.
According to Delsante et al.11, formation of the regular C15 structure (space group Fd-3 m) is expected for the RNi2 compounds with the enthalpy of formation ΔfHo at 300 K of less than − 40 kJ/mol. The enthalpies of formation ΔfHo of HoNi2 and ErNi2 equal to –48 and –50 kJ/mol, respectively, suggest the emergence of the regular C15 structure in these compounds, which was indeed confirmed in our earlier work 20. However, in the case of Ho0.5Er0.5Ni2 and Ho0.25Er0.75Ni2 solid solutions, this rule is not confirmed. Additional vacancies are induced and are responsible for the formation of the superstructure. Vacancies arise as structural defects resulting from differences in the atomic radii of elements comprising a solid solution. Owing to the difference in the atomic radii, Ho–Ni and Er–Ni bonds in the solid solutions differ in length; this fact has a direct impact on the formation of vacancies. Similar results were obtained for the Ho distribution in Tb1-xHoxNi2 solid solutions 22 and are in line with the data obtained for the other ternary Laves-phase solid solutions, e.g., Tb1-xDyxNi2 23 studied previously.
According to the data given in Fig. 1, small amounts of Ho2O3 and Er2O3 impurity phases are present in the Ho0.5Er0.5Ni2 and Ho0.25Er0.75Ni2 samples, the total content of which is not more than 3 wt. %. For Ho0.75Er0.25Ni2, the lattice parameter is equal to 7.1462 Å. For the two consecutive substitutions, the lattice parameter decreases as the Er content increases to x = 0.75. This is due to the fact that, in accordance with the lanthanide contraction, the radius of Er atoms (176 pm) is smaller than that of Ho (177 pm). It should be noted that the parent compounds, similarly to the Ho0.75Er0.25Ni2 compound, solidify with the formation of the cubic C15 crystal structure.
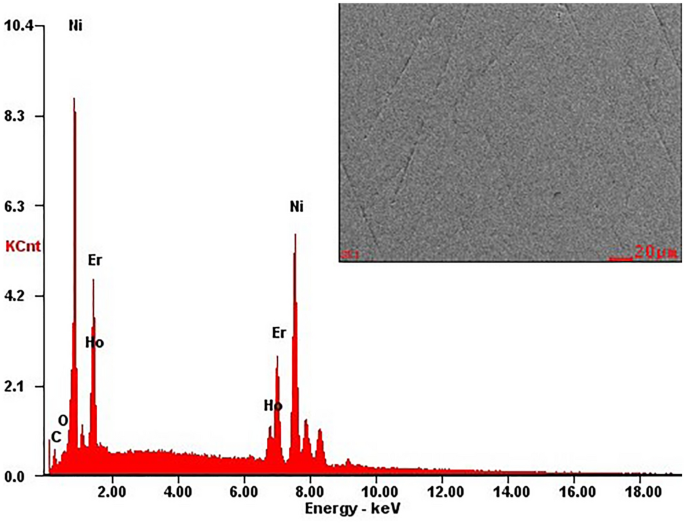
The typical SEM image and EDX studies of the characteristic microstructure of the polished section as representative of Ho0.25Er0.75Ni2 are shown in Fig. 2. The EDX analysis performed for large areas of Ho0.25Er0.75Ni2 sample showed that its chemical composition is consistent with the nominal one (the Ho, Er, and Ni contents are 8.07, 26.13, and 65.81 at.%, respectively). Similar results were also obtained for the other samples.
Evaluation of magnetocaloric effect by indirect method
In general, the heat capacity of metallic magnetic systems can be considered as the sum of the independent electron, lattice (phonon) and magnetic contributions:
The electron and phonon contributions to the heat capacity can be calculated by the formula:
where the first term represents an electron heat capacity and the second term corresponds to a phonon contribution in accordance with Debye’s model; γ is the Sommerfeld coefficient; ƟD is the Debye temperature; N = 3 is the number of atoms per formula unit; and R is the molar gas constant.
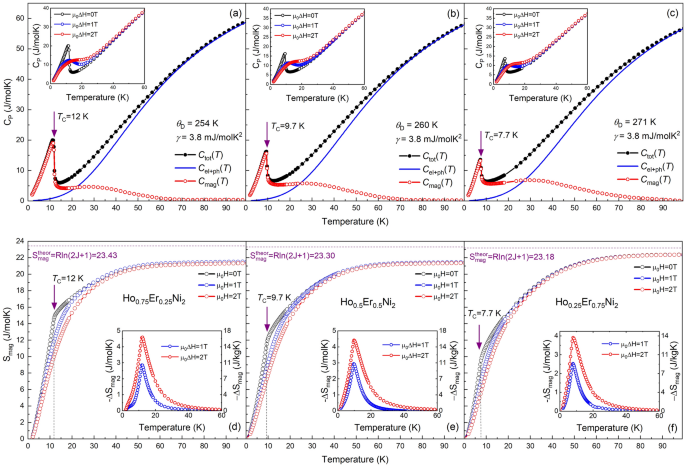
To isolate the electron–phonon contribution from the total heat capacity of measured Ho1-xErxNi2 solid solutions, the curves of the measured heat capacity for an isostructural non-magnetic compound, LaNi2 were used. It was found that, in the low-temperature range 1.8–4 K, the linear dependence of the CP/T vs T2 in LaNi2 can be fitted with the Sommerfeld coefficient γ = 6.6 mJ/molK and the Debye temperature ƟD = 242 K24. However, we have found that the best fittings for the wide temperature range 2–100 K, for all the studied samples, could be obtained by fixing the parameter γ = 3.8 mJ/molK2, while the Debye temperature ƟD of the Ho1−xErxNi2 system, similarly to that of the Dy1−xErxNi2 system25, increases as the Er content increases from 254 K for x = 0.25 to 271 K for x = 0.75. It should be noted that the Debye temperature values obtained are comparable with those of other known RNi2 compounds. By comparison, the ƟD values for TbNi2, DyNi2 and ErNi2 compounds were reported to be 261, 250 and 264 K, respectively26,27,28. Table 1 shows the Debye temperatures and the γ values calculated by this method.
Figures 3a–c show the temperature dependences of the total heat capacity, Ctot(T), of the Ho1-xErxNi2 solid solutions in zero magnetic field. Filled symbols correspond to the experimental data, and open symbols correspond to the magnetic part of heat capacity, Cmag(T), obtained after subtraction of the electron and phonon contribution, Cel+ph(T), which was estimated by Debye function (solid lines in Fig. 3a-c) according to the Eq. (2).
Total heat capacity Ctot(T) of Ho0.75Er0.25Ni2 (a), Ho0.5Er0.5Ni2 (b) and Ho0.25Er0.75Ni2 (c) measured in zero magnetic field. The calculated sum of electronic and phonon contributions Cel+ph as well as estimated magnetic contribution Cmag. The insets of (a)–(c) show the heat capacity as a function of temperature measured in zero, 1- and 2-T magnetic fields, respectively. Temperature dependences of the magnetic entropy Smag(T) for Ho0.75Er0.25Ni2 (d), Ho0.5Er0.5Ni2 (e) and Ho0.25Er0.75Ni2 (f) in zero, 1- and 2-T magnetic fields. The horizontal dotted lines correspond to the theoretical maximum value Smag = Rln(2 J + 1) and the vertical dotted lines correspond to the magnetic phase transition temperature TC. Insets show the magnetic entropy change ΔSmag measured for magnetic field changes of 1 and 2 T.
In the absence of magnetic field, the temperature dependence of the heat capacity shows a peak corresponding to magnetic phase transition typical of ferromagnetic compounds. The Curie temperatures TC of the Ho0.75Er0.25Ni2 (Fig. 3a), Ho0.5Er0.5Ni2 (Fig. 3b), and Ho0.25Er0.75Ni2 (Fig. 3c) compounds are 12.0, 9.7, and 7.7 K, respectively.
Insets in Fig. 3a–c show the heat capacity, as a function of temperature, measured in zero, 1- and 2-T magnetic fields. The feature observed for all of the studied compositions is the broadening of the Ctot(T) peak and reduction of its height, which takes place with the increasing applied magnetic field.
The magnetic part of the entropy Smag(T) was calculated by integrating the dependence Cmag(T)/T for each composition (Fig. 3d–f). This procedure is valid when assuming that the electronic and lattice contributions are field-independent and in the case of an adiabatic field change process, when ΔStot = 029. The fact that the dependence of entropy exhibits a strong tendency to saturation, but the entropy does not approach the theoretical maximum value Smag = Rln (2 J + 1) (where J is the total angular momentum of a rare earth ion) at the Curie temperatures can be explained by peculiarities in the ground-state level splitting by the crystal electric field (CEF) when several CEF levels are separated from others by a substantial energy gap30. Similar behavior was observed for other pseudo-binary Laves-phase compounds25,31,32. According to the theoretical calculations, the maximum magnetic entropy should equal to 23.2–23.4 J/molK. In the case of the tested solid solutions, the maximum value of Smag for Ho0.75Er0.25Ni2 and Ho0.5Er0.5Ni2 is 21.4 J/molK at 100 K and is 22.3 J/molK for Ho0.25Er0.75Ni2. This means that almost the total magnetic entropy associated with the magnetic process is utilized.
The temperature behaviour of the magnetic entropy in 1- and 2-T magnetic fields shows that the applied magnetic field leads to the decrease in Smag near TC. In particular, the maximum value of Smag for Ho0.75Er0.25Ni2 near TC decreases from 15.1 to 10.5 J/molK in the applied magnetic field. The temperature dependences of the isothermal magnetic entropy change ΔSmag(T) calculated using the heat capacity data according to the procedure reported in25 and caused by 1- and 2-T magnetic field change, are shown in insets in Fig. 3d–f. For a magnetic field change of 0–2 T, the experimental maximum − ΔSmag in the case of the Ho0.75Er0.25Ni2 compound reaches the highest value of 4.6 J/mol K (16.3 J/kg K) near 12.1 K and, as the Er content increases, becomes lower and equals to 3.9 J/mol K (13.7 J/kg K) for the Ho0.25Er0.75Ni2 sample near 8 K.
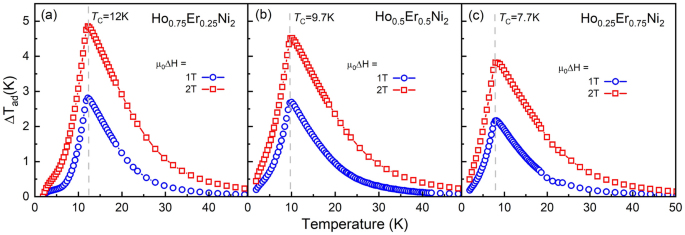
Figure 4a–c show dependences of the adiabatic temperature change, ΔTad, for Ho1−xErxNi2 with x = 0.25, 0.5, and 0.75, which were derived from the heat capacity data obtained in 1- and 2-T magnetic fields. As is seen, the increase in the applied magnetic field leads to an increase in the adiabatic temperature change near TC. Both at 1- and 2-T magnetic field changes, the highest magnetocaloric effect was observed for Ho0.75Er0.25Ni2. The maximum ΔTad for Ho0.75Er0.25Ni2 reaches 2.8 K (4.9 K) at 12.0 K, and, with increasing Er content, the maximum peak value of ΔTad decreases to 2.2 K (3.9 K) for Ho0.25Er0.75Ni2 at 7.7 K for a magnetic field change of 1 (2) T. Table 2 summarizes the data on the experimental isothermal magnetic entropy change ΔSmag(T) and adiabatic temperature change ΔTad(T) for low external magnetic field changes, which were estimated by the indirect method using the heat capacity data.
To compare the refrigeration properties of Ho1−xErxNi2 with those of the other previously investigated RNi2 compounds, the refrigerant capacities (RC), relative cooling power (RCP) and temperature averaged entropy change (TEC) were estimated. The first parameter is a measure of the amount of heat that can be transferred between the cold and hot sinks in one ideal refrigeration cycle and was estimated by integrating the ΔSmag(T) curve over the full width at half maximum33,34. It should be noted that, as the magnetic entropy change decreases owing to the Er doping in Ho1−xErxNi2, the RC also reduces, but it is still high, namely, ~ 45 J/kg and ~ 102 J/kg for a field change of 1 and 2 T, respectively.
The second parameter is defined as ǀΔSmagǀ(max) × δTFWHM, where δTFWHM denotes the full width temperature span of ǀΔSmagǀ vs. T curve at its half maximum35. As the Er content increases, the RCP values decrease from 65 J/kg for Ho0.75Er0.25Ni2 to 58 J/kg for Ho0.25Er0.75Ni2 at the 1-T magnetic field change and from 155 J/kg for Ho0.75Er0.25Ni2 to 133 J/kg for Ho0.25Er0.75Ni2 at the 2-T magnetic field change. It should be noted that, in the case of the Ho0.5Er0.5Ni2 solid solution, there are slight deviations for both the obtained RC and RCP values from the expected ones.
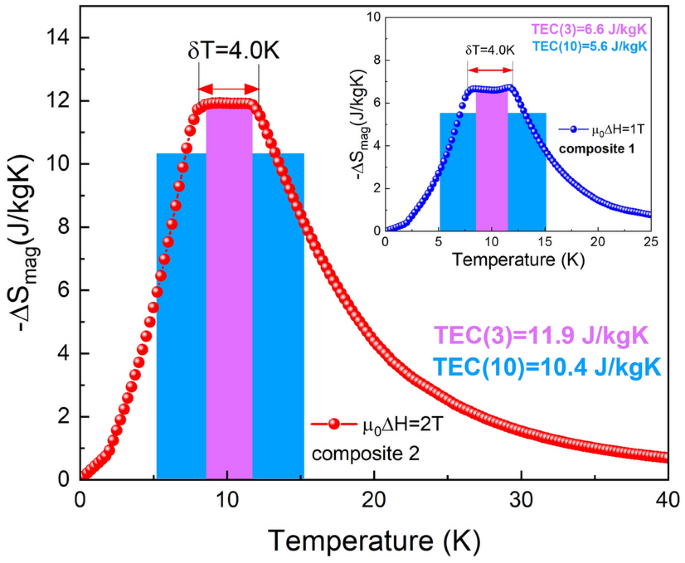
The third parameter, the temperature averaged entropy change (TEC), was introduced by Griffith et al.10 and the magnitude is calculated by the following formula:
where ΔTlift is the desired lift of temperature and Tmid is the temperature of the center of the TEC and is determined by maximizing the TEC value. Accordingly, two different ∆Tlift values of 3 and 10 K are chosen to calculate TEC for the Ho1−xErxNi2 solid solutions under study. The resulted values of TEC (3 K) and TEC (10 K) at µ0∆H = 1 T oscillate between 8.0–9.4 and 4.9–6.8 J/kgK and, at µ0∆H = 2 T, oscillate between 12.6–15.1 and 9.1–11.8 J/kgK, respectively.
The obtained values are of a high level and are comparable to those obtained for other promising low temperature magnetocaloric materials, such as TbNi236,37, DyNi237,38, ErNi2, HoNi220, Dy1−xErxNi225, Tb1−xHoxNi222, TmCoAl39, ErRu2Si240, or HoNi2B2C41.
Due to the fact that the ideal Ericsson cycle employs a constant value of ΔSmag in the temperature range of refrigeration, which is necessary for improving regeneration processes, composite materials were considered. It is expected that a composite material formed by at least two magnetic Ho1-xErxNi2 compounds differing in the Er concentration could exhibit a “table-like” behavior of MCE in a wider temperature range. In this context, according to a procedure proposed in Refs.20,42,43, numerical simulations were done to construct a composite material formed by Ho1−xErxNi2 compounds. The isothermal magnetic entropy change of a magnetic composite ǀΔSmagǀcomp based on N kinds of magnetic materials is equal to the sum of their magnetic entropy changes ǀΔSmagǀj weighted by a molar ratio yj. In our case, for a magnetic field change of 0–1 T (composite 1), optimal molar ratios are y1 = 0.599 for Ho0.25Er0.75Ni2, y2 = 0.046 for Ho0.5Er0.5Ni2, and y3 = 0.355 for Ho0.75Er0.25Ni2, while, in the case of a magnetic field change of 0–2 T (composite 2), two compounds are sufficient with y1 = 0.706 for Ho0.25Er0.75Ni2 and y2 = 0.294 for Ho0.75Er0.25Ni2.
Figure 5 shows the calculated isothermal magnetic entropy changes for the composite based on Ho1−xErxNi2 compounds, which are obtained for magnetic field changes of 1 and 2 T. It should be noted that, both in 1- and 2-T magnetic field changes, the maximum magnetic entropy change of the composite material exhibits an almost constant value of ǀΔSmagǀcomp that is around 6.7 J/kgK for µ0ΔH = 1 T and 12 J/kgK for µ0ΔH = 2 T. For both composites, calculated ǀΔSmagǀcomp remains almost unchanged in a temperature range of 8 to 12 K. These results suggest that, in order to design the appropriate composition of a refrigerant, it is necessary to evaluate the corresponding optimal molar ratios using the value of external magnetic field change at which the refrigerator should operate. To compare the magnetocaloric performance of the proposed composites with that of their constituents, the values of RC, RCP, and TEC have been calculated. The magnitudes computed by the methods described earlier for both composites are of a high level and are comparable to those of the individual solid-solution constituents; the value of RC(RCP) for composite 1 (µ0ΔH = 1 T) is equal to 57(67) J/kg and, for composite 2 (µ0ΔH = 2 T), it is 122(150) J/kg. The TEC(3) values obtained for both composites are comparable to their maximum isothermal magnetic entropy change values, which result directly from the scope of ∆Tlift values. In the case of TEC(10), the values are slightly smaller in comparison with TEC(3); however, they are still of a high level and comparable to those of the solid solution constituents (see Table 2).
Evaluation of the magnetocaloric effect with direct measurements
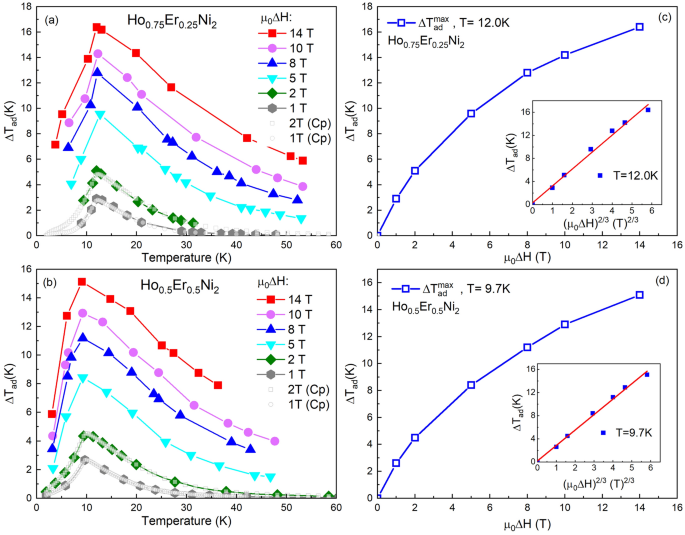
The adiabatic temperature change ΔTad caused by the magnetic field change µ0ΔH, i.e., the magnetocaloric effect, has been additionally determined by direct temperature measurements in the range of magnetic fields up to 14 T. Figures 6a,b show experimental ΔTad vs. the initial temperature, as obtained in the magnetizing process and for comparison, derived from heat capacity data, for Ho0.75Er0.25Ni2 and Ho0.5Er0.5Ni2, respectively. The initial field was zero in all cases. Note, that the results are very similar for both methods. As expected, the increase of the applied magnetic field leads to an increase in ΔTad. The maximum value of ΔTad at µ0ΔH = 14 T reaches 16.4 K at TC for Ho0.75Er0.25Ni2, and 15.1 K at TC for Ho0.5Er0.5Ni2. The maxima of ΔTad obtained at 1- and 2-T magnetic field changes by both direct and indirect methods have been detected at the same temperature and the determined values are in good agreement.
Temperature dependences of the adiabatic temperature change, ΔTad, as obtained from the heat capacity data (filled symbols) and from direct measurements (open symbols) for Ho0.75Er0.25Ni2 (a) and Ho0.5Er0.5Ni2 (b) at different magnetic field changes µ0ΔH and maximum adiabatic temperature change, ΔTadmax, for Ho0.75Er0.25Ni2 (c), Ho0.5Er0.5Ni2 (d) as a function of the magnetic field change, µ0ΔH. Insets show the ΔTad as a function of (µ0ΔH)2/3. Solid lines present the relation ΔTad = A(µ0ΔH)2/3, with A listed in Table 3.
Directly measured maximum ΔTad as a function of the final magnetic field is plotted in Fig. 6c,d. For both Ho0.75Er0.25Ni2 and Ho0.5Er0.5Ni2 solid solutions, ΔTad grows nonlinearly with increasing µ0ΔH. Characteristic quantity ΔTad/µ0ΔH decreases from 2.8 K/T at 1 T to 1.2 K/T at 14 T for Ho0.75Er0.25Ni2, and from 2.7 K/T at 1 T to 1.1 K/T at 14 T for Ho0.5Er0.5Ni2.
Experimental results can be interpreted within the framework of the thermodynamic Landau theory. According to this theory, the equation for the magnetization of paraprocess near the Curie temperature can be written as44
were α and β are the thermodynamic Landau coefficients and M is magnetization. The expression for MCE caused by an adiabatic change of magnetization is
Near the Curie temperature, the β coefficient is only weakly dependent on temperature and therefore the temperature derivative from Eq. (4) equals to
Substituting Eq. (6) into Eq. (5) we obtain
Integration of the expression(7) leads to
Thus, MCE must obey the law of proportionality to the squared magnetization in the region of paraprocess 44
where \(k = \frac{{\alpha_{1} T}}{{2_{M,P} }}\). This was confirmed experimentally by Weiss and Piccard 45. The magnetic field dependence of ΔT can be described by the equation of state following from the thermodynamic Landau theory
As is seen from Eq. (10), ΔT ~ H/ΔT1/2 or ΔT ~ H2/3. To check the applicability of the thermodynamic Landau theory for the description of our experimental results, the adiabatic temperature change ΔTad was plotted as a function of (µ0ΔH)2/3, as is shown in insets in Fig. 6c,d. The linear behavior of the dependences for both investigated compounds near their Curie temperature demonstrates a good agreement between the experimental results and thermodynamic Landau theory.
By plotting the maximum ΔTad value versus (µ0ΔH)2/3 and using an equation: ΔTad = A(µ0ΔH)2/3, where A is a characteristic parameter of magnetocaloric materials, one can obtain information about the magnetocaloric properties of investigated samples46. By fitting the experimental data, we find A = 2.9 K/T2/3 for Ho0.75Er0.25Ni2 and A = 2.6 K/T2/3 for Ho0.5Er0.5Ni2. These values are comparable with those obtained for the parent compounds and other binary Laves-phase compounds and are also comparable with the values of the most efficient magnetic refrigerants, such as Gd (A = 3.83 K/T2/3) and LaFe11.2Si1.8 (A = 2.16 K/T2/3)46. The data obtained by direct measurements are gathered in Table 3.
Discussion
The present study, by an example of Ho1−xErxNi2, develops the concept of magnetocaloric efficiency of the rare-earth Laves-phase solutions starting from their magneto thermodynamic properties and then proceeds illustrating their potentiality as components of magnetocaloric composites.
The analysis of the structural data obtained for the Ho1−xErxNi2 solid solutions confirms the similarity of the structures of the parent HoNi2 and ErNi2 binary compounds and the Ho diluted compound with x = 0.25, which have the regular C15 cubic structure (Laves phase). The subsequent introduction of Ho to x = 0.5 and 0.75 leads to the formation of a cubic superstructure that is due to a regular arrangement of vacancies at rare earth sites and decreases the crystal lattice symmetry (space group F-43 m). The superstructure is characterized by the doubled lattice parameter.
The measurements of the heat capacity were performed for the compounds, the phase and chemical compositions of which were well characterized. The appearance of Er atoms in the rare-earth sublattice results in the common magnetic dilution consisted in weakening the exchange interactions, which is accompanied by the decrease in the ordering temperature of the Ho1−xErxNi2 system. Thus, its linear variations, namely, decrease in the Curie temperature of the system from 12.0 K (for Ho0.75Er0.25Ni2) to 7.7 K (for Ho0.25Er0.75Ni2) are realized by the mutual substitutions of rare-earth components.
The magnetothermodynamics properties of the three-component solid solutions were characterized by indirect evaluation and direct measurements of magnetocaloric potentials in a wide range of magnetic fields. The possibility of precise tailoring the magnetocaloric potentials to a certain temperature range was demonstrated. As the Er content increases, the maximum magnetic entropy change decreases from 16.2 J/kgK for Ho0.75Er0.25Ni2 at 12 K and reaches 13.7 J/kgK at 7.7 K for Ho0.25Er0.75Ni2 for a magnetic field change of 2 T. The maximum adiabatic temperature change ΔTad for Ho0.75Er0.25Ni2 in the 2-T magnetic field change is equal to 4.9 K at 12 K, and with increasing Er content, the ΔTad value decreases to 3.9 K for Ho0.25Er0.75Ni2 in the vicinity of TC. The maximum values of the adiabatic temperature change, determined by the direct measurements, reach 16.4 K near 12.0 K for Ho0.75Er0.25Ni2 and 15.1 K near 9.7 K for Ho0.5Er0.5Ni2 at µ0ΔH = 14 T. The maximum values of ΔTad, obtained at 1- and 2-magnetic fields, obtained by direct and indirect methods are in good agreement. The directly measured adiabatic temperature changes near the Curie temperature in high magnetic fields were compared with the values obtained based on the Landau theory for the second-order phase transitions. It was demonstrated that, the magnetic field dependence of ΔTad obeys the (µ0ΔH)2/3 function with the parameter A, which characterizes intrinsic properties of refrigerants, and the Landau theory of second-order phase transitions is applicable for ΔTad description in high magnetic fields.
Additionally, the availability of the magnetocaloric potentials experimentally estimated for the individual three-component Ho1−xErxNi2 solid solutions allows us to simulate optimal molar ratios to construct the composites to be considered as a refrigerant material in magnetic refrigerators performing an Ericsson cycle at low temperatures. These theoretical results based on the real empirical data are very optimistic and are of interest to perform further experimental studies. The results of the simulation indicate that the proposed composite exhibits a high potential for the application in magnetic refrigeration devices, especially in the cryogenic temperature range.
Methods
Sample preparation, structural analysis, heat capacity, and direct magnetocaloric measurements
Ho1-xErxNi2 alloys with x = 0.25, 0.5, and 0.75 were prepared by repeated arc-melting of appropriate amounts of starting metals in a high-purity argon atmosphere at a pressure of 1.5 atm; starting metallic components of at least 99.9 wt.% (rare-earths) and 99.99 wt.% (Ni) purity were used. The obtained ingots were wrapped separately with Mo foil and subsequently subjected to homogenizing annealing in an argon-filled quartz tube. The annealing process was performed at 1123 K for one month; subsequently, the ingots were subjected to slow furnace cooling to room temperature to ensure their uniform cooling, exclude the fixation of high-temperature structural state of compounds, and to obtain their equilibrium state. The elemental composition was assessed by energy dispersive X-ray (EDX) spectroscopy with the simultaneous study of the sample’s microstructure by scanning electron microscopy (SEM) using an FEI Nova Nano SEM 230 scanning electron microscope (operating at an accelerating voltage of 20 kV) equipped with an energy-dispersive spectrometer (EDAX Genesis XM4). The crystal structure was determined by X-ray diffraction (XRD) analysis, which was carried out at room temperature using powdered samples and an Ultima IV Rigaku (Japan) diffractometer equipped with a “D/teX” high-speed semiconductor detector. X-ray diffraction patterns were taken in an angular range of 9–1000 at a step of 0.020 using CuKα radiation and a fluorescent correction regime.
Heat capacity was measured in the 2–100 K temperature range in zero, 1- and 2-T magnetic fields by a relaxation method using a PPMS-14 installation (Quantum Design, USA). The direct measurements of ΔTad were performed in the 4.2–50 K temperature range in magnetic fields up to 14 T using the original setup, which is based on the extraction method and allows us to perform direct measurements of the adiabatic temperature change 47. Steady magnetic fields up to 14 T were generated by a Bitter-type magnet, and a maximum field-change rate of ~ 6 T/s was obtained by moving the sample in and out of the applied magnetic field.
References
Weiss, P. & Piccard, A. Le ph´enom`ene magn´etocalorique. J. Phys. 7, 103–109 (1917).
Debye, P. Einige bemerkungen zur magnetisierung bei tiefertemperatur. Ann. Phys. 81, 1154–1160 (1926).
Giauque, W. F. A thermodynamic treatment of certain magnetic effects. A proposed method of producing temperatures considerably below 18 absolute. J. Am. Chem. Soc. 49, 1864–1870 (1927).
Tishin, A. M. & Spichkin, Y. I. Recent progress in magnetocaloric effect: Mechanisms and potential applications. Int. J. Refrig. 37, 223–229 (2014).
Liu, J., Gottschall, T., Skokov, K. P., Moore, J. D. & Gutfleisch, O. Giant magnetocaloric effect driven by structural transitions. Nat. Mater. 11, 620–626 (2012).
Gschneidner, K. A. Jr., Mudryk, Y. & Pecharsky, V. K. On the nature of the magnetocaloric effect of the first-order magnetostructural transition. Scr. Mater. 67, 572–577 (2012).
de Oliveira, N. A. & von Ranke, P. J. Theoretical aspects of the magnetocaloric effect. Phys. Rep. 489, 89–159 (2010).
Tegus, O., Brück, E., Buschow, K. H. J. & de Boer, F. R. Transition-metal-based magnetic refrigerants for room-temperature applications. Nature 415, 150–152 (2002).
Kitanovski, A. et al. Magnetocaloric Energy Conversion (Springer International Publishing, Switzerland, 2015).
Griffith, L. D., Mudryk, Y., Slaughter, J. & Pecharsky, V. K. Material-based figure of merit for caloric materials. J. Appl. Phys. 123, 034902 (2018).
Delsante, S., Stifanese, R. & Borzone, G. Thermodynamic stability of RNi2 Laves phases. J. Chem. Thermodynamics. 65, 73–77 (2013).
Latroche, M., Paul-Boncour, V. & Percheron-Guegan, A. Structural instability in R1−xNi2 compounds and their hydrides (R = Y, Rare Earth). Z. Phys. Chem. 179, 261–268 (1993).
Gratz, E. et al. Temperature- and pressure-induced structural transitions in rare-earth-deficient (R = Y, Sm, Gd, Tb) Laves phases. J. Phys. Condens. Matter 8, 8351 (1996).
Klimyenko, A. et al. Structure of LaNi2.286 and the LaNi system from LaNi1.75 to LaNi2.50. J. Less Common Met. 144, 133–141 (1988).
Lindbaum, A., Gratz, E. & Heathman, S. Pressure-induced order-disorder transitions in RNi2 compounds. Phys. Rev. B 65, 134114 (2002).
Latroche, M., Paul-Boncour, V., Percheron-Guegan, A. & Achard, J. C. Structure determination of Y0.95Ni2 by X-ray powder diffraction. J. Less Common Met. 161, L27–L31 (1990).
Kirchmayr, H. R. & Burzo, E. in: H. P. J. Wijn (Ed.), Landolt–Börnstein, New Series III/19d2, Berlin, (1990).
Wallace, W. E. & Segal, E. In Rare earth intermetallics (eds Alper, A. M. et al.) (Academic Press, New York, 1973).
Tishin, A. M. & Spichkin, Y. I. The magnetocaloric effect and its applications (Institute of Physics Publishing, Philadelphia, 2003).
Ćwik, J., Koshkid’ko, Y., Nenkov, K., Tereshina, E. A. & Rogacki, K. Structural, magnetic and magnetocaloric properties of HoNi2 and ErNi2 compounds ordered at low temperatures. J. Alloys Compd. 735, 1088–1095 (2018).
Hashimoto, T. et al. New application of complex magnetic materials to the magnetic refrigerant in an Ericsson magnetic refrigerator. J. Appl. Phys. 62, 3873 (1987).
Ćwik, J. et al. Magnetocaloric prospects of mutual substitutions of rare-earth elements in pseudobinary Tb1−xHoxNi2 compositions (x = 0.25–0.75). J. Alloys Compd. 886, 161295 (2021).
Ćwik, J., Koshkid’ko, Y., de Oliveira, N. A., Mikhailova, A. & Nenkov, K. Effect of composition changes on the structural, magnetic and thermodynamic properties in Tb1-xDyxNi2 intermetallic compounds. J. Alloys Compd. 769, 588–596 (2018).
Palewski, T. et al. Magnetic properties and specific heat of RNi2 compounds (R = Sc, Y, La, Lu, Ho). Phys. Met. Metallogr. 99, S113–S115 (2005).
Ćwik, J. et al. Experimental and theoretical analysis of magnetocaloric behavior of Dy1−xErxNi2 intermetallics (x = 0.25, 0.5, 0.75) and their composites for low-temperature refrigerators performing an Ericsson cycle. Phys. Rev. B 103, 214429 (2021).
Ćwik, J., Palewski, T. & Nenkov, K. Specific heat of the Tb1-xLaxNi2 solid solutions. Acta Phys. Pol. A 113, 343–346 (2008).
Ćwik, J. et al. Magnetic properties and magnetocaloric effect in Dy1-xScxNi2 solid solutions. J. Alloys Compd. 506, 626–630 (2010).
Ibarra, M. R., del Moral, A. & Abell, J. S. Magnetic anomalies of thermal expansion in (rare-earth) Ni2 intermetallic compounds. J. Phys. Chem. Solid. 45, 789–795 (1984).
Pecharsky, V. K., Gschneidner, K. A. Jr., Pecharsky, A. O. & Tishin, A. M. Thermodynamics of the magnetocaloric effect. Phys. Rev. B 64, 144406 (2001).
Lea, K. R., Leask, M. J. M. & Wolf, W. P. The raising of angular momentum degeneracy of f-Electron terms by cubic crystal fields. J. Phys. Chem. Solids 23, 1381–1405 (1962).
Ćwik, J. et al. Magnetic properties and specific heat of Dy1−xLaxNi2 compounds. J. Magn. Magn. Mater. 321, 2821–2826 (2009).
Gschneidner, K. A., Pecharsky, V. K., Gailloux, M. J. & Takeya, H. Utilization of the magnetic entropy in active magnetic regenerator materials. Adv. Cryog. Eng. 42, 465–474 (1996).
Gschneidner, K. A. Jr. et al. Recent developments in magnetic refrigeration. Mater. Sci. Forum 315–317, 69–76 (1999).
Franco, V. et al. Magnetocaloric effect: From materials research to refrigeration devices. Prog. Mater. Sci. 93, 112–232 (2018).
Gorria, P. et al. Relative cooling power enhancement in magneto-caloric nanostructured Pr2Fe17. J. Phys. D: Appl. Phys. 41, 192003 (2008).
Sánchez Llamazares, J. L. et al. Magnetic entropy change and refrigerant capacity of rapidly solidified TbNi2 alloy ribbons. J. Appl. Phys. 113, 17A912 (2013).
Ćwik, J. et al. Magnetocaloric effect in Laves-phase rare-earth compounds with the second-order magnetic phase transition: Estimation of the high-field properties. Acta Mater. 133, 230–239 (2017).
von Ranke, P. J., Pecharsky, V. K. & Gschneidner, K. A. Jr. Influence of the crystalline electrical field on the magnetocaloric effect of DyAl2, ErAl2, and DyNi2. Phys. Rev. B. 58, 12110 (1998).
Mo, Z. J. et al. Magnetic property and magnetocaloric effect in TmCoAl compound. Intermetallics 56, 75–78 (2015).
Samanta, T., Das, I. & Banerjee, S. Giant magnetocaloric effect in antiferromagnetic ErRu2Si2 compound. Appl. Phys. Lett. 91, 152506 (2007).
Li, L. et al. Large magnetic entropy change in Dy1-xHoxNi2B2C (x = 0–1) superconductors. Appl. Phys. Exp. 4, 093101 (2011).
Hashimoto, T. et al. A new method of producing the magnetic refrigerant suitable for the Ericsson magnetic refrigeration. IEEE Trans. Magn. 23, 2847–2849 (1987).
Smaili, A. & Chahine, R. Composite magnetic refrigerants for an ericsson cycle: New method of selection using a numerical approach. Adv. Cryog. Eng. 42, 445–450 (1996).
Belov, K. P. Magnetic Transitions, Consultants Bureau (Ed.), New York (1961).
Weiss, P. & Piccard, A. Sur un nouveau phe´nome`ne magne´tocalorique. Compt. Rend Ac. Sci. 166, 352–354 (1918).
Kuz’min, M. D. et al. Magnetic field dependence of the maximum adiabatic temperature change. Appl. Phys. Lett. 99, 012501 (2011).
Koshkid’ko, Y. et al. Magnetocaloric properties of Gd in fields up to 14 T. J. Magn. Magn. Mater. 433, 234–238 (2017).
Acknowledgements
The work was supported by the National Science Center, Poland through the OPUS Program under Grant No. 2019/33/B/ST5/01853.
Author information
Authors and Affiliations
Contributions
J.Ć.-Concept , Project administration, Resources, Methodology, Formal analysis, Writing - preparing original draft. Y.K.-Investigation, Formal analysis, Methodology. K.N., E.T.C., M.M., B.W and K.K.-Investigation, Formal analysis. All authors reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ćwik, J., Koshkid’ko, Y., Nenkov, K. et al. Magnetocaloric performance of the three-component Ho1-xErxNi2 (x = 0.25, 0.5, 0.75) Laves phases as composite refrigerants. Sci Rep 12, 12332 (2022). https://doi.org/10.1038/s41598-022-16738-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-16738-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.