Abstract
The evolutionary history and palaeoecology of orangutans remains poorly understood until today. The restricted geographic distribution of extant Pongo indicates specific ecological needs. However, it is not clear whether these needs were shared by the great diversity of fossil pongines known from the Miocene to the Pleistocene. Here we show how niche modelling of stable carbon and oxygen isotope data of the carbonate fraction of dental enamel can be used to reconstruct the paleoecology of fossil and modern pongines and associated mammal communities. We focus on Khoratpithecus ayeyarwadyensis, a Late Miocene pongine from Myanmar and the sister clade to extant orangutans, and compare it to its associated mammal fauna and other fossil and extant pongines. The results are consistent with a vertical position high up in the canopy of a forested habitat with purely C3 vegetation for K. ayeyarwadyensis as well as the contemporaneous Sivapithecus. Although their positions in the modelled isotopic niche space look similar to the ecological niche occupied by modern Pongo, a comparison of the modelled niches within the pongine clade revealed possible differences in the use of microhabitats by the Miocene apes.
Similar content being viewed by others
Introduction
Today, the only remaining genus of the Ponginae subfamily is the genus Pongo, including the three extant species of orangutans, whose distribution is highly restricted to forested areas of Borneo and Sumatra1 (Fig. 1). In contrast, paleontological data document a much greater diversity of pongine genera, with the earliest fossils recovered in Southeast Asia from the Chiang Muan Formation (Fm.) in Thailand and dating to 13–12.6 Ma2,3. Several different fossil pongine genera such as Khoratpithecus (Myanmar, Thailand), Sivapithecus (Pakistan, India, and Nepal), and Indopithecus (India) are known from the Miocene, as well as Gigantopithecus (China, Thailand, Vietnam) and early Pongo from the Pleistocene4,5. This high degree of diversification and endemism of the hominoid primates contrasts with the much more generalist character of the associated mammal fauna across different Southeast Asian sites6,7,8. The question now arises if this high pongine diversity also coincides with a diversity of subsistence strategies in this clade.
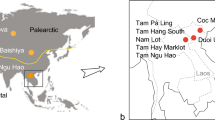
Location of fossil pongine localities from the Miocene to the Holocene in Southern Asia, Southeast Asia and Southern China including the present distribution of extant orangutans. The stable isotope data used in the study originates from the localities marked with a diamond (SIA = stable isotope analysis) and the Yinseik locality where K. ayeyarwadyensis was found is labelled with a star. A map version with labels for all the sites from which we used stable isotope data can be found in the Supplementary Information (Fig. SI 4). The map was created using QGIS 3.16.
Khoratpithecus is known from isolated teeth, two mandibles and a maxilla9,10,11,12. This genus has been first allocated to the pongines based on shared derived dental and mandibular characters with Pongo6,10. It has been hypothesized that Khoratpithecus may represent the sister-group of Pongo based on the lack of an insertion for the anterior digastric muscle, a feature exclusively shared between these genera, and structural similarities of their mandibular symphyses6,10 (e.g., strong symphyseal inclination and weak upper transverse torus). More recently, the analysis of a maxilla of Khoratpithecus has revealed several additional pongine features for this genus in its upper dentition (e.g., externally rotated canines) and subnasal anatomy (e.g., strong overlap of the premaxilla relative to the maxilla, antero-posteriorly convex nasoalveolar clivus, subhorizontal incisive foramen)11. Phylogenetic analyses5,13 strengthen the hypothesis of a pongine clade including Pongo and large Asian Neogene hominoids (Sivapithecus, Indopithecus, Gigantopithecus, Khoratpithecus, Ankarapithecus, and irregularly Lufengpithecus). In the most recent phylogenetic analysis5, Khoratpithecus ayeyarwadyensis is regularly found as the sister-group of Pongo. Among the fossil genera of Asian hominoids, only the pongine status of Lufengpithecus is highly controversial according to investigations of its skull anatomy (in particular the orbital region)14 and further comparisons of its craniofacial features with other Asian Miocene hominoids11. This hominoid probably represents a stem hominid.
As the genus Khoratpithecus is hypothesized to be the sister-clade to Pongo6, investigating its paleoecology is crucial to understand the evolutionary history of modern orangutans. There are currently three different known species of Khoratpithecus. The Middle Miocene K. chiangmuanensis9 and the Late Miocene K. piriyai6 have been found in Thailand, but teeth from these two species were not available for sampling. However, one specimen of the Myanmar species K. ayeyarwadyensis could be sampled for stable isotope analysis. It is, together with its associated mammal fauna, the focus of our study. The fossil remains have been found in the Irrawaddy Fm. at a locality southeast of Magway near Yinseik village dating to the Late Miocene (~ 9.5 Ma)12. Hence, it will be referred to as Yinseik locality hereafter.
The important phylogenetic role of the genus Khoratpithecus as a sister clade of modern Pongo made us particularly interested in its palaeoecology and habitat. We wanted to characterize this habitat concerning its palaeoseasonality, vegetation structure, and the niche partitioning of the mammal fauna associated with K. ayeyarwadyensis. In a second step, we used the available stable isotopic data of fossil and extant pongines (Fig. 1) to assess if there is evidence for ecological continuity within the pongine clade, an important question to enhance our understanding of the evolutionary ecology of the Ponginae. The response of fossil pongines to climatic changes and forest fragmentation additionally can provide information for conservation efforts of orangutan populations today, which are continuously put under stress due to anthropogenic disturbances and habitat loss15,16. Similar conditions of continuing forest fragementation have been hypothesised to be the cause of the extinction of Sivapithecus during the Late Miocene17.
To answer these questions, we performed stable isotopic analysis (SIA), and used these data to reconstruct the niche partitioning in this habitat, as well as palaeoseasonality and to get a first direct indication of the ecology of this fossil pongine. Carbon and oxygen isotopes from the carbonate fraction of dental enamel that we analysed are commonly used as proxies for vegetation type18,19, and canopy density20,21; temperature, humidity22, as well as vertical stratification23,24 in primates, respectively. In this study, we will discuss δ13C values corrected for isotopic enrichment from diet to bioapatite and for changes in the isotopic composition of CO2. These corrections are discussed in detail in the Methods section. With these data, we modelled the core ecological niches of the different taxonomic groups and could characterize the niche partitioning in the Yinseik mammal fauna, quantify niche width and niche packing.
The modelled ecological niches form the basis for our comparison of the Khoratpithecus fauna with a younger locality from the Irrawaddy Fm, which did not yield any Khoratpithecus fossils25. This is a similar approach as the comparison of the Sivapithecus and the post-Sivapithecus fauna performed by Nelson17. This comparison will enhance our understanding of the paleoecology of K. ayeyarwadyensis and the changes happening in its habitat especially regarding the introduction of C4 plants and the general opening of the landscape. Ecological core niches were also modelled for other fossil and extant pongines. These niches were compared to one another to see, if they are consistent with the hypothesis of ecological continuity in the Ponginae.
Material and methods
The fluvial deposits of the Irrawaddy Fm. represent one of the most important fossiliferous formations located in the Central Basin of Myanmar (Fig. 1). The hominoid-bearing locality of Yinseik from which the K. ayeyarwadyensis specimen and the associated mammal fauna analysed in this study originate is located close to the village Yinseik, east of the Irrawaddy River and about 20 km southeast of Magway (Magway Region). The section in the Yinseik area, which represents the base of the Irrawaddy Fm., is composed of about 100 m of fluviatile sediments with low dipping mostly constituted of poorly consolidated and coarse sandstones and hardened fine sandstones alternated with clayey sandstones and claystones. Oxidized conglomeratic layers also occur and are frequently fossiliferous. These deposits most probably represent a small lapse of time because they have yielded a homogeneous vertebrate fauna and have recorded a single normal magnetic polarity12. A 10.4–8.6 Ma age bracket (early Late Miocene) has been as inferred from biochronology. Two ages within this bracket, ~ 10 Ma and ~ 9 Ma, are compatible with the magnetostratigraphy12. There are great similarities in the composition of the associated mammal fauna of K. ayeyarwadyensis7 and of the contemporaneous faunas associated with K. piriyai6 and Sivapithecus8.
For this study, we sampled 44 teeth of various taxa, i.e. Rhinocerotidae (n = 9), Proboscidea (n = 8), Bovidae (n = 11), Suidae (n = 7), Giraffidae (n = 5), and one specimen of K. ayeyarwadyensis (MFI-K171) as well as one cervid and one anthracothere. The pongine specimen is a left hemi-mandible of K. ayeyarwadyensis, which is also the holotype of this species12. Sampling it for isotopic analysis was possible, as during preparation of the fossil a small enamel fragment of the m3 broke of, which was then analysed. We also included one modern bovid from the same area in our data set. Data from the Yinseik Equidae (n = 6) from the Yinseik locality has already been published by Jaeger et al.12.
Data from published studies with a similar scope and objective were used as reference (Table SI 3)12,17,25,26,27,28,29,30,31,32,33,34,35,36,37. These studies also analysed the carbonate fraction of dental enamel. However, the Indopithecus specimen was sampled using laser ablation instead of micro drilling. We use the normalized δ13C and δ18O values proposed by the authors26 to compare them to the other conventional CaCO3 data. However, especially the δ18O values might still be biased, due to the different methodologies. We complemented our data by the addition of Louys and Roberts24 data set on modern orangutans. Precise taxonomic information and specimen numbers of the whole data set used in this study are reported in Table SI 2.
Sample preparation
The enamel was sampled using a micro dremel tool to retrieve 6–10 mg of powdered sample during fieldwork in Myanmar conducted in 2017. We took bulk samples of most of the teeth, where the drilling was done in a band along the whole growth axis of the tooth. Values represent the average isotopic composition over the mineralization time of the tooth during ontogeny. For some high-crowned teeth, it was possible to conduct intra-individual serial sampling (IRWD-9, IRWD-17, IRWD-24, IRWD-31, IRWD-42, and PND-M1). In these cases, multiple samples were drilled from bands perpendicular to the growth axis of the tooth providing a continuous record of isotopic variation during the mineralization time of this tooth.
The powdered samples were pretreated and the carbonate fraction of the dental enamel was analysed in the laboratory of the Department of Geosciences (Biogeology working group) at the University of Tübingen (Germany). They were let to react with 1.35 ml of a NaOCl solution at a concentration of 2.5% for 24 h to remove all the organic matter. After rinsing them with Milli-Q H2O the samples reacted with 1.35 ml of 1 M acetic acid buffered solution (CH3COOH) for 24 h to remove all exogenous carbonates. The method for the pretreatment of the samples followed the methodology described in38,39,40. When the reaction was completed, the samples were dried at 40 °C. With each set of samples, two internal modern enamel standards (Elephant SRM (SRM = secondary reference material), Hippo SRM) were processed following the same pretreatment protocol. The internal standards were complemented by two international (IAEA-603, NBS-18) and one internal (LM = Laaser Marmor SRM) pure carbonate standards. Pure carbonate standards were not subjected to any pretreatment. All of the standards were measured after every 15 samples in the IRMS (isotope ratio mass spectrometer).
2.5–3 mg or 0.1 mg of sample for enamel and pure carbonates respectively was then reacted with phosphoric acid (H3PO4) at a concentration of ~ 99%. The CO2 gas that resulted from this reaction was then analysed with the Elementar IsoPrime 100 IRMS 5 times over a 15-min time span. These repeated measurements were then used to monitor measurement precision by calculating the mean and the standard deviation for each sample41. Measurement uncertainty, as assessed using the standards and is reported for each sample in Table SI 2. The measured isotopic ratios were then calibrated relative to VPDB (Vienna Pee Dee Belemnite) using the two internal enamel standards. They are reported using the δ-notation (in per mill) whose calculation is based on the following formula42,43 where jX is the heavier and iX the lighter isotope.
We also report an estimation of the CaCO3 content of each sample (Table SI 2). With the Ion Vantage software, we calculated an estimated elemental composition based on sample weight, peak area and the internal LM SRM. The obtained CaCO3 values are then scaled up, until one of the international pure carbonate standards (IAEA-603, NBS-18) reaches 100%.
Data correction
To enable the comparison of specimens from different species, sites, and time periods it was necessary to apply different corrections of the data, especially the δ13C values. All the δ13Capatite values were transformed to δ13Cdiet values. This was done using different enrichment factors (ε, in per mill) for different groups of animals. It is calculated using this formula42,44, where a stands for diet and b for apatite:
ε is based on the isotopic fractionation factor (⍺), which is derived from the δ-values as defined in (1). Isotopic fractionation from diet to apatite is not explainable by a single kinetic or equilibrium process42. We account for this complexity by the use of the terms apparent isotopic fractionation factor (⍺*) and apparent enrichment factor (ε*) in the rest of this paper. Using Δ (δ13Cdiet − δ13Capatite) decreases in accuracy when the isotopic differences among tissues are ≥ 10‰45, which is the reason why we decided to use ε instead.
We applied apparent enrichment factors based on the results of published studies of controlled feeding experiments of − 14‰ for large-bodied, ruminant herbivores45,46, − 11‰ for omnivores including suids46,47 and primates48.
For the comparison of animals from different time periods it is necessary to correct the δ13C values for changes of the δ13CCO2 values in the atmosphere caused by the Suess effect49. All values have been corrected to the pre-industrial values from 1850 of − 6.5‰50. The δ13CCO2 values of Miocene data points from sites that are older than 6 Ma were assumed to be − 6.1‰ and therefore a correction of 0.4‰ was applied to them51. Modern atmospheric δ13CCO2 values are − 8‰52,53, so all post 1930 samples were corrected by 1.5‰. Pre 1930 to 6 Ma old values are treated as equivalent to the pre-industrial ones54, and hence are not corrected. As the maximum age of the Chaingzauk fauna, from the other Miocene locality of the Irrawaddy Fm., is constrained to 6 Ma by its biostratigraphy25 no corrections were applied to the δ13C values from this site. The ε as well as the atmosphere correction used for each specimen are reported in Table SI 2 and Table SI 3 together with the calibrated data and corrected δ-values used in the analysis.
Statistical analysis
For this study, we applied different statistical methods, which we want to describe in the following section. Given the small sample sizes in our data set as well as the presence of outliers and not normally distributed data we decided to use the non-parametric Wilcox rank sum test when testing for differences of the medians between two groups throughout this study. The tests were run in R using the wilcox.test() function (package stats version 4.1.2, one of the base packages in R).
We re-ran the linear regression on minimum δ18O values over time (Ma = million years ago) published by Nelson55 using the lm() function (package stats version 4.1.2, one of the base packages in R) and added that data from the Yinseik equids to the figure in order to compare them to the regression line.
The lowest δ18O value (− 5.7‰) measured in the serially sampled modern bovid specimen (PND-M1) looked like an anomaly during visual inspection. We therefore wanted to test if it was a statistical outlier. Before that, it was necessary to test if the assumption of the Grubbs’ test that the data are normally distributed was fulfilled. Therefore we conducted a Shapiro–Wilk test of normality using the shapiro.test() function (package stats version 4.1.2, one of the base packages in R). The results of this test for the δ18O value of PND-M1 show that the p-value is bigger than 0.05 (W = 0.94899, p-value = 0.4094), indicating that we do not reject the null hypothesis that the data follow a normal distribution. The Grubbs’ test for outliers was run in R using the grubbs.test(data, opposite = T) function (package outliers56 version 0.15) to test, if the minimum value of the data set was an outlier.
To be able to quantify and better compare the niche width and niche partitioning of the different mammal communities we applied isotopic niche modelling. Until now, isotopic niches based on the niche concept of Hutchinson57,58 have been mostly limited to dietary or trophic niches based on carbon and nitrogen isotopic composition of collagen59. As we analysed the carbonate fraction of the fossil dental enamel and therefore have data on two proxies reflecting more general ecological characteristics of an individuals’ habitat, we will model more general ecological niches for the pongines and the associated mammal fauna using the R package SIBER (version 2.1.6)60. Nelson and Hamilton already adopted a similar approach focusing on the dietary transition and ecological niche of early humans61. In this study we use standard ellipse area corrected for small sample sizes (SEAc) that corresponds to a confidence interval (CI) of 40%60. These calculations are based on a maximum likelihood estimation in a Bayesion framework. This framework is well suited for small sample sizes in general as it counteracts their effects on the statistical power of the analysis to a certain extent. However, it should be noted that increasing the sample size especially for the Miocene hominoids would lead to more robust results.
Statistical tests and modelling (linear models and Bayesian niche modelling) were conducted using R version 4.1.2 (2021-11-01) “Bird Hippie”. Most figures were generated using Excel (2016) except for the maps for which we used QGIS (version 3.16) and the visualisation of the models, which were created using the plot functions in R (packages ggplot2 version 3.3.5, and SIBER version 2.1.6). All figures were further modified using GIMP (version 2.10.18).
Results and discussion
Paleoseasonality estimations for Late Miocene of Myanmar
Intra-individual variation of δ18O values is routinely used to infer paleoseasonality. In regions with monsoonal climate, the temperature effect on these values is overwritten with the amount effect22. Therefore, the wet season is characterized in these regions by lower δ18O values and the dry season by higher ones.
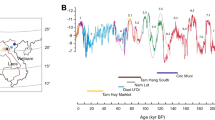
Sun and collaborators interpreted the lower δ18O values from the Shuitangba locality (~ 6.2 Ma) from the Yunnan province in southwest China, in comparison to modern reference data, as an indication of a wetter climate during the Late Miocene than today62. The same pattern is also present in the hipparions from the Siwaliks of Pakistan55. The minimum δ18O values of the carbonate fraction of their dental enamel measured through the serial sampling of hypsodont teeth rise over time, which is consistent with a decreasing amount of seasonal precipitation (Fig. 2). For the Late Miocene, seasonal precipitation regime with a dry season of five to six months (similar to the monsoonal forests in southern China today) was inferred from intra-individual serial sampling of equid dental enamel55.
Development of the minimum δ18O values from dental enamel of hipparions from the Siwaliks21 over time. The linear regression line. (y = − 1.1527x + 6.2827; adjusted R2 = 0.3216; p-value = 0.01611) based on these values shows a trend towards more positive δ18O values towards the end of the Miocene corresponding to lower seasonal maxima of precipitation. Minimum δ18O values from the Yinseik specimens were added in orange. All of them plot below the regression line among the lowest minima from the Siwalik hipparions. Icon obtained via PhyloPic and in public domain.
For our data set, we used a modern bovid from Myanmar as reference for the seasonal variation of the δ18O values in the study area today. The δ18O values of the modern bovid from Myanmar are significantly higher than the Miocene values from the Yinseik locality (Table 1). We tested this with a Wilcoxon rank sum test comparing the modern with the Miocene bovid, which was the individual whose δ18O values were closest to the modern specimen (W = 6, p-value = 0.0001248). This indicates a wetter climate in Miocene Myanmar than today and is clearly visible when plotting the δ18O values against the distance from the ERJ (enamel root junction) (Fig. 3). A comparison of the minimum δ18O values from the Siwaliks and the Yinseik locality also shows that the climate in Miocene Myanmar was likely even wetter than in the contemporaneous Siwaliks (Fig. 2). Only the bovid from the Yinseik locality plots among the equids from the Siwaliks and are near the regression line. The specimen did not have a high tooth crown preserved, due to tooth wear, and therefore our data might not cover the time period of maximum seasonal precipitation, which would explain the higher minimum δ18O value in comparison to the other mammals from the same locality. An alternative explanation would be differences in metabolism. High δ18O values are expected in water independent species, because they obtain their water mostly from their food and not meteoric water, which reduces the loss of body water since it is recycled. However, modern bovids are not considered to be such a water independent species.
Comparison of the intra-individual serial sampling of a Miocene rhino from the Irrawaddy Fm. with a modern bovid from the Central Basin in Myanmar. Distance from ERJ (enamel root junction) is plotted in reverse order to correctly represent the enamel mineralization from oldest (left) to youngest (right).
A Wilcoxon rank sum test showed that δ18O values of bulk samples from the Khoratpithecus fauna (Fig. 4a) are significantly lower in comparison with the Sivapithecus fauna (W = 4056, p-value = 1.245e−06) (Fig. 4c). This is also consistent with this interpretation. However, the amplitude of the δ18O values, defined as the difference between maximum and minimum values of one tooth, is smaller for the Miocene specimen than for the modern reference sample (Table 1). If we remove the outlier (PND-M1n) (tested with the Grubbs’ test for outliers (G = 2.5669, U = 0.6136, p-value = 0.0427)) of the modern bovid the amplitude drops from 5.3 to 3.5‰, which is still higher than the maximum amplitude from a Miocene specimen, the Giraffid (IRWD-42) with 2.8‰. This is consistent with a less pronounced difference in seasonal precipitation in the Miocene, as compared to the climate in modern day Myanmar.
Bayesian niche modelling of four mammal communities. The ellipses mark the SEA (standard ellipse area) or core ecological niche, which corresponds to a 40% CI. Summary statistics are reported in Table SI 1. (A) Yinseik fauna including K. ayeyarwadyensis (~ 9.5 Ma), (B) Chaingzauk fauna (6 4 Ma)25, (C) Siwalik fauna including Sivapithecus (~ 9 Ma)17, (D) later Siwalik fauna (~ 8 Ma)17. Icons from PhyloPic and in public domain or under CC 3.0 license (Rhinocerotidae, Hippopotamidae, and Tragulidae by Zimices).
In addition, we do not see the same pattern of covariation between the δ13C and the δ18O values. In the Miocene specimens, we see marginal positive correlations, whereas δ13C and δ18O values of the modern bovid are slightly negatively correlated. The stronger a positive or negative correlation is, the more seasonality effects an individuals diet, e.g. its quality or source. Hence, the results suggest a different influence of the seasonal extremes on the food resources in the Miocene than today. What the difference between a positive or negative covariation of δ13C and δ18O values reflects still needs to be characterised by further studies. The δ13Cdiet values have been corrected for variations of isotopic composition of the CO2 in the atmosphere as well as isotopic enrichment factors from diet to enamel. A detailed discussion can be found in the Methods section.
Paleoecology and niche partitioning of the Khoratpithecus mammal community
The δ13Cdiet values of the Khoratpithecus fauna from the Irrawaddy Fm. at the Yinseik locality span almost the entire range of modern C3 plants (− 33 to − 22‰)18, with Giraffidae and Rhinocerotidae having the lowest and Suidae having the highest values (Fig. 4a). When we correct the δ13C values of C3 plants from the modern values reported by Bender18 to preindustrial ones (used in this paper to compare data sets from different eras to one another) the cut-off point for C3 vegetation lies at − 20.5‰. Higher values would indicate the presence of C4 plants in an individual's diet.
A comparison of the range of δ13C values of the Yinseik data set (− 30.3 to − 21.3‰) to the contemporaneous Sivapithecus fauna from the Dhok Pathan and Khaur regions of the Siwaliks (− 29.0 to − 20.8‰) shows that, both K. ayeyarwadyensis and Sivapithecus lived in a mosaic landscape. The isotopic data from the two areas are consistent with a woodland forest with open patches that were mostly occupied by suids. In the Siwalik fauna the equids also have δ13C values indicating more open habitats. In contrast, the equids from the Yinseik locality lived in more closed parts of the habitat according to their δ13C values. Therefore, it seems that the Yinseik habitat was a bit more densely forested than the Siwalik one. None of the mammals from the Yinseik locality have δ13C values above the cut-off point of − 20.5‰ including the equids, indicating a pure C3 vegetation. Considerably higher δ18O values of the Sivapithecus fauna (maximum = 0.7‰, mean = − 4.5‰) in comparison to the Khoratpithecus fauna (maximum = − 3.3‰, mean = − 6.5‰) are consistent with a slightly cooler and more humid climate at the Yinseik locality than in the Siwaliks. This pattern was also present in the comparison of minimum δ18O values discussed in the previous section (Fig. 2).
The δ13C value of K. ayeyarwadyensis place its habitat in the more open parts of the forest. It does plot among the data points with the highest δ18O values similar to those of giraffids and equids. For the former, consumption of leaves or fruit with higher δ18O values due to evapotranspiration is the most probable reason for these values whereas drinking from evaporated water sources like ponds seem more likely for the latter. As expected, the δ18O values of browsers foraging on leaves closer to the forest floor like the rhinos are lower than the ones from more arboreal browsers like giraffids.
In primates folivory/frugivory as well as vertical stratification in habitat use have been discussed as drivers of oxygen isotope fractionation21,63. Recent studies however showed that vertical stratification probably is the primary driver of variations of δ18O values23,24. Hence, the δ18O value of K. ayeyarwadyensis is consistent with foraging high up in the canopy. The corresponding δ13C value is also consistent with this interpretation, because the canopy effect is less pronounced high up in the canopy leading to higher δ13C values of animals foraging there. A predominantly frugivorous diet, without any evidence for the consumption of hard objects has been reconstructed using dental microwear texture analysis and dental topography of K. piriyai and K. chiangmuanensis, the two closest allies of the Myanmar species64, a subsistence strategy consistent with our data. K. piriyai is known from fossil localities in Thailand contemporaneous to the Yinseik locality with K. ayeyarwadyensis discussed in this paper where it is associated with a similar mammal fauna6,7.
The two younger data sets from the Chaingzauk (6–4 Ma) locality in Myanmar (Fig. 4b) and the post-Sivapithecus (~ 8 Ma) layers from the Siwaliks (Fig. 4d) show similar trends. A slight shift towards more positive δ13C values in the Siwaliks (− 28.6 to − 19.7‰) and a drastic shift plus an increasing range for δ13C values in the Chaingzauk data (− 28.0 to − 11.3‰) both illustrate an ongoing opening of the landscape. As the Chaingzauk locality is 2–4 Ma younger than the post-Sivapithecus Siwalik data set, it becomes apparent, that the opening of the landscape was an ongoing process in South and Southeast Asia throughout the Late Miocene. The introduction of C4 plants results in the adaptation of the ecological niches of some groups of mammals, especially the suids and equids in the Siwalik and bovids and rhinos in the Chaingzauk community.
Assessing the ecological niches of fossil pongines
The ecological niches of Sivapithecus and K. ayeyarwadyensis inferred from the modelled isotopic niches and the comparison with associated mammal fauna look very similar to each other and to modern orangutans in some general characteristics. The ecological niches of the fossil pongines are consistent with predominantely frugivorous, arboreal forest dwellers, characteristics that can also be applied to orang-utans today. The direct comparison of the ecological niches of the various fossil and extant pongines however, indicates differences in their ecology and habitat use (Fig. 5). The facts that only one sample of Khoratpithecus was available and we do see a high variation in both the δ13C and δ18O values of the other pongine genera do limit the interpretations we can make in regard to this specific pongine. We can however give a first approximation of its ecology and show that it is consistent with the trends visible through the contemporaneous Sivapithecus, for which the sample size (n = 5) was large enough to model an isotopic niche with reasonable confidence60.
Bayesian niche modelling of fossil and extant pongines. The lines encompass the standard ellipse area or core ecological niche, corresponding to a confidence interval of 40%60. Icons are public domain (Gigantopithecus, Indopithecus) or Creative Commons 3.0 license [modern and Pleistocene Pongo by Gareth Monder, Khoratpithecus by Mateus Zica (modified by T. Michael Keesey), and Sivapithecus by Nobu Tamura (modified by T. Michael Keesey)] via PhyloPic.
Modern species of Pongo are the most frugivorous of all extant apes. Although the diets of the two Miocene pongines were reconstructed as predominantly frugivorous, the addition of some hard objects like seeds and nuts for Sivapithecus but not for Khoratpithecus64,65,66, highlights a difference in the dietary ecologies between the genera. Modern orangutans rely on a number of fallback foods (e.g. inner bark, pith, young leaves, and flowers)67,68 in times of fruit scarcity, which are tough, but not hard, and have therefore different microwear patterns69.
To test the similarity of habitat between Sivapithecus and Pleistocene Pongo, we conducted a Wilcoxon Rank Sum test to see if there is a difference in δ13Cdiet values between both pongines. The result shows a significant difference (W = 4.5, p-value = 0.01422). Therefore, although the ecological niche of Sivapithecus and K. ayeyarwadyensis within their respective mammal communities resemble that of modern Pongo, a comparison of these ecological variables with the other pongines on a broader scope (Fig. 5) reveals that the microhabitats they occupied or their habitat use differed significantly.
Although all groups of pongines lived in a forested habitat, K. ayeyarwadyensis, Sivapithecus and Indopithecus seem to be located in a more fragmented forest or higher up in the canopy where the canopy effect is less pronounced. The ecological niches of both modern and Pleistocene Pongo as well as Gigantopithecus are indicating a more closed canopy or foraging lower down in the canopy or even on the ground where the canopy effect is more pronounced. A difference in forest structure between the Miocene and Pleistocene is also suggested by palynological data, paleosol isotopic data, and climate and vegetation models52,70,71,72,73,74,75. The change in vegetation and landscape structure could be explained by the marked climate change during the end of the Neogene52.
The difference in isotopic niches between Miocene and the Pleistocene pongines as well as modern Pongo is likely not only related to the general structure of the habitats, but also to the nature of habitat use. The data on the Miocene pongines K. ayeyarwadyensis and Sivapithecus are consistent with using areas higher up in the canopy, as they have both higher δ18O and δ13Cdiet values. This correlates nicely with a lower body mass in comparison with the Pleistocene pongines. However, the estimated body mass of around 150 kg or more of Indopithecus, comparable with extant Gorilla76, is more similar to Gigantopithecus77 and makes an arboreal habitat less probable. Nevertheless, its isotopic niche is more similar to the Miocene pongines from Myanmar and Pakistan than to Gigantopithecus or Pongo. As the similar sized gorillas exploit arboreal resources and climb trees, a similar behaviour of Indopithecus would be possible. Alternatively, its isotopic niche would be also consistent with a more open habitat, which has already been suggested for Indopithecus78. In addition, the interpretation of the Indopithecus ecological core niche based on the data available for this study should be regarded as preliminary, especially for the δ18O values, given the fact that all the data come from one individual from which intra-individual serial samples were taken using laser ablation. Although we used the δ18O values corrected by an offset of 5.1‰ (“CO3 equivalent”)26 for our study, we cannot exclude that they are not biased due to the different sampling methodology.
Gigantopithecus seems to have occupied the most densely-forested habitats, given the low δ18O and δ13Cdiet values. Within this genus, there are two outliers with high δ13C values, which are two of the samples from the Early Pleistocene Longgudong Cave in Southern China28. These values are consistent with a more open habitat for these two individuals, although their δ18O values do not correspond to plants subjected to evaporative stress as food sources. However, Jiang and collaborators re-analysed one of these two samples and reported a value 1.6‰ lower79. It cannot be ruled out that the variation is partly caused by the different pretreatment method applied by Nelson28.
Wilcoxon Rank Sum test confirmed that Pleistocene and modern Pongo did not differ significantly in their δ13C values (W = 86.5, p-value = 0.2123). Hence, modern orangutans have not yet been pushed by human pressure into denser forest habitats or to the forest margins and more open spaces due to deforestation around a century ago, which is the time period our sample dates to. All the dates are reported in Table SI 2 and Table SI 3. These results also show that although their habitats became increasingly fragmented, pongines remained dependent on closed habitats and were unable to adapt and move into more open forested woodlands.
The data are consistent with shifts in δ18O consistent with differences in vertical stratification23,24. Decreasing δ13Cdiet values from the Miocene pongines (except in Indopithecus), to Pleistocene and modern Pongo, to Giganthopithecus are also consistent with this interpretation, as the canopy effect is more pronounced lower down in the canopy. This seems plausible for K. ayeyarwadyensis, Sivapithecus, Pleistocene Pongo and Gigantopithecus as this coincides nicely with increasing body mass in these three genera. The higher body mass of Pongo in comparison to both K. ayeyarwadyensis and Sivapithecus would make foraging high up in the canopy and close to terminal branches increasingly difficult. However, brachiation in conjunction with high hip flexibility in Pongo counters the negative impact of higher body mass during climbing to a certain extent, due to weight distribution over many smaller branches80,81,82.
At first it sound promising to relate the decreasing δ18O values from the Miocene to the Pleistocene genera (see in Fig. 5) to the increasing competition with cercopithecoid monkeys, which became increasingly abundant in Southeast Asia from the Late Miocene and especially the Plio-Pleistocene onward83,84,85,. Nevertheless, the higher body mass of pongines in comparison with the contemporaneous monkeys makes it very unlikely that the former had to resort to lower canopy layers for foraging due to increased competition86. On the contrary, it has been suggested that monkeys that are capable of eating unripe fruit create a pattern of ripe fruit availability that is more restricted to terminal branches and therefore forcing apes to adapt to exploit these resources80.
As discussed earlier on, it was necessary to assess to what extent changes in δ18O values might reflect climatic changes over time and geographical distance. Although climatic changes were responsible for part of the δ18O variation that we see between the different pongine genera (Fig. 5), we are confident that an ecological signal is also visible. Especially between the Miocene and Pleistocene taxa, the relative position of δ18O values of bovids, pongines and suids across time and space (see more detailed discussion in the Supplementary Information) and the overall consistency of our interpretations with body mass differences between the genera. The data are therefore consistent with the possiblity that there is a difference in the usage of the canopy between the two Miocene pongines Sivapithecus and K. ayeyarwadyensis, which could forage even higher up, and Pongo. Relative position of δ18O values of bovids, pongines and suids suggest that the difference between Pleistocene and extant Pongo however is probably predominantly caused by differences in climate between the islands of the Sunda Shelf and mainland Southeast Asia. This is consistent with the δ18O values of modern Pongo being well correlated well with δ18O values of precipitation from Sumatra and Borneo. These values are lower on the islands in the Sunda Shelf as compared to mainland Southeast Asia, predominantly caused by a more intense monsoon87. These preliminary interpretations should be further investigated with bigger data sets for a more robust assessment of the variations of δ18O values as they span a large spatio-temporal range and variation in these values can be caused by many different factors22.
Conclusions
Khoratpithecus ayeyarwadyensis, a Late Miocene pongine from Myanmar, lived in a woodland forest. The δ13C value is consistent with a microhabitat where the canopy effect is not strongly pronounced; so either in the lighter forest or higher up in the canopy, but not in the dense understory forest. Its high δ18O value is similar to those of sympatric giraffids and equids. Hence, the data are consistent with K. ayeyarwadyensis foraging higher up in the canopy where evapotranspiration leads to a depletion of 18O in food and water sources. Given that we could only sample one specimen of this genus, this only gives a first indication of the palaeoecology of K. ayeyarwadyensis, and should more specimen of this important pongine genus become available for stable isotope analysis, it would further improve our understanding of its palaeoecology and make our interpretations more robust. The precipitation regime in the habitat of K. ayeyarwadyensis was similar to the monsoon regime in present-day Myanmar, although seasonality was probably less pronounced. The high δ18O value is consistent with a diet consisting of plant tissues enriched in the heavier 18O isotope due to evapotranspiration like fruit or leaves. However, the results from dental microwear texture analysis of K. piriyai from Thailand suggest a predominantly frugivorous diet without the use of tough objects, so K. ayeyarwadyensis was likely also a frugivore and not a folivore. Overall, the habitats of these two Miocene hominoids seem to be similar to that of the modern Pongo, however the exploitation of these habitats and the ecological niches occupied by each species seem to be distinct. In the case of K. ayeyarwadyensis and Pongo they might use similar resources, but at different levels in the canopy.
The comparison of all Asian Miocene apes showed that the reconstructed ecological niches of Sivapithecus and K. ayeyarwadyensis seem to be superficially similar to the modern Pongo. They are all three predominantly frugivorous primates foraging high up in the canopy of forests with a seasonal monsoon-like precipitation regime. However, the density of the Miocene forest in the Siwalik and Irrawaddy region seems to be more fragmented with more deciduous vegetation. The overall climate was also drier than in modern Indonesia. Palynological and isotopic data on paleosols and benthic foraminifera as well as climate models suggest a similar development in vegetation structure and climate52,70,71,72,73,74,75. Gigantopithecus was adapted to foraging in denser forests, and given its immense body mass this was likely the the understory or forest floors. On the other hand, the data from modern and Pleistocene Pongo are consistent with using resources in the lower areas of the forest canopy.
As we compare pongine genera from different time periods with one another it was necessary to correct the δ13C values for changes in the isotopic composition of the atmospheric CO2. Such a correction is not possible for the δ18O values, as their variation is impacted by many different factors. We therefore assessed if some of the variation can be attributed to changes in ecology. We do however acknowledge, that the interpretations of variations and shifts in δ18O values are less robust, than the ones based on the δ13C values. Large scale investigations of δ18O variation over the spatio-temporal range of this study would improve the robustness of the interpretations.
As mentioned in the introduction, the present study contributes to improving our understanding of the evolutionary ecology of the fossil pongines that can provide valuable insight for current orangutan conservation. It seems that at least at the time from which the samples of modern Pongo originate, around a century ago, there was still enough suitable habitat available for the orangutans not to be pressured to leave their ancestral niche. However, the shifts in the environment we see in the isotopic niche modelling of the Sivapithecus and the associated K. ayeyarwadyensis fauna to later time periods after the decline and extinction of these Miocene ponginesillustrate the fate, which awaits the Southeast Asian apes if the habitats become more and more fragmented that of extinction.
Data availability
All data generated or analysed during this study are included in this published article (and its supplementary information files).
References
Spehar, S. N. et al. Orangutans venture out of the rainforest and into the anthropocene. Sci. Adv. 4, e1701422. https://doi.org/10.1126/sciadv.1701422 (2018).
Suganuma, Y. et al. Magnetostratigraphy of the Miocene Chiang Muan Formation, northern Thailand. Implications for revised chronology of the earliest Miocene hominoid in Southeast Asia. Palaeogeogr. Palaeoclimatol. Plaeoecol. 239, 75–86 (2006).
Coster, P. et al. A complete magnetic-polarity stratigraphy of the Miocene continental deposits of Mae Moh Basin, northern Thailand, and a reassessment of the age of hominoid-bearing localities in northern Thailand. Geol. Soc. Am. Bull. 122, 1180–1191 (2010).
Begun, D. R. The Miocene hominoid radiations. In A Companion to Paleoanthropology (ed. Begun, D. R.) 398–416 (Blackwell Publishing, 2013).
Pugh, K. D. Phylogenetic analysis of Middle-Late Miocene apes. J. Hum. Evol. 165, 1–33 (2022).
Chaimanee, Y. et al. Khoratpithecus piriyai, a Late Miocene Hominoid of Thailand. Am. J. Phys. Anthropol. 131, 311–323 (2006).
Chavasseau, O. et al. Advances in the biochronology and biostratigraphy of the continental Neogene of Myanmar. In Fossil Mammals in Asia. Neogene Biostratigraphy and Chronology (eds Wang, X. et al.) 461–474 (Columbia University Press, 2013).
Patnaik, R. Indian Neogene Siwalik Mammalian biostratigraphy. An overview. In Fossil Mammals in Asia Neogene Biostratigraphy and Chronology (eds Wang, X. et al.) 423–444 (Columbia University Press, 2013).
Chaimanee, Y. et al. A middle Miocene hominoid from Thailand and orangutan origins. Nature 422, 61–65 (2003).
Chaimanee, Y. et al. A new orang-utan relative from the Late Miocene of Thailand. Nature 427, 439–441 (2004).
Chaimanee, Y., Lazzari, V., Chaivanich, K. & Jaeger, J.-J. First maxilla of a late Miocene hominid from Thailand and the evolution of pongine derived characters. J. Hum. Evol. 134, 102636. https://doi.org/10.1016/j.jhevol.2019.06.007 (2019).
Jaeger, J.-J. et al. First Hominoid from the Late Miocene of the Irrawaddy formation (Myanmar). PLoS ONE 6, 1–14 (2011).
Begun, D. R. European hominoids. In The Primate Fossil Record (ed. Hartwig, W. C.) 339–368 (Cambridge University Press, 2002).
Kelley, J. & Gao, F. Juvenile hominoid cranium from the late Miocene of southern China and hominoid diversity in Asia. Proc. Natl. Acad. Sci. U.S.A. 109, 6882–6885 (2012).
Kettle, C. J., Maycock, C. R. & Burslem, D. New directions in dipterocarp biology and conservation: A synthesis. Biotropica 44, 658–660. https://doi.org/10.1111/j.1744-7429.2012.00912.x (2012).
Cannon, C. H., Morley, R. J. & Bush, A. B. G. The current refugial rainforests of Sundaland are unrepresentative of their biogeographic past and highly vulnerable to disturbance. Proc. Natl. Acad. Sci. U.S.A. 106, 11188–11193. https://doi.org/10.1073/pnas.0809865106 (2009).
Nelson, S. V. Isotopic reconstruction of habitat change surrounding the extinction of Sivapithecus, a Miocene hominoid, in the Siwalik Group of Pakistan. Palaeogeogr. Palaeoclimatol. Palaeoecol. 243, 204–222 (2007).
Bender, M. M. Variations in the 13C/12C ratios of plants in relation to the pathway of photosynthetic carbon dioxide fixation. Phytochemistry 10, 1239–1244 (1971).
Kohn, M. J. Carbon isotope compositions of terrestrial C3 plants as indicators of (paleo)ecology and (paleo)climate. Proc. Natl. Acad. Sci. 107, 19691–19695 (2010).
Bonafini, M., Pellegrini, M., Ditchfield, P. & Pollard, A. M. Investigation of the ‘canopy effect’ in the isotope ecology of temperate woodlands. J. Archaeol. Sci. 40, 3926–3935. https://doi.org/10.1016/j.jas.2013.03.028 (2013).
Krigbaum, J., Berger, M. H., Daegling, D. J. & McGraw, W. S. Stable isotope canopy effects for sympatric monkeys at Tai Forest, Cote d’Ivoire. Biol. Lett. 9, 20130466. https://doi.org/10.1098/rsbl.2013.0466 (2013).
Dansgaard, W. Stable isotopes in precipitation. Tellus 16, 436–468 (1964).
Fannin, L. D. & McGraw, W. S. Does oxygen stable isotope composition in primates vary as a function of vertical stratification or folivorous behaviour?. Folia Primatol. Int. J. Primatol. 91, 219–227. https://doi.org/10.1159/000502417 (2020).
Louys, J. & Roberts, P. Environmental drivers of megafauna and hominin extinction in Southeast Asia. Nature 586, 402–406. https://doi.org/10.1038/s41586-020-2810-y (2020).
Zin-Maung-Maung-Thein, et al. Stable isotope analysis of the tooth enamel of Chaingzauk mammalian fauna (late Neogene, Myanmar) and its implication to paleoenvironment and paleogeography. Palaeogeogr. Palaeoclimatol. Palaeoecol. 300, 11–22. https://doi.org/10.1016/j.palaeo.2010.11.016 (2011).
Patnaik, R., Cerling, T. E., Uno, K. T. & Fleagle, J. G. Diet and habitat of Siwalik primates Indopithecus, Sivaladapis and Theropithecus. Ann. Zool. Fenn. 51, 123–142. https://doi.org/10.5735/086.051.0214 (2014).
Pushkina, D., Bocherens, H., Chaimanee, Y. & Jaeger, J.-J. Stable carbon isotope reconstructions of diet and paleoenvironment from the late Middle Pleistocene Snake Cave in Northeastern Thailand. Naturwissenschaften 97, 299–309 (2010).
Nelson, S. V. The paleoecology of early Pleistocene Gigantopithecus blacki inferred from isotopic analyses. Am. J. Phys. Anthropol. 155, 571–578. https://doi.org/10.1002/ajpa.22609 (2014).
Qu, Y. et al. Preservation assessments and carbon and oxygen isotopes analysis of tooth enamel of Gigantopithecus blacki and contemporary animals from Sanhe Cave, Chongzuo, South China during the Early Pleistocene. Quat. Int. 354, 52–58. https://doi.org/10.1016/j.quaint.2013.10.053 (2014).
Bocherens, H. et al. Flexibility of diet and habitat in Pleistocene South Asian mammals. Implications for the fate of the giant fossil ape Gigantopithecus. Quat. Int. 434, 148–155 (2017).
Bacon, A.-M. et al. Nam Lot (MIS 5) and Duoi U’Oi (MIS 4) Southeast Asian sites revisited. Zooarchaeological and isotopic evidences. Palaeogeogr. Palaeoclimatol. Palaeoecol. 512, 132–144. https://doi.org/10.1016/j.palaeo.2018.03.034 (2018).
Jiang, Q.-Y., Zhao, L., Guo, L. & Hu, Y.-W. First direct evidence of conservative foraging ecology of early Gigantopithecus blacki (~2 Ma) in Guangxi, southern China. Am. J. Phys. Anthropol. https://doi.org/10.1002/ajpa.24300 (2021).
Ma, J. et al. Isotopic evidence of foraging ecology of Asian elephant (Elephas maximus) in South China during the Late Pleistocene. Quat. Int. 443, 160–167. https://doi.org/10.1016/j.quaint.2016.09.043 (2017).
Ma, J., Wang, Y., Jin, C., Hu, Y. & Bocherens, H. Ecological flexibility and differential survival of Pleistocene Stegodon orientalis and Elephas maximus in mainland southeast Asia revealed by stable isotope (C, O) analysis. Quat. Sci. Rev. 212, 33–44. https://doi.org/10.1016/j.quascirev.2019.03.021 (2019).
Janssen, R. et al. Tooth enamel stable isotopes of Holocene and Pleistocene fossil fauna reveal glacial and interglacial paleoenvironments of hominins in Indonesia. Quat. Sci. Rev. 144, 145–154. https://doi.org/10.1016/j.quascirev.2016.02.028 (2016).
Wang, W. et al. Sequence of mammalian fossils, including hominoid teeth, from the Bubing Basin caves, South China. J. Hum. Evol. 52, 370–379. https://doi.org/10.1016/j.jhevol.2006.10.003 (2007).
Suraprasit, K., Bocherens, H., Chaimanee, Y., Panha, S. & Jaeger, J.-J. Late Middle Pleistocene ecology and climate in Northeastern Thailand inferred from the stable isotope analysis of Khok Sung herbivore tooth enamel and the land mammal cenogram. Quat. Sci. Rev. 193, 24–42. https://doi.org/10.1016/j.quascirev.2018.06.004 (2018).
Bocherens, H., Fizet, M. & Mariotti, A. Diet, physiology and ecology of fossil mammals as inferred from stable carbon and nitrogen biogeochemistry. Implications for Pleistocene bears. Palaeogeogr. Palaeoclimatol. Palaeoecol. 107, 213–225 (1994).
Koch, P. L., Tuross, N. & Fogel, M. L. The effects of sample treatment and diagenesis on the isotopic integrity of carbonate in biogenic hydroxylapatite. J. Archaeol. Sci. 24, 417–429 (1997).
Wright, L. E. & Schwarcz, H. P. Correspondence between stable carbon, oxygen and nitrogen isotopes in human tooth enamel and dentine. Infant diets at Kaminaljuyú. J. Archaeol. Sci. 26, 1159–1170 (1999).
Szpak, P., Metcalfe, J. Z. & Macdonald, R. A. Best practices for calibrating and reporting stable isotope measurments in archaeology. J. Archaeol. Sci. Rep. 13, 609–616 (2017).
Coplen, T. B. Guidelines and recommended terms for expression of stable-isotope-ratio and gas-ratio measurement results. Rapid Commun. Mass Spectrom. 25, 2538–2560 (2011).
Bond, A. L. & Hobson, K. A. Reporting stable-isotope ratios in ecology. Recommended terminology, guidelines and best practices. Waterbirds 35, 324–331 (2012).
Craig, H. Carbon 13 in plants and the relationships between carbon 13 and carbon 14 variations in nature. J. Geol. 62, 115–149. https://doi.org/10.1086/626141 (1954).
Cerling, T. E. & Harris, J. M. Carbon isotope fractionation between diet and bioapatite in ungulate mammals and implications for ecological and paleoecological studies. Oecologia 120, 347–363 (1999).
Passey, B. H. et al. Carbon isotope fractionation between diet, breath CO2, and bioapatite in different mammals. J. Archaeol. Sci. 32, 1459–1470. https://doi.org/10.1016/j.jas.2005.03.015 (2005).
Howland, M. R. et al. Expression of the dietary isotope signal in the compound-specific δ13C values of pig bone lipids and amino acids. Int. J. Osteoarchaeol. 13, 54–65. https://doi.org/10.1002/oa.658 (2003).
Crowley, B. E. et al. Stable carbon and nitrogen isotope enrichment in primate tissues. Oecologia 164, 611–626. https://doi.org/10.1007/s00442-010-1701-6 (2010).
Keeling, C. D. The Suess effect: 13Carbon–14Carbon interrelations. Environ. Int. 2, 229–300. https://doi.org/10.1016/0160-4120(79)90005-9 (1979).
Marino, B. D., McElroy, M. B., Salawitch, R. J. & Spaulding, W. G. Glacial-to-interglacial variations in the carbon isotopic composition of atmospheric CO2. Nature 357, 461–466. https://doi.org/10.1038/357461a0 (1992).
Tipple, B. J., Meyers, S. R. & Pagani, M. Carbon isotope ratio of Cenozoic CO2 A comparative evaluation of available geochemical proxies. Paleoceanography https://doi.org/10.1029/2009PA001851 (2010).
Zachos, J., Pagani, M., Sloan, L., Thomas, E. & Billups, K. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science 292, 686–693 (2001).
Cerling, T. E., Harris, J. M., Leakey, M. G., Passey, B. H. & Levin, N. E. Stable carbon and oxygen isotopes in East African Mammals. Modern and fossil. In Cenozoic Mammals of Africa (ed. Werdelin, L.) 941–952 (University of California Press, 2010).
Friedli, H., Lötscher, H., Oeschger, H., Siegenthaler, U. & Stauffer, B. Ice core record of the 13C/12C ratio of atmospheric CO2 in the past two centuries. Nature 324, 237–238. https://doi.org/10.1038/324237a0 (1986).
Nelson, S. V. Paleoseasonality inferred from equid teeth and intra-tooth isotopic variability. Palaeogeogr. Palaeoclimatol. Palaeoecol. 222, 122–144 (2005).
Komsta, L. Processing data for outliers. R News 6, 10–13 (2006).
Hutchinson, G. E. Concluding remarks. In Cold spring Harbor Symposium on Quantitative Biology, edited by Q. Biology (1957).
Hutchinson, G. E. An Introduction to Population Ecology (Yale University Press, 1978).
Baumann, C., Bocherens, H., Drucker, D. G. & Conard, N. J. Fox dietary ecology as a tracer of human impact on Pleistocene ecosystems. PLoS ONE 15, e0235692. https://doi.org/10.1371/journal.pone.0235692 (2020).
Jackson, A. L., Inger, R., Parnell, A. C. & Bearhop, S. Comparing isotopic niche widths among and within communities: SIBER—Stable Isotope Bayesian Ellipses in R. J. Anim. Ecol. 80, 595–602. https://doi.org/10.1111/j.1365-2656.2011.01806.x (2011).
Nelson, S. V. & Hamilton, M. I. Evolution of the human dietary niche. Initial transitions. In Chimpanzees and Human Evolution (eds Muller, M. N. et al.) 286–310 (Harvard University Press, 2017).
Sun, F. et al. Paleoenvironment of the late Miocene Shuitangba hominoids from Yunnan, Southwest China: Insights from stable isotopes. Chem. Geol. 569, 120123. https://doi.org/10.1016/j.chemgeo.2021.120123 (2021).
Nelson, S. V. Chimpanzee fauna isotopes provide new interpretations of fossil ape and hominin ecologies. Proc. R. Soc. B: Biol. Sci. 280, 20132324. https://doi.org/10.1098/rspb.2013.2324 (2013).
Merceron, G., Taylor, S., Scott, R., Chaimanee, Y. & Jaeger, J.-J. Dietary characterization of the hominoid Khoratpithecus (Miocene of Thailand). Evidence from dental topographic and microwear texture analyses. Naturwissenschaften 93, 329–333. https://doi.org/10.1007/s00114-006-0107-0 (2006).
Kay, R. F. The nut-crackers—A new theory of the adaptations of the ramapithecinae. Am. J. Phys. Anthropol. 55, 141–151 (1981).
Nelson, S. V. The Extinction of Sivapithecus. Faunal and Environmental Changes Surrounding the Disappearance of a Miocene Hominoid in the Siwaliks of Pakistan (Brill Academic Publishers, 2003).
Kanamori, T., Kuze, N., Bernard, H., Malim, T. P. & Kohshima, S. Feeding ecology of Bornean orangutans (Pongo pygmaeus morio) in Danum Valley, Sabah, Malaysia: A 3-year record including two mast fruitings. Am. J. Primatol. 72, 820–840. https://doi.org/10.1002/ajp.20848 (2010).
Vogel, E. R. et al. Nutritional ecology of wild Bornean orangutans (Pongo pygmaeus wurmbii) in a peat swamp habitat. Effects of age, sex, and season. Am. J. Primatol. 79, 1–20. https://doi.org/10.1002/ajp.22618 (2017).
Louys, J. et al. Sumatran orangutan diets in the Late Pleistocene as inferred from dental microwear texture analysis. Quat. Int. 603, 74–81. https://doi.org/10.1016/j.quaint.2020.08.040 (2021).
Quade, J., Cerling, T. E. & Bowman, J. R. Development of Asian monsoon revealed by marked ecological shift during the latest Miocene in northern Pakistan. Nature 342, 163–166 (1989).
Hoorn, C., Ohja, T. & Quade, J. Palynological evidence for vegetation development and climatic change in the sub-Himalayan Zone (Neogene, Central Nepal). Palaeogeogr. Palaeoclimatol. Palaeoecol. 163, 133–161 (2000).
Morley, R. J. A review of the Cenozoic palaeoclimate history of Southeast Asia. In Biotic Evolution and Environmental Change in Southeast Asia (eds Gower, D. et al.) 79–114 (Cambridge University Press, 2012).
Morley, R. J. Assembly and division of the South and South-East Asian flora in relation to tectonics and climate change. J. Trop. Ecol. 34, 209–234. https://doi.org/10.1017/S0266467418000202 (2018).
Sepulchre, P. et al. Mid-tertiary paleoenvironments in Thailand. Pollen evidence. Clim. Past 6, 461–473 (2010).
Sepulchre, P., Jolly, D., Ducrocq, S., Chaimanee, Y. & Jaeger, J.-J. Mid-tertiary palaeoenvironments in Thailand. Pollen evidence. Clim. Past Discuss. 5, 709–734 (2009).
Fleagle, J. G., Janson, C. H. & Reed, K. E. Primate Communities (Cambridge University Press, 1999).
Fleagle, J. G. Primate Adaptation and Evolution 3rd edn. (Elsevier, 2013).
Pilbeam, D. Gigantopithecus and the origins of Hominidae. Nature 225, 516–519. https://doi.org/10.1038/225516a0 (1970).
Jiang, Q.-Y., Zhao, L.-X. & Hu, Y.-W. Isotopic (C, O) variations of fossil enamel bioapatite caused by different preparation and measurement protocols: A case study of Gigantopithecus fauna. Vertebr. PalAsiat. 58, 159–168 (2020).
Hunt, K. D. Why are there apes? Evidence for the co-evolution of ape and monkey ecomorphology. J. Anat. 228, 630–685. https://doi.org/10.1111/joa.12454 (2016).
Zihlman, A. L., Mcfarland, R. K. & Underwood, C. E. Functional anatomy and adaptation of male gorillas (Gorilla gorilla gorilla) with comparison to male orangutans (Pongo pygmaeus). Anat. Rec. Adv. Integr. Anat. Evol. Biol. 294, 1842–1855. https://doi.org/10.1002/ar.21449 (2011).
Thorpe, S. K. & Crompton, R. H. Orangutan positional behavior and the nature of arboreal locomotion in Hominoidea. Am. J. Phys. Anthropol. 131, 384–401. https://doi.org/10.1002/ajpa.20422 (2006).
Barry, J. C. The history and chronology of Siwalik cercopithecids. J. Hum. Evol. 2, 47–58 (1987).
Jablonski, N. G., Whitfort, M. J., Roberts-Smith, N. & Qinqi, X. The influence of life history and diet on the distribution of catarrhine primates during the Pleistocene in eastern Asia. J. Hum. Evol. 39, 131–157 (2000).
Takai, M., Saegusa, H., Thaung-Htike, & Zin-Maung-Maung-Thein,. Neogene mammalian fauna in Myanmar. Asian Paleoprimatol. 4, 143–172 (2006).
Houle, A., Chapman, C. A. & Vickery, W. L. Intratree vertical variation of fruit density and the nature of contest competition in frugivores. Behav. Ecol. Sociobiol. 64, 429–441. https://doi.org/10.1007/s00265-009-0859-6 (2010).
Vuille, M., Werner, M., Bradley, R. S. & Keimig, F. Stable isotopes in precipitation in the Asian monsoon region. J. Geophys. Res. 110, D23108 (2005).
Acknowledgements
This research was funded by the ANR (EVEPRIMASIA) and DFG (Grant BO 3478/7-)1, the Fondation Fyssen (Grant to O.C.) and the laboratory PALEVOPRIM. Sample preparation and analysis took place at the Eberhard-Karls Universität Tübingen in the laboratories of the AG Biogeologie. Thanks are due to P. Tung for conducting the isotopic analyses and to Catalina Labra Odde for proof reading the manuscript. We would also like to thank our reviewers for their time and effort they took with their feedback. It greatly increased the quality of our manuscript.
Author information
Authors and Affiliations
Contributions
S.G.H., O.C., and H.B. wrote the main manuscript text. S.G.H. prepared the figures and performed data analysis. O.C., J.-J.J., Y.C., A.N.S., and C.S. performed field work and sample collection. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Habinger, S.G., Chavasseau, O., Jaeger, JJ. et al. Evolutionary ecology of Miocene hominoid primates in Southeast Asia. Sci Rep 12, 11841 (2022). https://doi.org/10.1038/s41598-022-15574-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-15574-z
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.