Abstract
Polyoxometalates (POMs) as efficient catalysts can be used a wide range of chemical transformations due to their tunable Brønsted/Lewis-acidity and redox properties. Herein, we reported two hybrid and heterogeneous lacunary Keggin catalysts: (TBA)7[PW11O39] (TBA-PW11) and (TBA)8[SiW11O39]·4H2O (TBA-SiW11) (TBA+: tetrabutylammonium) in which [XW11O39]n− anions were coated by TBA+ cations. In this form, TBA+ can easily trap reactants on the surface of the catalysts and increase the catalytic reaction. Therefore, the catalytic performance of both POMs was tested in cyanosilylation of numerous compounds bearing-carbonyl group and trimethylsilyl cyanide under solvent-free conditions. TBA-PW11 is more effective than TBA-SiW11, conceivably due to the higher Lewis acidity of the P than the Si center and to the higher accessibility of the metal centers in the framework structure. Noteworthy, the recyclability and heterogeneity of both POMs catalysts were also examined, and the results confirmed that they remain active at least after three recycling procedures.
Similar content being viewed by others
Introduction
The cyanosilylation reaction (CYSR) is an imperative C–C bond-forming that provides access to many chemicals, containing an extensive range of biological compounds and poly-functionalized building blocks1,2. Cyanohydrin, as a known organic synthon, plays an essential role in chemistry and biology. Cyanohydrins are widely used for synthesizing various α-hydroxy compounds, which are mainly synthesized by the trimethylsilyl cyanide (TMSCN) toward CYSR using heterogeneous and homogeneous catalysts including organocatalyst, Lewis acid, base catalyst3,4,5,6,7,8,9,10,11,12 and POMs13,14,15,16,17,18. The employ of TMSCN as a CN source is more popular than other methods due to avoiding the unstable and toxic hydrogen cyanide (HCN) in cyanohydrins synthesis. In light of eco-friendly procedures, developing a safe, clean, and powerful pathway, which can efficiently catalyze CYSR, is an imperative area of present‐day research19. Although numerous papers have been reported in this field, some of which had limitations, such as, tedious separation and recycling problems, the presentation and promotion of a suitable method for synthesizing cyanohydrins are needed. Thus, investigating a mild and effective heterogeneous catalytic system for the CYSR of carbonyl compound and TMSCN under solvent-free conditions is still highly desirable.
Polyoxometalates (POMs) are known as anionic inorganic compounds with diverse structures and fascinating applications that arise from their various physicochemical properties and can be used in various fields such as magnetism20, medicine21, catalysis22,23,24 analytical chemistry25,26. POMs as a catalyst have several benefits such as redox and acid–base properties that can be fine-tuned by changing the chemical structures and compositions, POMs are oxidative and thermally stable compounds compared with organometallic complexes, and the catalytically active sites of POMs can be precisely controlled with an appropriate combination of transition metals and lacunary POMs as inorganic ligands. Generally, POMs can contain Brønsted and Lewis acid sites and are also referred as bifunctional catalysts due to the incorporation of redox and Lewis centers in one unit27,28. In addition, the anionic charge of POMs is delocalized over the oxygen atoms, therefore, surface basic oxygen atoms can act as Brønsted base and/or act as a Lewis base (nucleophile)29,30.
Up to now, Keggin with the total formula [XM12O40]n– (X = hetero atom, M = addenda atom) is the most well-studied type of POMs due to its unique structure and stability under different conditions. Lacunary Keggin can be prepared by removing one or more addenda atoms from the complete structure. The removal of one, two, or three addenda metals will respectively lead to the formation of mono-, di-, and tri-lacunary species. This operation is mainly controlled by the variation of the pH of the solution to tailor the desired structure. Lacunary Keggin possesses a higher negative charge than its complete form (anionic charge of the POM)/(number of non-hydrogen atoms of the POM)22. Up to now, Keggin-type POMs have been widely used as an oxidation catalyst31, and those containing P as the heteroatom showed higher catalytic activity. This behavior can be explained with different electronegativity of the heteroatoms, (P (2.19) > Si (1.90) > Al (1.61)). Fully, the lower electronegativity of the heteroatom leads the more polarized bond between this atom and the oxygen bridging atom as well as the addenda metal sites, resulting to an increase in the basicity of the POM28,32,33.
POMs are normally soluble in both water and polar organic solvents and counter-cations play an essential role in the solubility of POMs. For example, POMs with small cations such as Na+ or H+ are highly soluble in water and other polar organic solvents. On the other hand, POMs with large cations such as Cs+, tetrabutylammonium (TBA+), or dimethyldioctadecylammonium (DODA+) are insoluble in water and exhibit the low absorptive capacity for polar molecules. Therefore, the later groups can be categorized as heterogeneous catalysts34,35.
Following our attempt to investigate the synthesis and utility of POM-based catalysts for promoting organic transformations20,21,22,24,25,26,36,37 and applying heterogeneous nano-catalysts invaluable organic reactions38,39,40,41,42,43,44 herein, we wish to present two nano-sized organic–inorganic hybrid systems (TBA)7[PW11O39] (TBA-PW11) and (TBA)8[SiW11O39]·4H2O (TBA-SiW11) as heterogeneous catalysts to promote the synthesis of phenyl-trimethylsilanyloxy-acetonitrile derivatives by using aldehydes and ketones using TMSCN under solvent-free conditions (Fig. 1).
Results and discussion
Synthesis and characterization of catalysts
In this study, two heterogeneous nanocatalysts, TBA-PW11 and TBA-SiW11, were obtained by top-down approach with ultrasonic method upon 15 min of sonication. According to the SEM images, morphologies of TBA-PW11 and TBA-SiW11 can consider as rhombic and cubic (Fig. 2). Furthermore, the presence of O, C, and W, in the nanocatalysts is confirmed by the EDS spectrum (Fig. 2).
Also, there are several examples that the outer surface of anionic POMs can be surrounded by organic cations (like TBA+)45,46 and in the case of our catalysts, 1H NMR and 13C NMR spectra provide clear and direct evidence for the presence of TBA+. For example, in the 1H NMR, three peaks located separately around 1.35, 1.60, and 3.20 ppm can be assigned to the CH2 of TBA+ and the CH3 group located at 0.96 ppm (Fig. 3). Also, The 31P NMR spectrum of TBA-PW11 (Fig. S1) was in the normal range of diamagnetic phosphotungstate and showed one peak at − 13.21 ppm, corresponding to the P atoms in the lacunary anion47.
The IR spectra of POMs include characteristic metal–oxygen stretching vibrations that occur in the specific region (generally sharp bands between 400−1000 cm−1). The characteristic strong bands for X–O, W–Ot, W–Ob and W–Oc stretching vibrations of nanocatalysts are shown in Table 1 and Fig. S2, which can approve the exact structures of the final catalysts. Moreover, absorptions in the range 2873–2961 cm–1 correspond to the C–H stretching vibrations of TBA+. Specific bands at around 1700 and 3300 cm-1 assigned to water molecules48.
Catalytic activity
To extend the catalytic capacity of TBA-PW11 and TBA-SiW11 catalysts, herein, we study the achievement of both catalysts for the CYSR obtained from two reaction pathways. For this purpose, the reaction between 1 mmol of benzaldehyde (BA) and TMSCN (2 mmol) was selected as a model reaction and performed using TBA-SiW11 and TBA-PW11 (2 mol%), separately, without any solvents. Gratifyingly, the desired products were isolated and obtained in 65% and 96%, respectively, after 45 min. Encouraged by these results, different reaction conditions such as temperature, catalyst amount, and solvent were optimized (Table S1). In this context, the selected reaction was accomplished at different temperatures (r.t., 65 °C, and 90 °C) under S.F., which the CYSR led to the highest yield at 65 °C. Moreover, only 55% or 40% of product yield upon performing the reaction at r.t. (Table S1, entries 1 and 2), using TBA-PW11 or TBA-SiW11 as catalysts, respectively. Increasing the reaction temperature from 65 °C to 90 °C did not improve the reaction yields (Table S1, entry 14). After that, the effect of various solvents, such as CHCl3, MeOH, toluene, and THF, was examined on the CYSR. As evident, S.F. conditions demonstrate the higher activity, leading to a corresponding product yield of 96% or 65% for TBA-PW11 and TBA-SiW11, respectively (Table S1, entries 3 and 4), while, using other solvents was not suitable for CYSR due to the production of low-yield products in the range of trace to 78% (Table S1, entries 10–13). For finding the optimum catalyst amount, the CYSR was performed over a different amount of both catalysts from 1 to 3 mol%, and significant development of the product yield was observed from 45 to 96% for TBA-PW11 or from 35 to 80% for TBA-SiW11 (Table S1, entries 3, 4 and 6–9), noteworthy, the use of 3 mol% of both catalysts had no effect on improving the CYSR. Therefore, the best catalyst amount was achieved as 2 mol%. Finally, a blank test using BA and TMSCN without any solvent and catalyst at 65 °C was accomplished and resulted in only a trace yield of the final product after 3 h (Table S1, entry 5). Therefore, the best conditions for promoting CYSR of the BA and TMSCN concern 2 mol% of the TBA-PW11 as the best catalyst at 65 °C without any solvent.
Following, the performance of TBA-PW11 as the best catalyst was tested towards different substituted ketones and aldehydes (Table 2). As tabulated, various aldehyde-containing compounds with different electron densities could tolerate these reactions to provide the desired products in high yields. Generally, the aldehydes bearing electron‐withdrawing groups, for instance, nitro, bromo, and chloro groups, show the excellent activity with the highest yields, and the potential of the -para position was significant in advancing the reaction to the ortho and meta positions (Table 2, entries 1–4 and 7–9). In contrast, the aldehyde bearing electron-donating groups (methyl and methoxy groups) exhibit lower yields in longer reaction time (Table 2, entries 5 and 6). Moreover, ketone compounds with electron‐withdrawing groups show moderate to good reactivity and produce the corresponding phenyl-trimethylsilanyloxy-acetonitrile derivatives (Table 2, entries 10–13), being also above the corresponding yields for the ketones with electron‐donor substituents. However, the excellent reactivity of aldehydes compared to ketones is quite apparent. These behaviors are in agreement with the predictable effect of the substituent on the electrophilic character of the carbonyl groups to undergo attack by the -cyano group of TMSCN.
Aiming to evaluate the benefits of this study, the catalytic activity of the TBA-PW11 towards the CYSR of BA with TMSCN was compared with other literature, as shown in Table 3. As tabulated, TBA-PW11 shows high efficiency, in a shorter time, under solvent‐free conditions compared to TBA-SiW11 and other reported catalysts (Yield of 96%, in S.F. at 65 °C after 45 min, Table 3, entry 6).
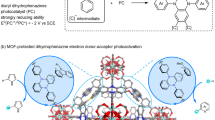
Commensurate with the experimental results and previously reported literatures, a possible CYSR mechanism is proposed and illustrated in Fig. 48,49. First, the carbonyl group in BA was activated by the coordinatively central P or Si atoms in catalysts (I) to nucleophilic attack of CN group in TMSCN (II). Finally, with the migration of the silyl group to the oxygen of intermediate (III), a carbon–carbon bond and then cyanohydrin (IV) is formed (Fig. 4). Notably, the products were replaced by BA, and the catalysts were continued to activate the BA in the next catalytic cycle.
Experimental
Chemicals and materials
The chemical compounds were purchased from Merck (Darmstadt, Germany, www.merckmillipore.com) and Sigma-Aldrich (St. Louis, MO, USA, www.sigmaaldrich.com) and used with no crystallization or purification. To conduct CYSR, aromatic aldehydes and ketones, TMSCN, toluene, methanol, chloroform, and THF were used.
Instrumentation
Melting points were determined with an electrothermal 9200 digital melting point apparatus (www.labnet.fi). Infrared (FT-IR) spectra were measured using KBr pellets containing the compounds (400–4000 cm−1) with a Bruker Tensor 27 FT-IR spectrometer. Also, the infrared spectra of catalysts were recorded in the range of 4000–400 cm–1 on a Thermo Nicolet/AVATAR 370 Fourier transform spectrophotometer (www.thermofisher.com) using KBr discs. A Thermo Finnigan Flash 1112EA elemental analyzer (www.thermofisher.com) was used for elemental analysis (C, H and N) compounds. The Spectro Arcos ICP-OES spectrometer model 76,004,555 in the range of 130–770 nm was measured metal content. 1H NMR, 13C NMR, and 31P NMR spectra were recorded in DMSO-d6 as the solvent on a Bruker FUM-300 spectrometer.
Preparation of catalysts
First, heterogeneous catalysts (TBA)7[PW11O39] (TBA-PW11) and (TBA)8[SiW11O39]·4H2O (TBA-SiW11) were synthesized and identified by FT-IR, elemental analysis, and NMR spectroscopy. Then, the top-down approach using ultrasonic technique were successfully synthesized the related nanocatalysts and characterized by FTIR and SEM–EDS.
Synthesis of (TBA)7[PW11O39] (TBA-PW11)
First, 72.5 g (0.22 mol) Na2WO4·2H2O and 7.16 g Na2HPO4·12H2O (0.02 mol) were dissolved in 100 mL water, which was heated to 70–80 °C. Dropwise HNO3 was added to adjust the pH of the solution to 3.0. The solution was concentrated to half of the initial volume by heating at 80 °C. Then, a hot water solution of TBABr (7 mmol, 7 mL) was added to the above mixture and stirred for a further 30 min. The resulted white precipitates were filtered off, washed twice with water and dried in vacuum.
Synthesis of (TBA)8[SiW11O39]·4H2O (TBA-SiW11)
First, Na2WO4·2H2O (182 g, 0.55 mol) is dissolved in 300 mL of boiling distilled water, and a solution of HCI (4 M, 165 mL) is added dropwise (in 30 min) to this. Next, 100 mL solution of sodium metasilicate (1.1 g, 50 mmol) was added quickly to the previous solution, and 50 mL of HCI (4 M) is also added. The pH is about 5 to 6. The solution is kept boiling for 1 h. After cooling to room temperature, the solution is filtered if it is not completely clear. Then, a hot water solution of TBABr (8 mmol, 7 mL) was added to the above solution, which is stirred magnetically for a further 30 min. The white precipitation of the product was collected by filtration and washed twice with water.
Synthesis of nano-sized TBA-PW11 and TBA-SiW11
The mixture solution of Ethanol (10 mL), water (15 mL) and TBA-PW11 (or TBA-SiW11) (0.03 g) was subjected to ultrasonication (200 W). After 15 min, nano-sized catalysts were collected by centrifugation and then washed with water (3 × 5 mL) under a vacuum.
Typical method for the CYSR of carbonyl compounds
In a tube, a mixture of a carbonyl compound (1 mmol), TMSCN (2 mmol), and 2 mol% of TBA-PW11 or TBA-SiW11 was prepared, and it was put in an oil bath. After that, the mixture was heated at 65 °C without any solvent, for the desired time. Upon completion of CYSR, both mentioned catalysts were separated by filtration, and the mixture's solvent was evaporated. Finally, the pure product was dissolved and achieved in CH2Cl2.
Catalyst recyclability
Moreover, for examining the heterogeneous nature of the TBA-PW11 and TBA-SiW11, both catalysts separated from the reaction after 20 min and kept the catalyst‐free reaction under a similar environment for 25 min more. After removing the TBA-PW11 and TBA-SiW11 catalysts from the reaction mixture, no noticeable rise in product yield was detected, which verifies the heterogeneous nature of both catalysts. Further, to explore the recyclability of both catalysts, the catalytic activities of the fresh and reused TBA-PW11 and TBA-SiW11 were studied and compared. For this purpose, after completing each reaction cycle, catalysts were separated by simple filtration, washed with EtOH, and dried. As exhibited in Fig. S3, TBA-PW11 and TBA-SiW11 catalysts could be effectively recycled three times. However, the TBA-SiW11 catalyst experienced a significant loss in catalytic activity compared to the TBA-PW11 catalyst.
Finally, to check the structural integrity, FTIR analysis of the fresh and recycled TBA-PW11 and TBA-SiW11 were recorded. As shown in Fig. 5A, no momentous changes in their patterns were detected. In addition, to elucidate whether the recycling process can result in any change in the catalyst's morphology and catalyst structure, the 1HNMR spectra and the SEM images of the recycled TBA-PW11 catalyst were recorded (Fig. 5B,C). These results support that the structure of the TBA-PW11 underwent several reactions was preserved, but some agglomeration is evident.
Characterization data
Spectral data for catalysts
TBA-PW11: Yield: 73% based on W. Anal. Calcd. for C112H252N7O39PW11: C, 30.75; W, 46.23; N, 2.24; P, 0.71; H, 5.81%. Found: C, 29.82; W, 45.78; N, 2.08; P, 0.67; H, 6.11%. FT-IR (KBr pellet, cm−1): 2961, 2937, 2873, 1484, 1461, 1381, 1155, 1106, 1053, 957, 888, 805, 754, 596, 517, 407. 1H NMR (d6-DMSO, 300 MHz, RT) [δ, ppm] 0.96 (t, TBA-CH3), 1.35 (h, TBA-CH2), 1.60 (dq, TBA-CH2), 3.20 (m, TBA-CH2). 13C NMR (d6-DMSO, 300 MHz, RT) [δ, ppm] 14.02, 19.70, 23.57, 58.00 (all singlets). 31P NMR (d6-DMSO, 300 MHz, RT) [δ, ppm] (− 13.21) (singlet).
TBA-SiW11: Yield: 68% based on W. Anal. Calcd. for C128H296N8O43SiW11: C, 32.81; W, 43.15; N, 2.39; Si, 0.60; H, 6.37%. Found: C, 33.15; W, 42.65; N, 2.45; Si, 0.58; H, 6.55%. FT-IR (KBr pellet, cm−1): 3410, 2960, 2937, 2873, 2732, 1633, 1484, 1472, 1462, 1380, 1152, 1108, 1061, 1007, 966, 920, 894, 804, 739, 532. 1H NMR (d6-DMSO, 300 MHz, RT) [δ, ppm] 0.95 (t, TBA-CH3), 1.33 (h, TBA-CH2), 1.58 (pd, TBA-CH2), 3.19 (m, TBA-CH2). 13C NMR (d6-DMSO, 300 MHz, RT) [δ, ppm] 13.99, 19.69, 23.55, 58.00 (all singlets).
Nano-TBA-PW11: FT-IR (KBr pellet, cm−1): 2961, 2937, 2873, 1484, 1381, 1155, 1108, 1059, 958, 888, 812, 754, 595, 516.
Nano-TBA-SiW11: FT-IR (KBr pellet, cm−1): 3414, 2961, 2932, 2873, 1627, 1484, 1381, 1155, 1106, 1059, 966, 920, 801, 738, 531.
Concluding remarks
In summary, two nano-sized organic–inorganic hybrid systems based on lacunary Keggin TBA-PW11 and TBA-SiW11 as heterogeneous catalysts were synthesized and characterized using a suite of analytical techniques. Due to the coexistence of the high negative charge of the above catalysts, they showed an excellent catalytic effect for cyanosilylation of various aldehydes and ketones, giving the corresponding cyanohydrin trimethylsilyl ethers with high yields in a short time. Notably, both catalysts were heterogeneous, but TBA-PW11 showed higher catalytic activity and recyclability towards the cyanosilylation of aldehydes under S.F. conditions (96%) in comparison with TBA-SiW11. Also, further studies are underway in our laboratory to extend the application of these family catalysts to other coupling reactions.
Data availability
All data generated or analysed during this study are included in this published article (and its Supplementary Information files).
References
Gregory, R. J. H. Cyanohydrins in nature and the laboratory: Biology, preparations, and synthetic applications. Chem. Rev. 99, 3649–3682 (1999).
Brunel, J.-M. & Holmes, I. P. Chemically catalyzed asymmetric cyanohydrin syntheses. Angew. Chem. Int. Ed. 43, 2752–2778 (2004).
Kurono, N. & Ohkuma, T. Catalytic asymmetric cyanation reactions. ACS Catal. 6, 989–1023 (2016).
North, M., Usanov, D. L. & Young, C. Lewis acid catalyzed asymmetric cyanohydrin synthesis. Chem. Rev. 108, 5146–5226 (2008).
Mahmoud, A. G., Mahmudov, K. T., da Silva, M. F. C. G. & Pombeiro, A. J. L. Reaction of sodium 2-(2-(2,4-dioxopentan-3-ylidene)hydrazinyl) benzenesulfonate with ethylenediamine on Cu(II) and Ni(II) centres: Efficient Cu(II) homogeneous catalysts for cyanosilylation of aldehydes. RSC Adv. 6, 54263–54269 (2016).
Ma, Z. et al. Effective cyanosilylation of aldehydes with copper(II)-based polymeric catalysts. Mol. Catal. 428, 17–23 (2017).
Gurbanov, A. V. et al. Copper(II) complexes with carboxylic- or sulfonic-functionalized arylhydrazones of acetoacetanilide and their application in cyanosilylation of aldehydes. J. Organomet. Chem. 834, 22–27 (2017).
Karmakar, A., Hazra, S., da Silva, M. F. C. G. & Pombeiro, A. J. L. Synthesis, structure and catalytic application of lead(II) complexes in cyanosilylation reactions. Dalton Trans. 44, 268–280 (2015).
Jia, Y., Zhao, S. & Song, Y.-F. The application of spontaneous flocculation for the preparation of lanthanide-containing polyoxometalates intercalated layered double hydroxides: Highly efficient heterogeneous catalysts for cyanosilylation. Appl. Catal. A Gen. 487, 172–180 (2014).
Zhang, Z., Lippert, K. M., Hausmann, H., Kotke, M. & Schreiner, P. R. Cooperative thiourea-brønsted acid organocatalysis: enantioselective cyanosilylation of aldehydes with TMSCN. J. Org. Chem. 76, 9764–9776 (2011).
Fuerst, D. E. & Jacobsen, E. N. Thiourea-catalyzed enantioselective cyanosilylation of ketones. J. Am. Chem. Soc. 127, 8964–8965 (2005).
Peng, D. et al. Enantioselective cyanoformylation of aldehydes catalyzed by a chiral quaternary ammonium salt and triethylamine. Synlett 2007, 2448–2450 (2007).
An, H. et al. Hybrid dimers based on metal-substituted Keggin polyoxometalates (metal = Ti, Ln) for cyanosilylation catalysis. Dalton Trans. 47, 9079–9089 (2018).
Kikukawa, Y. et al. Cyanosilylation of carbonyl compounds with trimethylsilyl cyanide catalyzed by an yttrium-pillared silicotungstate dimer. Angew. Chem. Int. Ed. 51, 3686–3690 (2012).
Hu, T.-P. et al. A novel silver(I)-Keggin-polyoxometalate inorganic–organic hybrid: A Lewis acid catalyst for cyanosilylation reaction. CrystEngComm 17, 5947–5952 (2015).
Suzuki, K. et al. Strategic design and refinement of Lewis acid-base catalysis by rare-earth-metal-containing polyoxometalates. Inorg. Chem. 51, 6953–6961 (2012).
Kamata, K. & Sugahara, K. Base catalysis by mono- and polyoxometalates. Catalysts 7, 345 (2017).
Li, S., Ji, P., Han, S., Hao, Z. & Chen, X. Two polyoxoniobates-based ionic crystals as Lewis base catalysts for cyanosilylation. Inorg. Chem. Commun. 111, 107666 (2020).
Lacour, M.-A., Rahier, N. J. & Taillefer, M. Mild and efficient trimethylsilylcyanation of ketones catalysed by PNP chloride. Chem. A Eur. J. 17, 12276–12279 (2011).
Babaei Zarch, M. et al. Single-molecule magnets within polyoxometalate-based frameworks. Dalton Trans. 50, 15047–15056 (2021).
Arefian, M., Mirzaei, M., Eshtiagh-Hosseini, H. & Frontera, A. A survey of the different roles of polyoxometalates in their interaction with amino acids, peptides and proteins. Dalton Trans. 46, 6812–6829 (2017).
ArabFashapoyeh, M. et al. Photochemical and electrochemical hydrogen evolution reactivity of lanthanide-functionalized polyoxotungstates. Chem. Commun. 54, 10427–10430 (2018).
Heravi, M. M. et al. H5BW12O40 as a green and efficient homogeneous but recyclable catalyst in the synthesis of 4 H-Pyrans via multicomponent reaction. Appl. Organomet. Chem. 32, e4479 (2018).
Lotfian, N., Heravi, M. M., Mirzaei, M. & Heidari, B. Applications of inorganic-organic hybrid architectures based on polyoxometalates in catalyzed and photocatalyzed chemical transformations. Appl. Organomet. Chem. 33, e4808 (2019).
Derakhshanrad, S., Mirzaei, M., Streb, C., Amiri, A. & Ritchie, C. Polyoxometalate-based frameworks as adsorbents for drug of abuse extraction from hair samples. Inorg. Chem. 60, 1472–1479 (2021).
Akbari, M., Mirzaei, M. & Amiri, A. Synergistic effect of lacunary polyoxotungstates and carbon nanotubes for extraction of organophosphorus pesticides. Microchem. J. 170, 106665 (2021).
Tao, M. et al. Lewis-acid-promoted catalytic cascade conversion of glycerol to lactic acid by polyoxometalates. Chem. Commun. 52, 3332–3335 (2016).
Zhong, J., Pérez-Ramírez, J. & Yan, N. Biomass valorisation over polyoxometalate-based catalysts. Green Chem. 23, 18–36 (2021).
Dey, C. Polyoxometalate clusters: Inorganic ligands for functional materials. J. Clust. Sci. https://doi.org/10.1007/s10876-021-02110-8 (2021).
Himeno, S., Takamoto, M., Santo, R. & Ichimura, A. Redox properties and basicity of Keggin-type polyoxometalate complexes. Bull. Chem. Soc. Jpn. 78, 95–100 (2005).
Zhang, T.-T., Li, G. & Cui, X.-B. Three new polyoxoniobates functioning as different oxidation catalysts. Cryst. Growth Des. 21, 3191–3201 (2021).
Tao, M. et al. Tailoring the synergistic bronsted-lewis acidic effects in heteropolyacid catalysts: Applied in esterification and transesterification reactions. Sci. Rep. 5, 13764 (2015).
Craig, M. J., Barda-Chatain, R. & García-Melchor, M. Fundamental insights and rational design of low-cost polyoxometalates for the oxygen evolution reaction. J. Catal. 393, 202–206 (2021).
Nisar, A., Lu, Y., Zhuang, J. & Wang, X. Polyoxometalate nanocone nanoreactors: Magnetic manipulation and enhanced catalytic performance. Angew. Chem. Int. Ed. 50, 3187–3192 (2011).
Gumerova, N. I. & Rompel, A. Polyoxometalates in solution: Speciation under spotlight. Chem. Soc. Rev. 49, 7568–7601 (2020).
Taleghani, S., Mirzaei, M., Eshtiagh-Hosseini, H. & Frontera, A. Tuning the topology of hybrid inorganic–organic materials based on the study of flexible ligands and negative charge of polyoxometalates: A crystal engineering perspective. Coord. Chem. Rev. 309, 84–106 (2016).
Mirzaei, M., Eshtiagh-Hosseini, H., Alipour, M. & Frontera, A. Recent developments in the crystal engineering of diverse coordination modes (0–12) for Keggin-type polyoxometalates in hybrid inorganic–organic architectures. Coord. Chem. Rev. 275, 1–18 (2014).
Sadjadi, S., Lazzara, G., Malmir, M. & Heravi, M. M. Pd nanoparticles immobilized on the poly-dopamine decorated halloysite nanotubes hybridized with N-doped porous carbon monolayer: A versatile catalyst for promoting Pd catalyzed reactions. J. Catal. 366, 245–257 (2018).
Sadjadi, S., Malmir, M., Lazzara, G., Cavallaro, G. & Heravi, M. M. Preparation of palladated porous nitrogen-doped carbon using halloysite as porogen: Disclosing its utility as a hydrogenation catalyst. Sci. Rep. 10, 2039 (2020).
Yekke-Ghasemi, Z. et al. Fabrication of heterogeneous-based lacunary polyoxometalates as efficient catalysts for the multicomponent and clean synthesis of pyrazolopyranopyrimidines. Inorg. Chem. Commun. 140, 109456 (2022).
Malmir, M., Heravi, M. M., Amiri, Z. & Kafshdarzadeh, K. Magnetic composite of γ-Fe2O3 hollow sphere and palladium doped nitrogen-rich mesoporous carbon as a recoverable catalyst for C-C coupling reactions. Sci. Rep. 11, 22409 (2021).
Sadjadi, S., Heravi, M. M. & Malmir, M. Pd@HNTs-CDNS-g-C3N4: A novel heterogeneous catalyst for promoting ligand and copper-free Sonogashira and Heck coupling reactions, benefits from halloysite and cyclodextrin chemistry and g-C3N4 contribution to suppress Pd leaching. Carbohydr. Polym. 186, 25–34 (2018).
Sadjadi, S., Malmir, M. & Heravi, M. M. A green approach to the synthesis of Ag doped nano magnetic γ-Fe2O3@SiO2-CD core–shell hollow spheres as an efficient and heterogeneous catalyst for ultrasonic-assisted A3 and KA2 coupling reactions. RSC Adv. 7, 36807–36818 (2017).
Sadjadi, S., Hosseinnejad, T., Malmir, M. & Heravi, M. M. Cu@furfural imine-decorated halloysite as an efficient heterogeneous catalyst for promoting ultrasonic-assisted A3 and KA2 coupling reactions: A combination of experimental and computational study. New J. Chem. 41, 13935–13951 (2017).
Nisar, A., Zhuang, J. & Wang, X. Cluster-based self-assembly: reversible formation of polyoxometalate nanocones and nanotubes. Chem. Mater. 21, 3745–3751 (2009).
Nie, Y.-M., Li, S.-H., Lin, M.-Y. & Yan, J. A micro-environment tuning approach for enhancing the catalytic capabilities of lanthanide containing polyoxometalate in the cyanosilylation of ketones. Chem. Commun. 56, 3809–3812 (2020).
Maksimovskaya, R. I. & Maksimov, G. M. 31P NMR studies of hydrolytic conversions of 12-tungstophosphoric heteropolyacid. Coord. Chem. Rev. 385, 81–99 (2019).
Niu, Y. et al. A new sandwich polyoxometalate based on Keggin-type monolacunary polyoxotungstoborate anion, [Zr(α-BW11O39)2]14−. Inorg. Chem. Commun. 12, 853–855 (2009).
Aguirre-Díaz, L. M., Iglesias, M., Snejko, N., Gutiérrez-Puebla, E. & Monge, M. Á. Indium metal–organic frameworks as catalysts in solvent-free cyanosilylation reaction. CrystEngComm 15, 9562 (2013).
Karmakar, A., Paul, A., Sabatini, E. P., da Silva, M. F. C. G. & Pombeiro, A. J. L. Pyrene carboxylate ligand based coordination polymers for microwave-assisted solvent-free cyanosilylation of aldehydes. Molecules 26, 1101 (2021).
Han, D., Yan, X.-L. & Liu, J. Porous Gd III-organic framework as a dual-functional material for cyanosilylation of aldehydes and ablation of human liver cancer cells. Z. Anorg. Allg. Chem. 645, 422–427 (2019).
Zhang, Z. et al. Insight into the catalytic properties and applications of metal–organic frameworks in the cyanosilylation of aldehydes. RSC Adv. 5, 79355–79360 (2015).
Han, Q., Sun, X., Li, J., Ma, P. & Niu, J. Novel isopolyoxotungstate [H2W11O38]8– based metal organic framework: As Lewis acid catalyst for cyanosilylation of aromatic aldehydes. Inorg. Chem. 53, 6107–6112 (2014).
Ma, Z. et al. Multinuclear Zn(II)-arylhydrazone complexes as catalysts for cyanosilylation of aldehydes. J. Organomet. Chem. 912, 121171 (2020).
Wang, Z., Fetterly, B. & Verkade, J. G. P(MeNMCH2CH2)3N: An effective catalyst for trimethylsilycyanation of aldehydes and ketones. J. Organomet. Chem. 646, 161–166 (2002).
Acknowledgements
Authors are grateful to Iran National Science Foundation (INSF) for the financial support provided by the post-doctoral project (99023684). We also appreciate the Alzahra University Research Council and the Ferdowsi University of Mashhad, Mashhad, Iran, for their help and supports.
Author information
Authors and Affiliations
Contributions
M.Malmir: Methodology, Formal analysis, Investigation, Software, Data curation, Writing-original draft, Preparing the revised file. M.M.H.: Conceptualization, Funding acquisition, Supervision, Project administration, Visualization. Z.Y.-G.: Methodology, Formal analysis, Investigation, Software, Data curation, Writing-original draft, Preparing the revised file. M.Mirzaei: Methodology, Conceptualization, Funding acquisition, Supervision, Main idea, Writing-review and editing, Project administration, Visualization.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Malmir, M., Heravi, M.M., Yekke-Ghasemi, Z. et al. Incorporating heterogeneous lacunary Keggin anions as efficient catalysts for solvent-free cyanosilylation of aldehydes and ketones. Sci Rep 12, 11573 (2022). https://doi.org/10.1038/s41598-022-15831-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-15831-1
This article is cited by
-
Efficient oxidation of sulfides to sulfoxides catalyzed by heterogeneous Zr-containing polyoxometalate grafted on graphene oxide
Scientific Reports (2023)
-
Polyoxometalate-based boron dipyrromethene (BODIPY) conjugates: syntheses, characterization and photophysical properties
Journal of the Iranian Chemical Society (2023)
-
Structural, physico-chemical properties of a hybrid material based on Anderson-type polyoxomolybdates
Journal of the Iranian Chemical Society (2023)
-
Crystal structure, structural characterization and Hirshfeld surface analysis of three hydrogen bonded pyridine derivative crystals
Journal of the Iranian Chemical Society (2023)
-
Molecular Speciation of Isopolyoxomolybdates and Isopolyoxotungstates with Silicic Acid in Aqueous Solution Using ESI–MS
Journal of Solution Chemistry (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.