Abstract
To examine the outcomes of preterm infants born to women with preterm premature rupture of membranes (PPROM) at periviable gestational age. This is an observational retrospective cohort study analyzing data collected on singleton deliveries complicated by prolonged premature rupture of membranes occurring between 17 and 33 weeks of gestation. Neonatal outcomes including birth weight, Apgar score, retinopathy of prematurity, intraventricular hemorrhage, bronchopulmonary dysplasia, necrotizing enterocolitis, hearing impairment and mortality were evaluated. Ninety-four preterm infants who were born after a prolonged premature rupture of membranes of at least 7 days were included in the study. Median gestational week at onset of membrane rupture was 27.1 ± 4.2 weeks (range 17–33) and median latency period in days was 16 ± 21.8 (range 7–105). The cohort was stratified by gestational week (GW) at onset of PPROM (group 1: 17–23, group 2: 24–27, and group 3: 28–33). We found that the survival rate to discharge within neonates born after prolonged rupture of membrane at gestational week less than 24 weeks is 79.2% and 88.9% in group 2. These neonates did not show an increased rate of major morbidities compared to neonates born following membrane rupture at gestational week 24 to 27. We described a high survival rate to discharge without major morbidities following prolonged preterm membrane rupture of at least 7 days of latency before viability.
Similar content being viewed by others
Introduction
Preterm premature rupture of membranes (PPROM) defined, as the spontaneous rupture of membranes before 37 weeks of gestation, is a relatively common complication in pregnancy. It occurs in about 5 to 7% of pregnancies1 and accounts for one-third of preterm births2,3. The incidence of PPROM, at periviable gestational age (broadly defined as 20 0/7 through 25 6/7 weeks of gestation)4 is less common, affecting approximately four in 1000 pregnancies5. PPROM exposes the fetus to infection, abruptio placentae, cord prolapse, and intrauterine death, during the latency period (time between onset of PPROM to birth)5. The neonatal outcome is generally poor as a result of preterm delivery, inflammatory response, and a certain degree of pulmonary hypoplasia stemming from the reduction in amniotic fluid at a very early gestational age (GA)6,7. The optimal management of pregnancies complicated by PPROM remains a challenge. The issue of expectant management vs. immediate delivery, especially in cases of PPROM occurring at less than 30 gestational week (GW), is controversial6,7,8,9,10,11. The last Cochrane on this issue recommended a policy of expectant management between 25 and 37 weeks of pregnancy with careful monitoring to achieve better outcomes for the mother and her baby12.
The current approach in Israel to pregnancies presenting with PPROM falls into three categories as a function of GW13. Before 23 weeks, the recommendation is to terminate the pregnancy; between 23 + 0 and 23 + 6 weeks, the decision on pregnancy termination is made following consultation between the medical team and the parents; and above 24 weeks, expectant management, including corticosteroids and antibiotics administration, is the general rule.
Although these gestational age-based guidelines are essential to health care providers in the maternal/neonatal treatment decision-making, counselling parents on the rate of neonatal survival and long-term disabilities is a challenging complex issue, and present women with dilemmas in view of individual circumstances and patient values.
The primary aim of this study was to describe the outcomes, especially survival rate, of preterm infants born following management of prolonged PPROM occurring from 17 to 33 weeks with PROM of more than 7 days.
Materials and methods
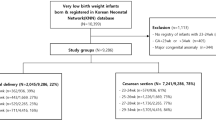
This is a retrospective analysis of data on singleton deliveries complicated by prolonged PPROM (≥ 1 week) occurring between 17 and 33 weeks of gestation, conducted at the Mayanei HaYeshua Medical Center (MHMC) between January 2014 and December 2019. The local ethics committee of the Mayanei Hayeshua Medical Center approved the present study (0015-19-MHMC) and waived the need for informed consent, due to the retrospective character of the study. The study was carried out in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki. We included preterm babies born following prolonged PPROM and admitted to the Neonatal Intensive Care Unit (NICU). Data were extracted from medical records, included were maternal data on age, medical history and comorbidities (e.g. gravidity, parity, previous preterm delivery, hypothyroidism, diabetes mellitus, gestational diabetes mellitus, hypertension, epilepsy, thrombophilia, the use of selective serotonin reuptake inhibitors (SSRIs), group B streptococcus (GBS) colonization), chorioamnionitis, maternal fever, GW at PPROM onset, mode of delivery and duration of latency. Neonatal data on GA, birth weight (BW), gender, APGAR score at 1 and 5 min, intubation and days of ventilation, mode of ventilation or assisted ventilation, nitric oxide (NO) inhalation, morbidities such as retinopathy of prematurity (ROP), intraventricular hemorrhage (IVH), periventricular leukomalacia (PVL), bronchopulmonary dysplasia (BPD), necrotizing enterocolitis (NEC) stage 2–3, patent ductus arteriosus (PDA), blood stream infection (BSI), hearing impairment, pulmonary hypertension, pneumothorax and central line blood stream infection (CLABSI)), medication (surfactant, diuretics and steroid treatment), and survival at discharge were recorded.
PPROM was diagnosed using the AmniSure ROM Test (Qiagen Sciences LLC, Germantown, MD, USA) which is based on a report of watery leakage from the vagina, confirmed by sterile speculum examination and the observation of either fluid accumulation in the posterior vaginal fornix or direct leakage from the cervical canal with pressure from uterine fundus or upon coughing. Cases of PPROM latency period less than 7 days before birth and PPROM occurring after 35 + 0 gestational weeks were excluded.
Statistical analysis
Categorical variables are reported as frequencies and percentages. Descriptive statistics (means and standard deviations) were calculated for demographic data. Normality was assessed by Shapiro–Wilk tests. Fisher’s exact tests or Chi square tests were used to compare categorical variables between groups as appropriate and followed by a post-hoc Bonferonni correction when appropriate. Multiple comparisons of continuous variables among groups were performed using the Kruskal–Wallis test followed by post-hoc Bonferonni pairwise comparison or the one-way analysis of variance (ANOVA) followed by post-hoc Tukey HSD test, when appropriate. The Mann–Whitney U test was applied to compare continuous variables when appropriate. A Spearman’s rank correlation coefficient was used to test for correlation between GW at onset of PPROM, GA at delivery, duration of latency period, Apgar scores and BW. Binary logistic regression analysis was conducted to investigate whether variables (GA at delivery, GW at onset of PPROM, BW, Apgar scores and latency period length) could predict neonatal survival to discharge.
IBM SPSS Statistics for Windows, version 25, was used for statistical data analysis and p-values < 0.05 were considered statistically significant.
Results
Ninety-four cases of PPROM (≥ 7 days) deliveries were included in the study period. None of the mothers underwent a prior amniocentesis. They were all admitted at onset of PPROM and were kept under observation at the maternal high-risk pregnancy ward until delivery. Demographic and clinical characteristics of the mothers and preterm infants are presented in Table 1. Briefly, the average maternal age was 28.4 ± 5.4 and the average GA at delivery was 30.8 ± 3.1 weeks (range 23–34). Median GW at onset of PPROM was 27.1 ± 4.2 weeks (range 17–33) and median length of latency period in days was 16 ± 21.8 (7–105). Corticosteroids, magnesium and antibiotics were administrated to almost all the mothers (97.9–98.9%).
The cohort was further stratified by GW at onset of PPROM, as group 1 (17–23 weeks), group 2 (24–27) and group 3 (28–33) (Fig. 1, Table 1). There were no significant differences in maternal comorbidities including hypothyroidism, diabetes mellitus, gestational diabetes mellitus, hypertension, epilepsy, thrombophilia, maternal fever, group B streptococcus (GBS) colonization and maternal use of selective serotonin reuptake inhibitors (SSRIs), between the groups. However, the rate of chorioamnionitis, per Chi square analysis, was significantly higher in-group 2 (p ≤ 0.001) and lower in-group 3 (p = 0.006) after Bonferonni correction. Furthermore, mothers in group 3 had a significant lower rate of cesarean delivery (32.6%, p = 0.006) and significantly more mothers in group 1 (29.2%) had a previous history of preterm delivery, (p = 0.004) than the ones in the other groups after Bonferonni correction. By Kruskal–Wallis test with post hoc comparison, the median length of latency period, in group 3, was significantly shorter than in group 2 and 1 (12 ± 5.7 vs 17 ± 14.8 and vs 49 ± 28.1, p < 0.001, respectively).
By one-way ANOVA test with post hoc analysis, GA and BW in group 3 were significantly higher than in group 2 and 1 (p < 0.001). Similarly, Apgar score at 1 and 5 min in group 3 were significantly higher than in group 1 and 2, (p < 0.001 and p < 0.05, respectively). We found that the survival rate to discharge in neonates born to prolonged PPROM-complicated pregnancy at GW < 24 is 79.2%, compared to 88.9% in group 2 (p < 0.451) and 100% in group 3 (p < 0.012). We further compared neonatal outcomes between group 1 and group 2, the next closest group in terms of GW at PPROM onset (Table 2). There were no significant differences between group 1 and 2 for most of the reported parameters with the exception of use of surfactant (70.8% vs 37%, respectively, p = 0.025, two-sided Fisher’s exact test) and NO inhalation treatments (25% vs 3.7%, respectively, p = 0.042, two-sided Fisher’s exact test). Also, mean Apgar at 5 min in group 1 was significantly lower than in group 2, (7.6 ± 1.4 vs 8.3 ± 1.9, p = 0.029).
The neonates who did not survive in group 1 had a lower GW at PPROM (20 ± 2.3 vs 22 ± 1.2, p = 0.036) and were more susceptible to infection (40% of CLABSI cases, p = 0.036) than those who survived (Table 3). In group 2, the neonates who did not survive had a significantly lower Apgar score at 5 min than those who did survive (6 ± 2 vs 8.6 ± 1.7, respectively, p = 0.036), suffered from more PPHTN and pneumothorax (66.7% vs 4.2%, respectively, p = 0.025), and had a higher rate of CLABSI infection (66.7% vs 4.2%, respectively, p = 0.025) (Table 3).
Spearman’s correlation was computed to assess the relationship between the following variables, GW at PPROM, GA, Apgar score at 1 and 5 min, latency period length and BW within groups 1 and 2. There were strong positive correlations between latency period and BW (rs = 0.662, n = 51, p < 0.001), latency period and GA (rs = 0.704, n = 51, p < 0.001) and between GA and BW (rs = 0.812, n = 51, p < 0.001). Additionally, there were moderate positive correlations between GA and Apgar score 1 min (rs = 0.458, n = 51, p = 0.001), BW and Apgar score 5 min (rs = 0.465, n = 51, p = 0.001) and between Apgar score 1 min and 5 min (rs = 0.52, n = 51, p < 0.001). Finally, the latency period length was inversely related to GW at PPROM onset (rs = − 0.457, n = 51, p = 0.001) (Fig. 2). In binary logistic regression analysis, none of these variables (GA at delivery, GW at onset of PPROM, BW, Apgar score 1 and 5 min, and latency period length) remained significant in predicting survival to discharge within groups 1 and 2.
Per two-sided Fisher exact test, the rate of neonates affected by major morbidities, including ROP, IVH grade 3–4, NEC stage 2–3, BPD and hearing impairment, were similar between groups 1 and 2 (Table 2). Among the 19 surviving neonates in group 1, 63.2%12 were affected by one, two or three major morbidities (37%, 21% and 5.3% respectively) (Table 3) and none of the discharged neonates were affected by more than three major morbidities. In group 2, 33.3%8 of the surviving neonates were affected by one or two major morbidities (25% and 8.3%, respectively) (Table 3). None of the neonates in group 2 were affected by three or more major morbidities.
However, it worth noting that the prevalence of illness with irreversible damages (ROP, IVH 3–4 and hearing impairment) among the surviving neonates was only 31.6% (affected by one or two illnesses) in group 1 and only half of it, 16.7%, (affected by one illness) in group 2. None of the neonates in none of the groups was affected by all three major morbidities.
Discussion
We found that the survival rate to discharge in neonates born to prolonged PPROM-complicated pregnancy at GW < 24 is 79.2%, a definitively non-negligible number. These neonates did not present with an increased rate of major morbidities compared to neonates born to PPROM-complicated pregnancy at GW 24 to 27.
Though advances in perinatal and neonatal practices have led to improved neonatal survival rates, they remain widely variable throughout the literature14,15. Our results differ from other studies that reported ranges of survival rates at periviable PPROM from as low as 24% to as high as 70%3,16,17,18. Sim et al. showed an overall neonatal survival rate to discharge of 33.8%, and stratification of patients into early (12 to 19 + 6 weeks of gestation) and late pre-viable PPROM (20 to 23 + 6 weeks of gestation) revealed a 3.6-fold increase in survival rate in the latter group (12.2% versus 43.8%, p < 0.001)16. Additionally, the reported neonatal survival rate to discharge was 20% in the retrospective study done by Linehan et al. in PPROM diagnosed between 14 and 23 + 6 weeks of gestation17. Esteves et al. found that neonatal survival rate to discharge of PPROM at GW between 18 and 20 was 18.7% and between 22 and 24 was 42.8%18.
Another finding from our study was that 37.7% of the neonates born following PPROM below GW 24 and survived to discharge were not affected with major morbidities (i.e. ROP, IVH grade 3–4, NEC, BPD and hearing impairment). This finding is in the range of previously reported rates of 17.8%18 and 55%19 neonatal survival without major morbidities in a similar population. Alternatively, when only ROP, IVH stage 3–4 and hearing impairment where taken into account as irreversible morbidities, the rate of discharged neonates without those major morbidities was almost twice higher than reported.
It is worth noting that most of the mothers hospitalized in our center are orthodox religious Jews who tend to reject gynecological guidelines and refuse terminating the pregnancy as it conflicts with their personal values. Hence, these mothers benefited from expectant management of their PPROM that led in many cases to life birth. Neonate survival depends on both the GW at which membrane rupture occurs and at what GA the baby is born5. The same was true in our study as the infants who did not survive were from a lower GW at the time of PPROM.
We speculate that the high survival rate in our study differ from the one reported in the literature due to several reasons. First, we specifically recruited prolonged PPROM complicated pregnancies (latency period of at least 7 days), that might have led to a subset of PPROM cases with better odds of neonatal survival in excluding fetal distress or emergent medical indications. Secondly, the prolonged latency period allowed for medication therapy management that included administration of two courses of corticosteroids, antibiotics and magnesium, with the ultimate goal of reaching advanced gestational age at delivery. Indeed, 50% of the mother’s in group 1 had a latency period of at least 7 weeks.
Although several studies have shown that prolonged PPROM is associated with an increased risk of infant death and morbidity1,3,14,16,18, we could not see an increase in the rate of major morbidity in group 1 compared with group 2. Conversely, other studies have found that prolonged PPROM did not worsen neonatal outcomes20 and was associated with a decreased risk of neonatal sepsis21. In addition, along with higher GW at rupture, increased length of latency period increased the probability of neonatal survival22.
Finally, we reviewed cases of PPROM from 2014 until 2019, a more recent period than the ones indicated in previously published studies on cohorts recruited until 20153,15,17,19. Advances in neonatal care might have enhanced survival rates and reduced major morbidities.
Strengths and limitations
There are few limitations to our study: the retrospective nature of the study and secondly, the fact that the study was probably not powered enough to detect statistically significant differences, between group 1 and 2, for any of the major morbidities. On the other hand, the strength of this study is its relatively large sample of singletons born preterm after a prolonged PPROM started at gestational week 17 as data on outcomes of neonates born following prolonged early preterm premature rupture of membranes are limited.
Conclusion
The survival rate to discharge was 79.2% in neonates born to women who had prolonged PPROM before 24 weeks of at least 7 days of latency. About one third of these infants were discharged alive without major morbidities. Our findings provide new insights to physicians when counseling women with prolonged PPROM at periviable GA.
Data availability
The data that support the findings of this study are available on request from the corresponding author.
References
Waters, T. P. & Mercer, B. M. The management of preterm premature rupture of the membranes near the limit of fetal viability. Am. J. Obstet. Gynecol. 201, 230–240. https://doi.org/10.1016/j.ajog.2009.06.049 (2009).
Waters, T. P. & Mercer, B. M. Preterm PROM: Prediction, prevention, principles. Clin. Obstet. Gynecol. 54, 307–312 (2011).
Walker, M. W., Picklesimer, A. H., Clark, R. H., Spitzer, A. R. & Garite, T. J. Impact of duration of rupture of membranes on outcomes of premature infants. J. Perinatol. 34, 669–672. https://doi.org/10.1038/jp.2014.73 (2014).
Gibson, K. S. & Brackney, K. Periviable premature rupture of membranes. Obstet. Gynecol. Clin. N. Am. 47, 633–651. https://doi.org/10.1016/j.ogc.2020.08.007 (2020).
American College of Obstetricians and Gynecologists, Society for Maternal-Fetal Medicine. Obstetric Care consensus No. 6: Periviable Birth. Obstetr. Gynecol. 130, e187–e199. https://doi.org/10.1097/AOG (2017).
Williams, O., Michel, B., Hutchings, G. & Debauche, C. Hubinont C Two-year neonatal outcome following PPROM prior to 25 weeks with a prolonged period of oligohydramnios. Early Hum. Dev. 88, 657–661. https://doi.org/10.1016/j.earlhumdev.2012.01.012 (2012).
Armstrong-Wells, J. et al. Inflammatory predictors of neurologic disability after preterm premature rupture of membranes. Am. J. Obstet. Gynecol. 212, e1–e9. https://doi.org/10.1016/j.ajog.2014.09.016 (2015).
Crane, J. M. et al. Canadian Perinatal Network (CPN) Collaborative Group (Appendix) maternal and perinatal outcomes of pregnancies delivered at 23 weeks’ gestation. J. Obstet. Gynaecol. Can. 37, 214–224. https://doi.org/10.1016/S1701-2163(15)30307-8 (2015).
Pasquier, J. C. et al. Intentional early delivery versus expectant management for preterm premature rupture of membranes at 28–32 weeks’ gestation: A multicentre randomized controlled trial (MICADO STUDY). Eur. J. Obstet. Gynecol. Reprod. Biol. 233, 30–37. https://doi.org/10.1016/j.ejogrb.2018.11.024 (2019).
Schmitz, T. et al. Preterm premature rupture of the membranes: Guidelines for clinical practice from the French College of Gynaecologists and Obstetricians (CNGOF). Eur. J. Obstet. Gynecol. Reprod. Biol. 236, 1–6. https://doi.org/10.1016/j.ejogrb.2019.02.021 (2019).
van der Ham, D. P. et al. Management of late-preterm premature rupture of membranes: The PPROMEXIL-2 trial. Am. J. Obstet. Gynecol. 207(276), e1–e10 (2012).
Bond, D. M. et al. Planned early birth versus expectant management for women with preterm prelabour rupture of membranes prior to 37 weeks’ gestation for improving pregnancy outcome. Cochrane Database Syst. Rev. 3, CD004735 (2017).
Boettcher, L. B. & Clark, E. Neonatal and childhood outcomes following preterm premature rupture of membranes. Obstet. Gynecol. Clin. N. Am. 47, 671–680. https://doi.org/10.1016/j.ogc.2020.09.001 (2020).
Mercer, B. M. Preterm premature rupture of the membranes. Obstetr. Gynecol. 10, 178–193. https://doi.org/10.1016/s0029-7844(02)02366-9 (2003).
Sim, W. H., Ng, H. & Sheehan, P. Maternal and neonatal outcomes following expectant management of preterm prelabor rupture of membranes before viability. J. Matern. Fetal Neonatal Med. 33, 533–541. https://doi.org/10.1080/14767058.2018.1495706 (2020).
Linehan, L. A. et al. Neonatal and maternal outcomes following midtrimester preterm premature rupture of the membranes: A retrospective cohort study. BMC Pregn. Childbirth 16, 25. https://doi.org/10.1186/s12884-016-0813-3 (2016).
Esteves, J. S., de Sá, R. A., de Carvalho, P. R. & Coca Velarde, L. G. Neonatal outcome in women with preterm premature rupture of membranes (PPROM) between 18 and 26 weeks. J. Matern. Fetal Neonatal Med. 29, 1108–1112. https://doi.org/10.3109/14767058.2015.1035643 (2016).
Wagner, P. et al. Outcome of pregnancies with spontaneous PPROM before 24+0 weeks’ gestation. Eur. J. Obstet. Gynecol. Reprod. Biol. 203, 121–126. https://doi.org/10.1016/j.ejogrb.2016.05.018 (2016).
Manuck, T. A., Maclean, C. C., Silver, R. M. & Varner, M. W. Preterm premature rupture of membranes: Does the duration of latency influence perinatal outcomes? Am. J. Obstet. Gynecol. 201, e1–e4146. https://doi.org/10.1016/j.ajog.2009.07.045 (2009).
Drassinower, D., Friedman, A. M., Običan, S. G., Levin, H. & Gyamfi-Bannerman, C. Prolonged latency of preterm premature rupture of membranes and risk of neonatal sepsis. Am. J. Obstet. Gynecol. 214, e1–e7436. https://doi.org/10.1016/j.ajog.2015.12.031 (2016).
LeMoine, F., Moore, R. C., Chapple, A., Moore, F. A. & Sutton, E. Neonatal survivability following previable PPROM after hospital readmission for intervention. AJP Rep. 10, e395–e402. https://doi.org/10.1055/s-0040-1721421 (2020).
Author information
Authors and Affiliations
Contributions
J.H. contributed to the conception, design and development of the project. He also contributed to data collection, analysis and interpretation and drafted the manuscript. L.M. and R.M. contributed to the project development, data analysis and interpretation, manuscript writing and editing. H.A. and D.L. contributed to data collection. All the authors have made substantial contributions to the concept, data interpretation, drafting or critical revision of the manuscript for intellectual content and have approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Herzlich, J., Mangel, L., Halperin, A. et al. Neonatal outcomes in women with preterm premature rupture of membranes at periviable gestational age. Sci Rep 12, 11999 (2022). https://doi.org/10.1038/s41598-022-16265-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-16265-5
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.