Abstract
The ascomycete fungus Ophidiomyces ophiodiicola (Oo) is the causative agent of ophidiomycosis (Snake Fungal Disease), which has been detected globally. However, surveillance efforts in the central U.S., specifically Texas, have been minimal. The threatened and rare Brazos water snake (Nerodia harteri harteri) is one of the most range restricted snakes in the U.S. and is sympatric with two wide-ranging congeners, Nerodia erythrogaster transversa and Nerodia rhombifer, in north central Texas; thus, providing an opportunity to test comparative host–pathogen associations in this system. To accomplish this, we surveyed a portion of the Brazos river drainage (~ 400 river km) over 29 months and tested 150 Nerodia individuals for the presence of Oo via quantitative PCR and recorded any potential signs of Oo infection. We found Oo was distributed across the entire range of N. h. harteri, Oo prevalence was 46% overall, and there was a significant association between Oo occurrence and signs of infection in our sample. Models indicated adults had a higher probability of Oo infection than juveniles and subadults, and adult N. h. harteri had a higher probability of infection than adult N. rhombifer but not higher than adult N. e. transversa. High Oo prevalence estimates (94.4%) in adult N. h. harteri has implications for their conservation and management owing to their patchy distribution, comparatively low genetic diversity, and threats from anthropogenic habitat modification.
Similar content being viewed by others
Introduction
Emerging infectious diseases (EIDs) have become a serious threat to wildlife with several outbreaks across broad taxonomic groups1. Prime examples of fungal infections in wildlife across the globe include chytridiomycosis caused by Batrachochytrium dendrobatidis2,3,4 or B. salamandrivorans5,6,7 in amphibians, as well as white-nose syndrome caused by Pseudogymnoascus destructans infections in bats8,9,10,11. Similarly, the ascomycete fungus Ophidiomyces ophiodiicola (Oo) is the causative agent of ophidiomycosis (Snake Fungal Disease), an EID in North America and Europe that was also recently detected in Asia12,13,14,15. Signs of infection first appear as dermatitis, and if not cleared, infections will advance to lesions, ulcers, and tissue necrosis—the latter of which can precede death12,16.
In North America, Oo was first detected in 2008 among wild populations of eastern massasaugas (Sistrurus catenatus) from Illinois14. At the population level, low genetic diversity and climatic factors in combination with a disease that had signs similar to ophidiomycosis were later thought to have contributed to the decline of an isolated population of timber rattlesnakes (Crotalus horridus) in New Hampshire12,17. The pathogen has since been detected across the contiguous United States in numerous species throughout the Midwest12, East Coast18,19,20,21, California22, Arizona (E. Nowak, unpublished data), and most recently, Texas. Prior to this study, two reports from the Texas Parks and Wildlife Department confirmed Oo infections in Nerodia harteri harteri, the Brazos water snake (or Brazos River Watersnake), in 2016 and in an Eastern Patch-nosed Snake (Salvadora grahamiae) in 2017 (Dryad repository: https://doi.org/10.5061/dryad.t76hdr83p). Owing to a lack of surveillance efforts, however, little is known regarding the host–pathogen associations of ophidiomycosis in the state.
Confirmed infections of Oo across multiple snake genera suggest low host specificity21,23. Thus, this pathogen presents a potential concern regarding biodiversity and conservation in Texas, as the state harbors the highest diversity of snakes in the U.S.24,25,26,27 with 76 documented species and 115 varieties—if subspecies are included—seven of which are considered state threatened species28. Nerodia harteri is among those species and also is one of two snake species endemic to Texas. Both of its subspecies N. h. harteri and N. h. paucimaculata, the Concho water snake (or Concho River Watersnake)29, show high affinity for water bodies and only inhabit disjunct stretches of the Brazos and Colorado River basins, respectively30,31,32.
Historically, both subspecies of N. harteri have been affected by anthropogenic influences such as habitat destruction and variable water flow regimes from dam releases31,33. Nerodia h. paucimaculata was listed as federally threatened in 198634 and then delisted in 2011 owing to a recovery in their populations35. Nerodia h. harteri was initially petitioned for federal listing in 198435 and, although the U.S. Fish and Wildlife Service found that listing was not warranted after a 12-month review in 198536, the snake remained on the candidate species list until 199437. More recent studies detected declines in overall abundance and evidence of low genetic diversity in N. harteri30,33. In contrast, two non-threatened sympatric congeners, Diamond-backed water snakes (N. rhombifer) and Blotched water snakes (N. erythrogaster transversa), have much larger ranges29,38, attain larger sizes, can be more fecund, and are more common39; thus, providing a suitable system to test differences in host–pathogen associations among species that differ in their ecological attributes and conservation status.
Factors that contribute to the probability of Oo infection and mortality among individuals and species vary considerably, although some studies have shown climate and season are associated with incidence and severity of infection12,17,18,40,41,42. However, interspecific43 and demographic factors18 associated with pathogen prevalence are still understudied. One study presented evidence of interspecific Oo prevalence differences in Georgia (USA)43, and another documented vertical transmission of Oo from infected mothers to neonates among viviparous and oviparous snakes44. However, there are no published studies assessing the role of interspecific and demographic factors in the distribution of Oo in Texas snake populations. Assessments of Oo prevalence (i.e., the proportion of Oo-positive snakes among a subset of a population), geographic distribution of the pathogen, and pathogen-associated abiotic (i.e., temperature and rainfall) and biotic factors (i.e., species and demography) are needed in this region—especially among endemic species with small populations like N. harteri17.
We address the paucity of surveillance data for Oo in Texas by conducting the first population-level survey of Oo prevalence among Nerodia species within the Brazos River basin. Our goals were to: (1) specifically survey areas with historically abundant populations of N. h. harteri; (2) estimate the geographic distribution of Oo in the upper Brazos River basin; and (3) test for associations between biotic and abiotic factors and Oo infection prevalence estimates among Nerodia inhabiting those areas. We predicted that N. h. harteri would be more susceptible to Oo infections on account of their comparatively smaller population sizes. Our study seeks to increase understanding of the distribution of Oo in this region of the U.S. and comparative Oo infection associations among sympatric Nerodia. We also aim to provide key ecological and epidemiological data for the future management of threatened N. h. harteri populations.
Results
In total, we captured, visually inspected, and swabbed 150 snakes (Table 1). We collected 76 N. rhombifer (33 males, 41 females, 2 undetermined sex), 34 N. e. transversa (11 males, 15 females, 8 undetermined sex), and 40 N. h. harteri (17 males, 20 females, 3 undetermined sex). During our surveys, we recaptured six snakes: one N. rhombifer, four N. e. transversa, and one N. h. harteri. The N. rhombifer and N. e. transversa were sampled, released, and recaptured during the same sampling trip, and the single N. h. harteri was recaptured 14 days after the initial capture. The average snout-vent length (SVL) for N. rhombifer, N. e. transversa, and N. h. harteri was 63.78 cm (SD = 30.06), 51.20 cm (SD = 21.42), and 40.31 cm (SD = 17.16), respectively. The average mass for N. rhombifer, N. e. transversa, and N. h. harteri was 249.26 g (SD = 231.20), and 143.78 g (SD = 134.17), and 62.30 g (SD = 62.22), respectively.
We detected potential signs of infection (SOI, see Methods for definition; Fig. 1) on 30 N. rhombifer (26 adults, 1 subadult, 3 juveniles), 8 N. e. transversa (5 adults, 3 subadults), and 13 N. h. harteri (11 adults, 2 subadults). We detected Oo on 39 of the 51 snakes with SOI (20 N. rhombifer, 6 N. e. transversa, and 13 N. h. harteri). We also detected Oo on 30 snakes not exhibiting any apparent SOI (16 N. rhombifer, 7 N. e. transversa, and 7 N. h. harteri) (Table 1). We detected Oo on 11 of the 13 snakes with SOI that were subset for skin biopsies. When we compared Oo detections from swab and biopsy extractions, there were 11 out of 11 detections from swab extractions and 9 out of 11 detections from the skin biopsy extractions. Both swab and skin biopsy qPCR tests agreed on the two negatives that were observed in this subset.
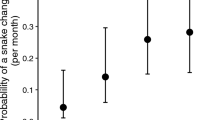
Our estimated Oo prevalence for N. rhombifer, N. e. transversa, and N. h. harteri was 47.4% (CI: 36.5–58.5%), 38.2% (CI: 23.6–55.2%), and 50.0% (CI: 35.0–65.0%), respectively, and overall, our uncorrected Oo prevalence was 46.0% (CI: 38.2–54.0%) (Table 1). The false-negative rate was 0 for swab samples and 0.153 for the skin biopsies, and the Bayesian approximation of corrected Oo prevalence was 48.2% (CI: 40.5–56.4%) among sampled Nerodia species. Adult N. h. harteri showed the highest estimate of Oo prevalence (94.4%; CI: 69.3–99.2%; Fig. 2; Table 1). We captured more snakes in May and June during their peak activity (Fig. 3).
Number of wild-caught adults and total sample size (all) for three Nerodia species tested for the presence of Ophidiomyces ophiodiicola via qPCR indicated by gray bars. Closed circles represent estimates of prevalence for each species and a subset of adults, with black lines showing the 95% confidence intervals.
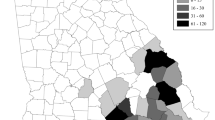
Geographically, we detected Oo at 13 of the 21 sites (Fig. 4) and in all eight counties sampled. Our county-level prevalence estimates ranged from 25.0% (Hill Co.) to 75.0% (Bosque Co.) (Table 2). We found a significant association between signs of infection and Oo occurrence (Fishers Exact Test, P < 0.001) but not between species and SOI (P > 0.20) nor between sex and Oo occurrence (P > 0.40). We also detected significant differences in body condition (BCI) values among adult N. e. transversa with and without signs of infection (Kruskal–Wallis test, P < 0.01; Table 3).
Map of sampling sites demarcated with open circles. Cross-hatching indicates the historical range of Nerodia harteri harteri at the county level within the Brazos River drainage designated by the shaded area. Each pie chart represents the relative proportion of Nerodia that tested positive or negative for Ophidiomyces ophiodiicola via qPCR. The size of the pie chart represents the relative number of snakes collected per site. QGIS version 3.16.3 (https://www.qgis.org/en/site/) was used to generate the map.
The most parsimonious model explaining Oo occurrence included species and life stage with temporal autocorrelation accounted for by sample month as a first order autoregressive [AR(1)] random effect (Supplementary Table S1). The model indicated that N. h. harteri were more likely to be infected than N. rhombifer (β = –1.28; SE = 0.64), while juvenile snakes (β = − 2.87; SE = 0.66) and subadults (β = − 2.00; SE = 0.57) were less likely to be infected than adults (Supplementary Tables S2 and S3).
Our modeled average probability of infection for N. rhombifer, N. e. transversa, and N. h. harteri was 20.7% (CI: 9.20–40.3%), 25.7% (CI: 11.1–48.9%), and 48.4% (CI: 25.9–71.6%), respectively (Supplementary Table S4). The modeled average probability of infection at the juvenile, subadult, and adult stage was 11.2% (CI: 4.0–27.7%), 23.2% (CI: 9.6–46.2%), and 69.1% (CI: 48.9–83.9%), respectively (Supplementary Table S5). Adult N. h. harteri had the highest value of estimated probability of infection (82.7%, CI: 60.9–93.6%) (Supplementary Table S6).
Discussion
Ophidiomycosis has been increasingly detected in North America across a broad range of host species of snakes, which includes some taxa of conservation concern14,17,23,45. The initiation of pathogen surveillance efforts is an important step in determining the host–pathogen associations among potentially susceptible taxa. In this study, we addressed a major wildlife disease surveillance gap in Texas snakes by focusing on the upper Brazos River drainage, because it hosts the endemic and threatened Brazos water snake. To provide comparative Oo prevalence data, we also sampled two more common and widespread sympatric congeners. Owing to the negative consequences of small population sizes (e.g., compromised immunity due to inbreeding depression), we predicted that N. h. harteri could be more susceptible to Oo infections; thus, we used estimates of Oo prevalence and associated SOI among these sympatric species to test this prediction. Previous Oo surveys have reported prevalence estimates ranging from 1.8 to 66.5%18,46,47,48,49. We observed moderately high prevalence across all samples (46.0%) as well as at the species level (38.2–50.0%). Prevalence estimates among adults were higher in N. h. harteri than in N. rhombifer, which partially supports our prediction regarding the greater susceptibility of N. h. harteri to Oo infections.
Detection of ophidiomycosis
Signs of infection were a strong indicator of Oo occurrence, which confirms Oo infections in Nerodia populations from the upper Brazos River and its tributaries, and is potentially more widespread in this region given the range size of N. rhombifer and N. e. transversa. Even though we did not detect snakes that were moribund owing to severe infections in this population sample, some exhibited signs of an advancing infection (see Fig. 1). We have, however, also confirmed the presence of Oo on emaciated, moribund snakes from other parts of Texas—specifically, Coluber constrictor, N. e. transversa, and Agkistrodon piscivorus (Dryad repository: https://doi.org/10.5061/dryad.t76hdr83p). Therefore, we are confident that the advanced infections observed in our study area likely represent progressing ophidiomycosis.
Subsampling for histopathological detection of Oo infections, and confirmation of ophidiomycosis, would be useful, albeit not tractable for N. h. harteri owing to its low abundance, conservation status, and the lack of detectable moribund individuals in this study. We have demonstrated that using a single swab with multiple strokes across the body is cost-efficient and suitable for distinguishing between SOI owing to Oo or other reasons, and serves as an alternative to using multiple swabs per snake. The use of multiple swabs decreases the probability of false-negatives47 and would be beneficial in populations with lower Oo prevalence than we estimated in this region. In 12 snakes that exhibited potential SOI but did not test positive for the presence of Oo, it is likely those represent an injury, a cleared infection, other infection type, or a false negative. A larger subsample would allow for better estimates of false-negatives and our estimates of Oo prevalence likely represent minimum values. However, considering our estimated false-negative rate for these data (~ 15%), we can assume the number of true false-negatives in this sample is relatively low (2 samples), and the estimate of previously injured but not Oo infected snakes is approximately 6.70% in our overall sample.
Abiotic and biotic predictors of Oo detection
With the exception of adult N. e. transversa, our analyses did not detect differences in BCI between snakes with or without the presence of Oo, or with or without signs of infection (Table 3). These results are consistent with other reports that compared body condition among infected and non-infected snakes in laboratory settings16 and in the wild18,46. On the other hand, some studies have shown general body condition decreased with increased severity of ophidiomycosis14,40,42. We did not observe that general pattern, which might be an artifact of the aquatic habitat these species use leading to decreased encounters of moribund or dead individuals. Additionally, capturing dying snakes is presumably difficult, as they are less active than healthy snakes. For instance, behavioral observations of wild eastern massasauga rattlesnakes showed that infected individuals moved less and remained concealed more often than their uninfected counterparts50. Conversely, other studies of free-ranging pygmy rattlesnakes (Sistrurus miliarus) showed severity of Oo infection fluctuated and was not related to the probability of capture40. Therefore, the lack of moribund individuals in our sample could result from low rates of mortality among infected individuals, disease induced changes in behavior, or simply attributable to the stochastic nature of encounters in dynamic aquatic habitats.
Our results showed adult snakes were more likely to be infected than subadults and juveniles (Fig. 2; Supplementary Table S5). In general, Nerodia emerge from hibernation in early spring and are active through early summer38; thus, we consider it possible that seasonal patterns and reproductive behavior might have contributed to age-class differences in Oo prevalence. We sampled snakes with lesions as early as March (Fig. 3), before the mating season. Nerodia mate in mid to late spring and parturition occurs during late summer to early fall38,51,52,53. Surveys of a geographically proximal snake, N. h. paucimaculata, have found both single and small group brumation, but not large communal groups, likely owing to warmer, shorter winters in this region39,54. Therefore, the infected snakes we captured in March and April may have been infected through environmental transmission12 or possibly through secondary contact with infected snakes during brumation. Infected snakes collected in June might have acquired Oo via environmental transmission or contact with other infected snakes during reproduction events throughout April and May. The lower infection prevalence among the subadults and juveniles might have resulted from insufficient time in the environment to acquire infections and the lack of secondary contact during reproduction events. Although, a previous study has reported variable neonate mortality associated with Oo infection vertically transferred from the dam44. Thus, it is also possible that infected neonates are being removed from the population before an Oo infection can be detected.
Even though we did not include season as a factor in our analyses, our results are preliminarily consistent with other surveys for Oo suggesting that infected snakes are more likely to be encountered during the spring owing to fungal exposure during brumation through the winter months18,40,48,55. These observations highlight concerns that climatic factors (i.e., cooler, wetter weather) could act synergistically with Oo and increase the severity of infection17,42. Yet, mean monthly low temperatures and mean monthly precipitation were less parsimonious explanations to Oo occurrence in this study than species and life stage. The differences in Oo infection and severity among Texas snakes from different habitat types (i.e., terrestrial vs. aquatic) has yet to be tested; thus, expanded long-term surveillance of Oo infections and severity across years and habitat types will be informative in detecting patterns of seasonality.
Host identity
Consistent with our observations, other studies have shown that Oo prevalence differs intra-generically among Nerodia43. In our study, N. h. harteri exhibited higher Oo prevalence compared to N. rhombifer, which is concerning owing to the conservation status and rarity of N. h. harteri. With respect to N. h. harteri and N. e. transversa, the binomial 95% confidence intervals for Oo prevalence estimates overlapped, which could be interpreted as non-significance (Fig. 2); however, their relative abundance and distribution should be taken into consideration. For example, field surveys have reported that N. h. harteri were found in only ~ 300 km of stream and in two reservoirs within the Brazos River Drainage31,33. These reports concluded that both subspecies of N. harteri are amongst the most range-restricted snakes in the U.S. In contrast, the range of N. rhombifer spans 12 states in the U.S. and 9 states in Mexico38,56, and N. e. transversa is found in much of the eastern U.S.38. Both snakes are generally more frequently encountered than N. h. harteri, which we captured at only 7 of 21 sites. This patchy distribution is congruent with estimates from previous studies30,31,33. Comparatively, we captured N. rhombifer at 15 of 21 sites and N. e. transversa at 14 of 21 sites (Supplementary Fig. S1). Consequently, achieving captures at a rate needed to confer narrow confidence intervals for Oo prevalence estimates is difficult for relatively rare species like N. h. harteri. Yet, the number of N. h. harteri we captured during our survey (N = 40) was consistent with previous population surveys conducted over similar time intervals30,33; therefore, uncertainty of Oo prevalence estimates could also be remedied by long-term surveillance efforts for this taxon.
Conservation implications
Our survey data also suggest that N. h. harteri might now inhabit a more limited area within its historical range (Supplementary Fig. S1). Combined with a moderately high prevalence of Oo (47.4% overall, Table 1), this finding highlights the need for continued Oo surveillance and renewed conservation action planning. Previous studies have shown that both subspecies of N. harteri have low genetic diversity, and N. h. paucimaculata populations exhibit bottleneck signatures30,57. The application of additional sampling and genetic markers are needed, however, to facilitate robust tests for population contractions and their timing in N. harteri30,57. For instance, spatial ecology studies have shown N. h. paucimaculata can exhibit high site fidelity and are unlikely to move more than 1 km unless driven by stochastic factors such as variable water flow32. It is likely that N. h. harteri movement among sites is likewise rare, given their low detection probability, the barriers to movement within the Brazos River (i.e., dams or unsuitable habitat), and evidence of genetic population structure in N. h. paucimaculata, which shares many ecological attributes with N. h. harteri30,57.
Low genetic diversity, low dispersal potential, and high site fidelity are likely to increase inbreeding in populations of N. harteri. Inbreeding depression has been documented and implicated as a viable threat to snakes58 and in other systems59,60,61,62,63. Both inbreeding depression and an infection consistent with ophidiomycosis were attributed to population declines of timber rattlesnakes in New Hampshire17. Estimates of mortality among snakes infected with Oo vary12,14,40,41, and the contribution from inbreeding depression to increased risk of mortality from ophidiomycosis is still unknown. Conservation stakeholders should consider that inbreeding depression might act synergistically with other reported natural and anthropogenic stressors contributing to population declines in N. h. harteri and indeed, N. harteri sensu lato.
Our results have implications regarding the conservation status of N. h. harteri and indicate that ophidiomycosis is present in Brazos water snake populations. In the early 1980s, a proposal was submitted to the U.S. Fish and Wildlife Service to list both subspecies of N. harteri as endangered or threatened owing to their endemism and habitat loss64. However, it was not listed because the snake was consistently present in suitable habitat within reservoirs34,36. A population survey conducted from 2006 to 2008 reported the absence of snakes in areas where they were once historically abundant and concluded that interspecific competition, altered flow regimes, and negative effects from invasive species were likely contributing factors to low abundance33. Currently, N. h. harteri is listed by the state of Texas as threatened28, has a G1 (Critically) imperiled status (i.e., a very high risk of extinction65), and a Near Threatened status from the IUCN66. We have shown that ophidiomycosis is another potential threat to N. h. harteri populations and possibly other snake species in Texas—especially those with small population sizes. Thus, also initiating Oo surveillance in the Colorado River drainage among populations of N. h. paucimaculata is necessary to elucidate the host–pathogen associations for the species.
We marked snakes to avoid artificial inflation of snake counts and skewed estimates of pathogen prevalence, but this study was not designed to explicitly track disease outcomes. Thus, we cannot derive conclusions regarding neutral or negative population-level effects of ophidiomycosis among the few individuals we recaptured within short timeframes (< 15 days). We have, however, highlighted the need for mark-recapture studies within infected populations to determine the impacts of ophidiomycosis in these and other Texas snake populations, similar to a study conducted for pygmy rattlesnakes in Florida40, albeit over a longer period (i.e., greater than the average life span of a species). Texas encompasses broad ecological regions, each with unique vegetation cover and climatic patterns. Therefore, statewide surveillance will be useful in evaluating the wider distribution of the pathogen and assessing risks to the other seven snake species currently threatened in the state (e.g., Cemophora coccinea copei, Cemophora coccinea lineri [Texas endemic], Coniophanes imperialis, Drymobius margaritiferus, Leptodeira septentrionalis, Pituophis ruthveni, and Tantilla cucullata). Additionally, the deeper temporal dynamics of Oo in Texas are unclear. A retrospective survey of preserved specimens—similar to those conducted in eastern massasaugas67 and other fungal pathogen systems68,69—could also better elucidate seasonal and historical trends of Oo prevalence in Texas snakes, which remain largely understudied at the population-scale in this region of North America.
Methods
Study area
In total, we sampled 21 sites distributed within Paint Creek, the Clear Fork of the Brazos, and sections of the Upper Brazos River basin (Fig. 4 and Supplementary Fig. S1); because of their patchy distribution and rarity, the majority of our effort focused on sites and counties where N. h. harteri populations were present historically30,31,33. All procedures involving snakes were carried out in accordance with guidelines and regulations approved by the Texas State University Office of Research Integrity & Compliance (IACUC Protocol #26). Capture and sampling of snakes was approved by the Texas Parks and Wildlife Department (Scientific permits SPR-0316-059, SPR-0220-025, and SPR-0102-191), and the reporting in this manuscript follows the ARRIVE guidelines (https://arriveguidelines.org).
Data collection and Oo qPCRs
We collected snakes from 16 April 2018 through 6 October 2020. Our sampling trips were carried out opportunistically and depended on weather conditions without rainfall and with ambient air temperatures above 21 °C; thus, we were unable to implement a structured temporal sampling design. Instead, we conducted the majority of our sampling trips during the spring and early summer months when Nerodia activity is known to be higher38,52. Flooding in our study area also prevented surveys in June, July, and August of 2019, however, activity generally decreases after June. In 2020, surveys were delayed until May owing to mandatory travel restrictions attributable to the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) outbreak.
To maximize the probability of capturing snakes present at a site, we utilized active and passive capture methods. Our active capture method consisted of kayaking navigable waterways and walking along shorelines to visually locate individuals and then capturing them by hand. Our passive capture method consisted of deploying Gee’s galvanized funnel crawfish traps (78.7 cm × 22.9 cm) along the shoreline32,70 in areas identified as suitable Nerodia habitat (i.e., near chunk rock, edges of riffles, and large rip-rap). All traps were sanitized prior to deployment and after retrieval with a 1% bleach solution.
We handled each snake with fresh, sterile nitrile gloves. After capture, each snake was visually inspected for signs of Oo infection (see Fig. 1). We defined signs of Oo infection (SOI) as the presence of dermatitis, gross lesions, or any general scale abnormality (e.g., signs of inflammation, crust, nodules, or pus discharge)12,16. We also photographed all potential visible signs of infection. To decrease costs, reduce handling time, minimize animal stress, and increase the surface area sampled, we swabbed the entire body of each snake using several strokes with a single sterile cotton-tipped swab (Medical Wire, MW113). We swabbed all surfaces of the head and then swabbed down the snake along the dorsum and the venter towards the tail. We immediately placed the swabs into a sterile 2 mL centrifuge tube with an O-ring screw cap. Of the snakes that exhibited SOI during 2018, we randomly chose a subset (N = 13) and collected skin biopsies (i.e., scale clips) from the affected area and placed the sample in 95% ethanol for downstream DNA extraction and qPCR testing. We kept tubes with swabs and skin biopsies on ice in the field and during transportation back to the laboratory, where they were stored at − 20 °C until processing.
After swabbing, we recorded sex (determined via visual inspection and everting the hemipenes), snout-vent length (SVL), and mass for each captured snake. To minimize cross contamination of equipment, we placed each snake in a sterile plastic bag prior to weighing it. We sterilized all non-disposable equipment with a 1% bleach solution prior to processing other individuals. We then marked the venter of each snake with a unique identifier using a cautery unit in case of future recapture71. We used SVL to categorize individuals into species-specific life stages (see Supplementary Table S7)38,51,52,53,72,73. For each county sampled, we retrieved monthly low temperature and monthly precipitation data from the National Centers for Environmental Information-National Oceanic and Atmospheric Administration climate database.
Molecular analysis
For the swab DNA extractions, we used the PrepMan Ultra Sample Preparation Reagent (Applied Biosystems, Foster City, CA, USA) that is designed to rapidly extract fungal and bacterial DNA from complex samples (see Supplementary information for full protocol)15,69,74. We extracted genomic DNA from all skin biopsies using the GeneJET Genomic DNA Purification Kit (K0722; Thermo Scientific). To detect Oo via qPCR, we used the primers and probe designed by Allender et al.75. Triplicate reactions were run in 25 µL total volumes, which were composed of 12.5 µL 2X TaqMan Fast Advanced Master Mix (Applied Biosystems), 2.75 µL nuclease-free water, 0.9 µM of each primer, 0.25 µM of probe, 400 ng BSA, and 5 µL of non-diluted extracted DNA (variable concentrations). For our standards, we used a dynamic range of 1 × 10−1–1 × 10−5 ng/µL of gDNA and substituted gDNA with nuclease-free water for the negative controls. We used the following fast cycling parameters: initial incubation at 95 °C for 20 s, followed by 50 cycles of 95 °C for 1 s, and 60 °C for 20 s (data capture step) on a QuantStudio 3 0.1 μL block real-time instrument (Applied Biosystems).
Previous studies have used internal transcribed spacer 1 (ITS1) copy number estimates75 and a dynamic range of known DNA concentrations76 to infer positive Oo infection and copy number via qPCR. The lower limit of sensitivity for the assay proposed by Allender et al.75 was ~ 10 ITS1 copies. However, ITS1 copy number variation among strains and isolates has been documented in other fungal pathogens77, and currently, there are no published data indicating whether or not Oo ITS1 copy number varies across strains or regional isolates of Oo. The assay proposed by Bohuski et al.76 utilized a tenfold serial dilution of genomic DNA from 500,000 to 50 fg, and their reported detection limit was 5 fg. The latter approach, however, does not provide a quantity for the number of nuclear equivalents in solution.
Since the development of these initial assays, the Oo genome has been sequenced and the haploid genome size is estimated to be 21.9 Mb78. Therefore, we combined the methods utilized by Allender et al.75 and Bohuski et al.76 by using genome size as an estimate of the number of nuclei in solution (i.e., genomic equivalents). To estimate genomic equivalents, we updated the equation that Allender et al.75 employed by substituting the molecular mass of the ITS1 region with the molecular mass of the nuclear genome. Therefore, we estimated the mass of one Oo genomic equivalent at approximately 2.36 × 10−5 ng or 23.6 fg using the following equation:
Our positive controls consisted of tenfold serial dilutions spanning a dynamic range of 500,000 fg to 50 fg (i.e., ~ 21,186 genomic equivalents to ~ 2.1186 genomic equivalents). We conducted reactions using duplicate positive and negative controls. To compensate for increased variance at the lower concentrations of the dynamic range, the 500 fg and the 50 fg standard reactions were run in quadruplicate.
Statistical analysis
Using the equation: mass/SVL2, we calculated body condition index (BCI)18,79,80. We also estimated prevalence among counties, species, and across our total sample size. We estimated 95% binomial confidence intervals with a logistic parameterization for all categorical data (i.e., county and species) using the R package “binom”81. We compared the skin biopsy qPCR Oo detections to the swab detections and estimated the false-negative rate from swabs as well as the false negative-rate of skin biopsies using the R package “cutpointr”82. We then used Bayesian estimates of “corrected” Oo prevalence with 95% credible intervals and used our false-negative rate estimate to set our informative priors for lower sensitivity rate83. We conducted four Fisher’s Exact Tests to examine the associations between: (1) SOI and Oo occurrence; (2) species and SOI; (3) sex and Oo occurrence; and (4) life stage and Oo occurrence. We were concerned with comparison-wise error only; therefore, we did not apply correction to P-values obtained from our analyses84. We also used a Kruskal–Wallis non-parametric analysis to test for differences in median BCI values of snakes with positive and negative qPCR results as well as differences in BCI and SOI. To minimize the effects of snake size disparity between life stages and across species, we tested for differences in BCI values only within each life stage, and within species, but not across life stages or species.
To evaluate the contributions of predictive factors to the probability of the Oo occurrence, we constructed 14 logistic regression mixed effects models (two intercept-only models) with the binomial family distribution using the “glmmTMB” package85. We considered the following explanatory variables: species, life stage, mean annual temperature, and mean annual precipitation. Eight of the models included sampling site as a random effect as well as sample month as an AR(1) variable to account for temporal autocorrelation in the model. Six models included only sample month as an AR(1) variable. To avoid overparameterization, we constructed the models with only one or two fixed effects. We then computed AICc for each candidate model to determine the most parsimonious model using the “MuMIn” package86. Our analyses did not consider the effects of variable interactions owing to low sample size. We were unable to collect snakes during several months of the 2019 summer season and most of the 2020 spring season, and thus, we did not estimate seasonal Oo prevalence. Using the most parsimonious AICc model, we inferred infection probability via estimated marginal means (EMM) obtained from an inverse logit transformation using the “emmeans” package87. Post hoc analysis of the EMM for each predictor of was conducted using Tukey’s HSD method.
All statistical analyses were conducted using R (R version 3.6.1, www.r-project.org, accessed 5 July 2019). We used the Thermo Fisher ConnectTM Cloud Dashboard Software (Thermo Fisher Scientific) for qPCR data processing and analysis, and we created distribution maps using QGIS open-source software (QGIS version 3.16.3, QGIS Development Team, QGIS Geographic Information System, Open Source Geospatial Foundation, https://www.qgis.org/en/site/, accessed 15 Jan 2019).
Data availability
The raw data generated and analyzed for this study are available from the Dryad depository (https://doi.org/10.5061/dryad.t76hdr83p).
References
Fisher, M. C. et al. Emerging fungal threats to animal, plant and ecosystem health. Nature 484, 186–194 (2012).
Fisher, M. C., Gow, N. A. & Gurr, S. J. Tackling emerging fungal threats to animal health, food security and ecosystem resilience. Philos. Trans. R. Soc. B 371, 20160332. https://doi.org/10.1098/rstb.2016.0332 (2016).
Lips, K. R. Overview of chytrid emergence and impacts on amphibians. Philos. Trans. R. Soc. B 371, 20150465. https://doi.org/10.1098/rstb.2015.0465 (2016).
Lips, K. R., Diffendorfer, J., Mendelson, J. R. III. & Sears, M. W. Riding the wave: Reconciling the roles of disease and climate change in amphibian declines. PLoS Biol. 6, e72 (2008).
Caruso, N. M. & Lips, K. R. Truly enigmatic declines in terrestrial salamander populations in great smoky mountains national park. Divers. Distrib. 19, 38–48 (2013).
Martel, A. et al. Recent introduction of a chytrid fungus endangers western palearctic salamanders. Science 346, 630–631 (2014).
van der Spitzen Sluijs, A. et al. Rapid enigmatic decline drives the fire salamander (Salamandra salamandra) to the edge of extinction in the Netherlands. Amphib.-Reptil. 34, 233–239 (2013).
Blehert, D. S. et al. Bat white-nose syndrome: An emerging fungal pathogen?. Science 323, 227–227 (2009).
Thogmartin, W. E., King, R. A., McKann, P. C., Szymanski, J. A. & Pruitt, L. Population-level impact of white-nose syndrome on the endangered Indiana bat. J. Mammal. 93, 1086–1098 (2012).
Fisher, M. C., Garner, T. W. & Walker, S. F. Global emergence of Batrachochytrium dendrobatidis and amphibian chytridiomycosis in space, time, and host. Annu. Rev. Microbiol. 63, 291–310 (2009).
Martel, A. et al. Batrachochytrium salamandrivorans sp. Nov. causes lethal chytridiomycosis in amphibians. Proc. Natl. Acad. Sci. 110, 15325–15329 (2013).
Allender, M. C., Raudabaugh, D. B., Gleason, F. H. & Miller, A. N. The natural history, ecology, and epidemiology of Ophidiomyces ophiodiicola and its potential impact on free-ranging snake populations. Fungal Ecol. 17, 187–196. https://doi.org/10.1016/j.funeco.2015.05.003 (2015).
Grioni, A. et al. Detection of Ophidiomyces ophidiicola in a wild Burmese python (Python bivittatus) in Hong Kong SAR, China. J. Herpetol. Med. Surg. 31, 283–291 (2021).
Allender, M. C. et al. Chrysosporium sp. infection in eastern massasauga rattlesnakes. Emerg. Infect. Dis. 17, 2383–2384. https://doi.org/10.1136/vr.b4816 (2011).
Franklinos, L. H. V. et al. Emerging fungal pathogen Ophidiomyces ophiodiicola in wild European snakes. Sci. Rep. 7, 1–7. https://doi.org/10.1038/s41598-017-03352-1 (2017).
Lorch, J. M. et al. Experimental infection of snakes with Ophidiomyces ophiodiicola causes pathological changes that typify snake fungal disease. mBio 6, 1–9. https://doi.org/10.1128/mBio.01534-15 (2015).
Clark, R. W., Marchand, M. N., Clifford, B. J., Stechert, R. & Stephens, S. Decline of an isolated timber rattlesnake (Crotalus horridus) population: Interactions between climate change, disease, and loss of genetic diversity. Biol. Cons. 144, 886–891. https://doi.org/10.1016/j.biocon.2010.12.001 (2011).
Chandler, H. C. et al. Ophidiomycosis prevalence in Georgia’s eastern indigo snake (Drymarchon couperi) populations. PLoS ONE 14, e0218351 (2019).
Guthrie, A. L., Knowles, S., Ballmann, A. E. & Lorch, J. M. Detection of snake fungal disease due to Ophidiomyces ophiodiicola in Virginia, USA. J. Wildl. Dis. 52, 143–149. https://doi.org/10.7589/2015-01-007 (2016).
Last, L. A., Fenton, H., Gonyor-McGuire, J., Moore, M. & Yabsley, M. J. Snake fungal disease caused by Ophidiomyces ophiodiicola in a free-ranging mud snake (Farancia abacura). J. Vet. Diagn. Invest. 28, 709–713. https://doi.org/10.1177/1040638716663250 (2016).
Lorch, J. M. et al. Snake fungal disease: An emerging threat to wild snakes. Philos. Trans. R. Soc. B 371, 20150457. https://doi.org/10.1098/rstb.2015.0457 (2016).
Haynes, E. et al. First report of ophidiomycosis in a free-ranging California Kingsnake (Lampropeltis californiae) in California, USA. J. Wildl. Dis. 57, 246–249 (2021).
Burbrink, F. T., Lorch, J. M. & Lips, K. R. Host susceptibility to snake fungal disease is highly dispersed across phylogenetic and functional trait space. Sci. Adv. 3, 1–10. https://doi.org/10.1126/sciadv.1701387 (2017).
Dixon, J. R. Amphibians and Reptiles of Texas: With Keys, Taxonomic synopses, Bibliography, and Distribution Maps 3rd edn. (Texas A&M University Press, 2000).
McKeown, S. A Field Guide to Reptiles and Amphibians in the Hawaiian Islands (Diamond Head Publishing, 1996).
Powell, R., Conant, R. & Collins, J. T. Peterson Field Guide to Reptiles and Amphibians of Eastern and Central NORTH AMERICA (Houghton Mifflin Harcourt, 2016).
Stebbins, R. C. & McGinnis, S. M. Peterson Field Guide to Western Reptiles and Amphibians (Houghton Mifflin Harcourt, 2018).
Texas Administrative Code. State‐listed threatened species in Texas. 31 TAC §65.175. (2020).
Dixon, J. R., Werler, J. E. & Forstner, M. R. J. Texas Snakes: A Field Guide Revised. (University of Texas Press, 2020).
Rodriguez, D., Forstner, M. R. J., McBride, D. L., Densmore, L. D. III. & Dixon, J. R. Low genetic diversity and evidence of population structure among subspecies of Nerodia harteri, a threatened water snake endemic to Texas. Conserv. Genet. 13, 977–986 (2012).
Scott, N. J., Maxwell, T. C., Thornton, O. W., Fitzgerald, L. A. & Flury, J. W. Distribution, habitat, and future of Harter’s water snake, Nerodia harteri Texas. J. Herpetol. 23, 373–389 (1989).
Whiting, M. J., Dixon, J. R. & Greene, B. D. Spatial ecology of the Concho water snake (Nerodia harteri paucimaculata) in a large lake system. J. Herpetol. 31, 327–335 (1997).
McBride, D. L. Distribution and status of the Brazos water snake (Nerodia harteri harteri) Master of Science thesis, Tarleton State University (2009).
United States Office of the Federal Register. Endangered and threatened wildlife and plants; determination of Nerodia harteri paucimaculata (Concho water snake) to be a threatened species Final rule. Fed. Regist. 51, 31412–31422 (1986).
United States Office of the Federal Register. Endangered and threatened wildlife and plants; removal of the Concho water snake from the federallist of endangered and threatened wildlife and removal of designated critical habitat. Fed. Reg. 76, 66779–66804 (2011).
United States Office of the Federal Register. Endangered and threatened wildlife and plants; findings on petitions and initiation of status review. Fed. Reg. 50, 29238–29239 (1985).
United States Office of the Federal Register. Endangered and threatened wildlife and plants; animal candidate review for listing as endangered or threatened species. Fed. Reg. 59, 58982–59028 (1994).
Gibbons, J. W. & Dorcas, M. E. North American Watersnakes: A Natural History (University of Oklahoma Press, 2004).
Werler, J. E. & Dixon, J. R. Texas Snakes: Identification, Distribution, and Natural History (University of Texas Press, 2000).
Lind, C. M., McCoy, C. M. & Farrell, T. M. Tracking outcomes of snake fungal disease in free-ranging pigmy rattlesnakes (Sistrurus miliarius). J. Wildl. Dis. 54, 352–356. https://doi.org/10.7589/2017-05-109 (2018).
McBride, M. P. et al. Ophidiomyces ophiodiicola dermatitis in eight free-ranging timber rattlesnakes (Crotalus horridus) from Massachusetts. J. Zoo Wildl. Med. 46, 86–94. https://doi.org/10.1638/2012-0248R2.1 (2015).
McCoy, C. M., Lind, C. M. & Farrell, T. M. Environmental and physiological correlates of the severity of clinical signs of snake fungal disease in a population of pigmy rattlesnakes Sistrurus miliarius. Conserv. Physiol. 5, cow077. https://doi.org/10.1093/conphys/cow077 (2017).
Haynes, E. et al. Ophidiomycosis surveillance of snakes in Georgia, USA reveals new host species and taxonomic associations with disease. Sci. Rep. 10, 1–15 (2020).
Stengle, A. G. et al. Evidence of vertical transmission of the snake fungal pathogen Ophidiomyces ophiodiicola. J. Wildl. Dis. 55, 961–964 (2019).
Britton, M., Allender, M. C., Hsiao, S.-H. & Baker, S. J. Postnatal mortality in neonate rattlesnakes associated with Ophidiomyces ophiodiicola. J. Zoo Wildl. Med. 50, 672–677 (2019).
Allender, M. C., Hileman, E., Moore, J. & Tetzlaff, S. Detection of Ophidiomyces, the caustive agent of snake fungal disease, in the eastern massasauga (Sistrurus catenatus) in Michigan, USA, 2014. J. Wildl. Dis. 52, 694–698. https://doi.org/10.7589/2015-12-333 (2016).
Hileman, E. T. et al. Estimation of Ophidiomyces prevalence to evaluate snake fungal disease risk. J. Wildl. Manag. 82, 173–181. https://doi.org/10.1002/jwmg.21345 (2018).
McKenzie, J. M. et al. Field diagnostics and seasonality of Ophidiomyces ophiodiicola in wild snake populations. EcoHealth 16, 141–150 (2019).
Snyder, S. D., Sutton, W. B. & Walker, D. M. Prevalence of Ophidiomyces ophiodiicola, the causative agent of Snake Fungal Disease, in the Interior Plateau Ecoregion of Tennessee, USA. J. Wildl. Dis. 56, 907–911 (2020).
Tetzlaff, S. J. et al. Snake fungal disease affects behavior of free-ranging massasauga rattlesnakes (Sistrurus catenatus). Herpetol. Conserv. Biol. 12, 624–634 (2017).
Aldridge, R. D., Flanagan, W. P. & Swarthout, J. T. Reproductive biology of the water snake Nerodia rhombifer from Veracruz, Mexico, with comparisons of tropical and temperate snakes. Herpetologica 51, 182–192 (1995).
Greene, B. D., Dixon, J. R., Whiting, M. J. & Mueller, J. M. Reproductive ecology of the Concho water snake Nerodia harteri paucimaculata. Copeia 1999, 701–709 (1999).
Kofron, C. P. Reproduction of aquatic snakes in south-central Louisiana. Herpetologica 35, 44–50 (1979).
Green, B. D. Life History and Ecology of the Concho Water Snake, Nerodia harteri paucimaculata. Dissertation (Texas A&M University, 1993).
McKenzie, C. M. et al. Ophidiomycosis in red cornsnakes (Pantherophis guttatus): potential roles of brumation and temperature on pathogenesis and transmission. Vet. Pathol. 57, 825–837 (2020).
Gregoire, D. R. Nerodia rhombifer (Hallowell, 1852): U.S. geological survey, nonindigenous aquatic species database, Gainesville, FL, Retrieved from 27 Oct 2009 https://nas.er.usgs.gov/queries/FactSheet.aspx?SpeciesID=2577.
Janecka, M. J., Janecka, J. E., Haines, A. M., Michaels, A. & Criscione, C. D. Post-delisting genetic monitoring reveals population subdivision along river and reservoir localities of the endemic Concho water snake (Nerodia harteri paucimaculata). Conserv. Genet. 22, 1005–1021 (2021).
Madsen, T., Stille, B. & Shine, R. Inbreeding depression in an isolated population of adders Vipera berus. Biol. Cons. 75, 113–118 (1996).
Carter, J. et al. Variation in pathogenicity associated with the genetic diversity of Fusarium graminearum. Eur. J. Plant Pathol. 18, 573–583 (2002).
Charlesworth, D. & Willis, J. H. The genetics of inbreeding depression. Nat. Rev. Genet. 10, 783 (2009).
Keller, L. F. & Waller, D. M. Inbreeding effects in wild populations. Trends Ecol. Evol. 17, 230–241 (2002).
Nieminen, M., Singer, M. C., Fortelius, W., Schöps, K. & Hanski, I. Experimental confirmation that inbreeding depression increases extinction risk in butterfly populations. Am. Nat. 157, 237–244 (2001).
Roelke, M. E., Martenson, J. S. & O’Brien, S. J. The consequences of demographic reduction and genetic depletion in the endangered Florida panther. Curr. Biol. 3, 340–350. https://doi.org/10.1016/0960-9822(93)90197-v (1993).
United States Office of the Federal Register. Endangered and threatened wildlife and plants: findings on petitions involving the Yacare Caiman and Harter’s water snake. Fed. Reg. 49, 21089–21090 (1984).
NatureServe. NatureServe Explorer: An Online Encyclopedia of Life [web application]. Version 7.0. NatureServe, Arlington, Virginia., http://www.natureserve.org/explorer (2020).
Hammerson, G. A. Nerodia harteri (Trapido, 1941). The IUCN red list of threatened species 2007. https://doi.org/10.2305/IUCN.UK.2007.RLTS.T62238A12583490.en (2007).
Allender, M. C. et al. Hematology in an eastern massasauga (Sistrurus catenatus) population and the emergence of Ophidiomyces in Illinois, USA. J. Wildl. Dis. 52, 258–269 (2016).
Becker, C. G., Rodriguez, D., Lambertini, C., Toledo, L. F. & Haddad, C. F. Historical dynamics of Batrachochytrium dendrobatidis in Amazonia. Ecography 39, 954–960 (2016).
Rodriguez, D., Becker, C. G., Pupin, N. C., Haddad, C. F. B. & Zamudio, K. R. Long-term endemism of two highly divergent lineages of the amphibian-killing fungus in the Atlantic forest of Brazil. Mol. Ecol. 23, 774–787. https://doi.org/10.1111/mec.12615 (2014).
Fitch, H. S. Collecting and Life-History Techniques. In Snakes: Ecology and Evolutionary Biology (eds Seigel, Richard A. et al.) 143–164 (Macmillan, 1987).
Winne, C. T., Willson, J. D., Andrews, K. M. & Reed, R. N. Efficacy of marking snakes with disposable medical cautery units. Herpetol. Rev. 37, 52–54 (2006).
Greene, B. D., Dixon, J. R., Mueller, J. M., Whiting, M. J. & Thornton, O. W. Jr. Feeding ecology of the Concho water snake, Nerodia harteri paucimaculata. J. Herpetol. 28, 165–172 (1994).
Lacki, M. J., Hummer, J. W. & Fitzgerald, J. L. Population patterns of copperbelly water snakes (Nerodia erythrogaster neglecta) in a riparian corridor impacted by mining and reclamation. Am. Midl. Nat. 153, 357–369 (2005).
Hyatt, A. D. et al. Diagnostic assays and sampling protocols for the detection of Batrachochytrium dendrobatidis. Dis. Aquat. Org. 73, 175–192 (2007).
Allender, M. C., Bunick, D., Dzhaman, E., Burrus, L. & Maddox, C. Development and use of a real-time polymerase chain reaction assay for the detection of Ophidiomyces ophiodiicola in snakes. J. Vet. Diagn. Invest. 27, 217–220. https://doi.org/10.1177/1040638715573983 (2015).
Bohuski, E., Lorch, J. M., Griffin, K. M. & Blehert, D. S. TaqMan real-time polymerase chain reaction for detection of Ophidiomyces ophiodiicola, the fungus associated with snake fungal disease. BMC Vet. Res. 11, 1–10. https://doi.org/10.1186/s12917-015-0407-8 (2015).
Longo, A. V. et al. ITS1 copy number varies among Batrachochytrium dendrobatidis strains: Implications for qPCR estimates of infection intensity from field-collected amphibian skin swabs. PLoS ONE 8, e59499 (2013).
Ohkura, M. et al. Genome sequence of Ophidiomyces ophiodiicola, an emerging fungal pathogen of snakes. Genome Announc. 5, 1–2 (2017).
Falk, B. G., Snow, R. W. & Reed, R. N. A validation of 11 body-condition indices in a giant snake species that exhibits positive allometry. PLoS ONE 12, e0180791 (2017).
Garrow, J. S. & Webster, J. Quetelet’s index (W/H2) as a measure of fatness. Int. J. Obes. 9, 147–153 (1985).
Dorai-Raj, S. binom: Binomial confidence intervals for several parameterizations. https://CRAN.R-project.org/package=binom (2014).
Thiele, C. & Hirschfeld, G. cutpointr: Improved estimation and validation of optimal cutpoints in R. J. Stat. Softw. 98, 1–27 (2021).
Diggle, P. J. Estimating prevalence using an imperfect test. Epidemiol. Res. Int. 2011, 1–5 (2011).
Bender, R. & Lange, S. Adjusting for multiple testing: When and how?. J. Clin. Epidemiol. 54, 343–349 (2001).
Brooks, M. et al. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 9, 378–400 (2017).
Barton, K. MuMIn: Multi-model inference. R package version 1.43.6 (2019).
Lenth, R., Singmann, H., Love, J., Buerkner, P. & Herve, M. emmeans: Estimated marginal means, aka least-squares means, R package version 1.4.8. https://CRAN.R-project.org/package=emmeans (2020).
Acknowledgements
We thank Dustin McBride for logistical advice. We thank Jeremiah Leach, Daniel Puckett, Gabriella Solis, Thomas Marshall, Shashwat Sirsi, Michaela Bowlsby, Cheyenne N. Gonzales, Arya J. Sanjar, Juliette Garza, Robert Tyler, Stephen Roussos, Jeremy Weaver, Mark Pyle, Kasey Jobe, and Nathan Rains for assistance the field. We thank Toriann Molis, Mario B. Sarmiento, Clarissa Rivera, Gabriella Solis, Mireya A. Escandon, Stephanie Monroe, Carlos Baca, and Rebecca M. Brunner for assistance in the laboratory. This project was funded by Texas Parks and Wildlife Department and the U.S. Fish and Wildlife Service through the State Wildlife Grant Program (TX T-164-R-1 to D.R. and M.R.J.F.), the Conservation License Plate Program (contract #531961 to S.J.M.), and startup funds to D.R. from Texas State University.
Author information
Authors and Affiliations
Contributions
D.R., P.C., and M.R.J.F. conceived and designed the study. S.F.H., S.J.M., M.R.J.F. and, D.R. attained funding for the study. S.F.H., J.R.Y., P.C., S.J.M., M.R.J.F., and D.R. participated in surveys and sample collection. S.F.H. and D.R. performed molecular diagnostics. S.F.H., C.G.B., and, P.C. designed and performed the statistical analyses. S.F.H. and D.R. co-wrote the draft and constructed the figures. S.F.H., C.G.B., J.R.Y., P.C., M.R.J.F., S.J.M., and, D.R. contributed to the interpretation of the results and writing the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Harding, S.F., Becker, C.G., Yates, J.R. et al. Comparative host–pathogen associations of Snake Fungal Disease in sympatric species of water snakes (Nerodia). Sci Rep 12, 12303 (2022). https://doi.org/10.1038/s41598-022-16664-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-16664-8
This article is cited by
-
Characterisation, prevalence and severity of skin lesions caused by ophidiomycosis in a population of wild snakes
Scientific Reports (2024)
-
Pilot survey reveals ophidiomycosis in dice snakes Natrix tessellata from Lake Garda, Italy
Veterinary Research Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.