Abstract
There is inconclusive evidence on the association between ambient air pollution and pulmonary tuberculosis (PTB) incidence, tuberculosis-related hospital admission and mortality. This review aimed to assess the extent to which selected air pollutants are associated to PTB incidence, hospital admissions and mortality. This was a systematic review of studies published in English from January 1st, 1946, through May 31st, 2022, that quantitatively assessed the association between PM2.5, PM10, NO2, SO2, CO, O3 and the incidence of, hospital admission or death from PTB. Medline, Embase, Scopus and The Cochrane Library were searched. Extracted data from eligible studies were analysed using STATA software. Random-effect meta-analysis was used to derive pooled adjusted risk and odds ratios. A total of 24 studies (10 time-series, 5 ecologic, 5 cohort, 2 case–control, 1 case cross-over, 1 cross-sectional) mainly from Asian countries were eligible and involved a total of 437,255 tuberculosis cases. For every 10 μg/m3 increment in air pollutant concentration, there was a significant association between exposure to PM2.5 (pooled aRR = 1.12, 95% CI: 1.06–1.19, p < 0.001, N = 6); PM10 (pooled aRR = 1.06, 95% CI: 1.01–1.12, p = 0.022, N = 8); SO2 (pooled aRR = 1.08, 95% CI: 1.04–1.12, p < 0.001, N = 9); and the incidence of PTB. There was no association between exposure to CO (pooled aRR = 1.04, 95% CI: 0.98–1.11, p = 0.211, N = 4); NO2 (pooled aRR = 1.08, 95% CI: 0.99–1.17, p = 0.057, N = 7); O3 (pooled aRR = 1.00, 95% CI: 0.99–1.02, p = 0.910, N = 6) and the incidence of PTB. There was no association between the investigated air pollutants and mortality or hospital admissions due to PTB. Overall quality of evidence was graded as low (GRADE approach). Exposure to PM2.5, PM10 and SO2 air pollutants was found to be associated with an increased incidence of PTB, while exposure to CO, NO2 and O3 was not. There was no observed association between exposure to these air pollutants and hospital admission or mortality due to PTB. The quality of the evidence generated, however, remains low. Addressing the tuberculosis epidemic by 2030 as per the 4th Sustainable Development Goal may require a more rigorous exploration of this association.
Similar content being viewed by others
Introduction
Pulmonary tuberculosis (PTB), a bacterial infection of the lungs caused by Mycobacterium tuberculosis is one of the top 10 causes of death worldwide and the leading cause of death from a single infectious agent1. PTB remains a global health emergency despite the significant progress that has been made worldwide in its control over the past two and a half decades2. Much still needs to be done to end the tuberculosis epidemic by 20303 as per the World Health Organisation’s (WHO) 4th sustainable development goal (SDG). This includes addressing important predisposing factors to tuberculosis infection such as smoking, diabetes, human immunodeficiency virus (HIV) and social determinants of health such as poverty, malnutrition, poor ventilation and over-crowding among others1,4. A multi-faceted and multi-sectorial approach to tuberculosis prevention, case identification, management and control of its health and social determinants is therefore required4,5.
Air pollution, currently on several global health agendas, has rapidly become a global problem with the increasing global urbanisation, transportation-related emissions, and increased energy consumption. Air pollution could therefore be an important factor to address on the journey to ending tuberculosis as there are growing concerns of its association to increased tuberculosis-related hospital admissions and deaths6,7.
There is a well-known association between different air pollutants and cardio-respiratory diseases8,9,10,11,12,13,14,15. However, there is still no conclusive evidence of an association between PTB and outdoor air pollution despite its well-known association to indoor pollution from activities such as smoking and biomass fuel burning16,17,18 The review by Popovic et al. indicated a possible association between PM2.5 and PTB outcomes (incidence, hospital admissions and mortality) and reported the contrasting findings from several earlier studies on the association between PM10, NO2, and SO2 and PTB19, but did not synthesise these findings to determine to what extent these air pollutants are associated to PTB. Also, several studies have been published on this subject after the review by Popovic et al. This review therefore had as objectives to determine if there is an association between the selected air pollutants (PM2.5, PM10, NO2, SO2, CO, O3) and PTB incidence, hospital admissions and mortality, and to what extent, by systematically reviewing and quantitatively combining published evidence on this topic.
Methods
This was a systematic literature review and meta-analysis of articles published in English from January 1st, 1946, through May 31st, 2022, that quantitatively assessed the association between ambient air pollution and PTB. The study protocol for this review was registered with the international prospective register of systematic reviews (PROSPERO) with trial registration number CRD42020165888 and has been published20. This review was reported according to The RepOrting standards for Systematic Evidence Syntheses (ROSES) for systematic review21 as presented in Additional file 1.
Deviations from the protocol
There were no deviations from the published study protocol.
Search for articles
A comprehensive search strategy (Additional file 2) combining medical subject headings (MeSH) and free-text searches for the appropriate keywords was developed by the authors and used to search the databases: Medline, Embase, Scopus and The Cochrane Library. The keywords ‘air pollution’, ‘carbon monoxide’, ‘nitrogen dioxide’, ‘sulphur dioxide’, ‘ozone’, and ‘particulate matter’ were combined with the keywords ‘tuberculosis’, ‘incidence’, ‘mortality’, ‘hospital admission’ and their respective synonyms, using the Boolean operator ‘AND’ in the search strategy. The search was run by the principal investigator (CAD), all searches were limited to the language English and grey literature search was not conducted given the lack of relevant studies from preliminary searches of the grey literature. Search dates of interest were January 1st, 1946, through May 31st, 2022. The search language was in English, and all the database searches were done on the same day, June 5th, 2022. The search was run twice to ensure replicability of results and the same results were obtained with each search run.
Article screening and study eligibility criteria
Screening process
Articles returned by the search were saved on Zotero Version 5.0 reference management software and duplicates of the studies were manually removed by the principal investigator (CAD) with the assistance of the reference management software. More articles were added to the search output by the principal investigator by reviewing the reference list of relevant articles. The titles and abstracts of all the remaining articles were then independently screened for eligibility according to the set eligibility criteria by each of the two independent reviewers (CAD and BMK). The full texts of all the articles retained after the title and abstract screen, were then independently reviewed by the same two independent reviewers (CAD and BMK) for eligibility and inclusion to the analysis. The two independent reviewers compared their findings at the end of both the title and abstract screening and the full text review stages of the article selection process to ensure concordance in their final selection. There were no reviewer disagreements at all stages of the study selection process and no third reviewer to settle discordances as had been planned in the study protocol, was therefore needed due to concordance in the findings of the two independent reviewers.
Eligibility criteria
The following criteria were used during the article selection process to determine the eligible studies.
The following studies were included:
-
1.
Population: Studies focused on adults aged 18 and above with PTB
-
2.
Exposure: Studies that reported direct measurements on any of the air pollutants; carbon monoxide (CO), nitrogen dioxide (NO2), sulphur dioxide (SO2), ozone (O3), particulate matter ≤ 2.5 µm (PM2.5) and/or particulate matter ≤ 10 µm (PM10) in any country, region, city or locality;
-
3.
Outcomes: Studies that reported measures of association on the risk of PTB incidence, hospital admission and/or mortality from PTB;
-
4.
Study design/Other: Cross-sectional, case–control, cohorts, case-crossover, ecological and time-series studies that reported on the association between ambient air pollution and PTB.
The following studies were excluded:
-
1.
Population: Studies that reported on respiratory diseases other than PTB
-
2.
Exposure: Studies that reported on other forms of air pollution such as indoor air pollution
-
3.
Outcomes: Studies that reported outcomes related to PTB in combination with other respiratory diseases. Studies that reported on measures of effect/association other than risk ratios and odds ratios or that provided data from which these measures could not be calculated.
-
4.
Other: Conference abstracts, editorials, letters, opinion papers, unpublished studies, same studies published in different journals with the same or a different title.
Study validity assessment
Assessment of study quality of each included study was done by both independent reviewers (CAD and BMK) using the respective Study Quality Assessment Tools of the National Health Institute/National Heart, Lung and Blood Institute (NHI/NHLBI)22 depending on their study designs. There was no discordance in the overall rating of the quality of the eligible studies. Study quality indicators were included in the meta-regression.
The overall quality of the evidence provided by the studies with regards to the primary outcome of interest was assessed and graded as very low, low, moderate or high, using the Grading of Recommendations Assessment, Development and Evaluation (GRADE)23.
Data coding and extraction strategy
Data on the publication details, study methods and outcomes of interest were extracted from the eligible studies into a Microsoft excel office 365 data extraction sheet (Additional file 3) by the principal investigator (CAD) and independently rechecked by a second reviewer (BMK) for accuracy. The following data were extracted: First author, year of publication, study location, study design, socio-demographic and clinical characteristics of study participants, study duration, number of tuberculosis cases and new tuberculosis cases, annual incidences of tuberculosis, mean and median concentration data on air pollutants of interest (CO, NO2, SO2, O3, PM2.5 and PM10), data on incidence, hospital admission and mortality from tuberculosis, including measures of effect/association (risk ratios, odds ratios and percentage change in the incidence of PTB) and their respective confidence intervals, and confounders reported by the respective studies and if studies adjusted for confounders or not. PM2.5 and PM10 air pollutants were measured in µg/m3 and NO2, SO2 and O3 in parts per billion (ppb) and CO in parts per million (ppm). For studies that reported air pollutant concentrations in units other than the above, the Air Pollution Information System24, was used to convert air pollutant concentrations to appropriate units, taking into consideration the average yearly temperatures reported for the various cities or countries. The average annual outdoor temperature obtained from public sources was used for studies that did not report them. In studies where several measures of effect were reported for different quintiles or levels of exposure to air pollutants, the largest numerical estimates of the measures of effect were considered, to quantify the maximum extent of the association of air pollutants to PTB. When protective effects were observed among the measures of effect, the lowest numerical measures of effect were used. Default measures of effect reported by the studies were considered. Adjusted measures of effect were chosen over crude measures, and both single-pollutant models and multi-pollutant models were reported as appropriate. All data was transferred to STATA version 14.0 statistical software for analysis.
Potential effect modifiers/reasons for heterogeneity
Between-study heterogeneity was anticipated given the differences in study designs, settings, duration, sample sizes, and population characteristics based on review of existing literature.
Data synthesis and presentation
Meta-analyses were done through random effects models to account for the possibility of between-study heterogeneity. Risk ratios and odds ratios on the incidence of PTB following exposure to the selected air pollutants, and their respective confidence intervals from the various studies, were log-transformed, and the corresponding standard errors derived. Pooled summary estimates for the respective log-transformed measures of association were computed and presented on forest plots. Studies were pooled according to their study designs with ecologic studies and studies that used time-series analysis pooled together, separate from cohort and case–control studies. Heterogeneity between studies was assessed using the Cochrane’s Q test, and the I2 test statistic was reported as a measure of the extent of this heterogeneity. The Begg’s and Egger’s statistical tests were used for the statistical assessments of publication bias and small study effect25,26. All statistical tests and plots were done on STATA version 14.0 statistical software.
Ethics approval and consent to participate
This systematic review does not require ethical approval as it entails a synthesis of data collected from several primary studies. No primary data collection from patients will be done for this systematic review.
Results
Review descriptive statistics
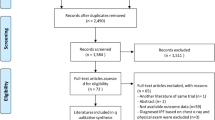
Figure 1 summarises the study selection process.
A total of 12,652 records were returned by the search. Following removal of duplicates, screening of titles and abstracts, addition of studies from the reference list of relevant studies, full-text reviews, 24 eligible studies were retained. Figure 1 summarises the PRISMA flow chart of the study selection process. The studies excluded following full-text review and the reasons for exclusion are presented in Additional file 4.
Narrative synthesis including study validity assessment
Most studies were from Asian countries and a total of 437,255 tuberculosis cases were reported across the 22 studies that reported the number of tuberculosis cases over their study periods (1996–2019)7,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46. Of the 24 studies included in the review, 10 were time series, 5 were cohort studies (3 retrospective, 2 prospective), 5 were ecologic, 2 were case–control studies (1 nested, 1 retrospective), 1 was a retrospective case cross-over and 1 was cross-sectional. Average male participation was at 64.9% (N = 13 studies)7,27,29,30,31,32,33,34,35,37,40,43,46, mean age of 46.3 years (N = 7 studies)27,30,31,32,37,40,43 and average annual tuberculosis incidence was 45.3 per 100,000 population (N = 10 studies)7,27,29,32,35,36,45,46,47,48. Study and participant characteristics are summarised on Table 1.
The average of the annual mean concentrations of the various air pollutants are presented on Table 2.
Twelve studies were of good quality, eleven of fair quality and one of poor quality (Additional file 6). The overall quality of evidence for the association of all 6 air pollutants to the incidence of PTB was graded as low based on the study limitations affecting generalisability of the findings, and some inconsistency across the studies due to the significantly elevated between-study heterogeneity (Additional file 7).
Data synthesis
Association between air pollutants and pulmonary tuberculosis Incidence
PM2.5
There was a significant association between exposure to PM2.5 and incidence of pulmonary tuberculosis (PTB), pooled adjusted RR = 1.12 (95% CI: 1.06–1.19), p < 0.001, N = 6, I2 = 72.4%7,29,38,39,43,49. There was no evidence of publication bias (Begg’s test, p = 0.133 and Egger’s test, p = 0.203). Begg’s test, p = 1. Likewise, Xiong et al.46 reported an association (RR = 3.10, 95% CI: 1.10–8.79) for a 50 µg/m3 increase in PM2.5 concentration. The study by Lai et al.32 (RR = 1.39, 95% CI: 0.95–2.03) which was cohort in design did not find a significant association. Jassal et al.28 reported an odds ratio of 25.3 (95% CI: 3.38–29.1).
PM10
There was a significant association between exposure to PM10 and incidence of PTB, pooled adjusted RR = 1.06 (95% CI: 1.01–1.12), p = 0.022, N = 8, I2 = 97.6% (Begg’s test, p = 0.536 and Egger’s test, p = 0.204)7,29,35,39,40,43,44,49. The studies by Lai et al.32 (HR = 0.95, 95% CI: 0.78–1.17) and Hwang et al.27 (male RR = 1.00, 95% CI: 0.96–1.05 and female RR = 1.01, 95% CI: 0.98–1.05) did not find a significant association. Likewise, the pooled adjusted OR was 1.03 (95% CI: 1.01–1.04), p = 0.001, N = 3, I2 = 0% (Begg’s test, p = 1 and Egger’s test, p = 0.211) (Fig. 2)31,34,37.
Forest plot showing the individual and pooled risk ratios and odds ratios for pulmonary tuberculosis incidence for PM2.5 and PM10. The dashed line on the Forest plot represents the overall pooled estimate. The grey squares and horizontal lines represent the vaccine acceptance rate of each study and their 95% confidence intervals. The size of the grey square represents the weight contributed by each study in the meta-analysis. The diamond represents the pooled vaccine acceptance rate and its 95% confidence intervals.
CO
There was no significant association between exposure to CO and the incidence of PTB, pooled adjusted RR = 1.04 (95% CI: 0.98–1.11), p = 0.211, N = 4, I2 = 87.4% (Begg’s test, p = 0.734 and Egger’s test, p = 0.355)39,43,47,49. The studies by Lai et al.32 (HR = 1.89, 95% CI: 0.78–4.58) and Hwang et al.27 (male RR = 0.99, 95% CI: 0.95–1.03 and female RR = 1.01, 95% CI: 0.98–1.04) had similar findings. The pooled adjusted OR was 1.22 (95% CI: 0.84–1.76), p = 0.305, N = 3, I2 = 78.5% (Begg’s test, p = 1 and Egger’s test, p = 0.364) (Fig. 3)31,34,37. However, Xiong et al.46 (RR = 1.436, 95% CI: 1.004–2.053) reported a significant association for a 100 µg/m3 increase in CO concentration.
Forest plot showing the individual and pooled risk ratios and odds ratios for pulmonary tuberculosis incidence for CO and NO2. The dashed line on the Forest plot represents the overall pooled estimate. The grey squares and horizontal lines represent the odds ratios of each study and their 95% confidence intervals. The size of the grey square represents the weight contributed by each study in the meta-analysis. The diamond represents the pooled odds ratio and its 95% confidence intervals.
NO2
There was no association between exposure to NO2 and the incidence of PTB, pooled adjusted RR = 1.08 (95% CI: 0.99–1.17), p = 0.057, N = 7, I2 = 98% (Begg’s test, p = 1 and Egger’s test, p = 0.437) (Fig. 3)7,35,39,40,43,48,49. Lai et al.32 (HR = 1.33, 95% CI: 1.04–1.70) found a significant association, while Hwang et al.27 (male RR = 1.00, 95% CI: 0.96–1.05 and female RR = 1.01, 95% CI: 0.98–1.05) did not. Likewise, the pooled adjusted OR was 1.05 (95% CI: 0.95–1.17), p = 0.322, N = 3, I2 = 72.4% (Begg’s test, p = 0.296 and Egger’s test, p = 0.145) (Fig. 3)31,34,37. However, Xiong et al.46 (RR = 1.8, 95% CI: 1.11–2.91) reported a significant association for a 5 µg/m3 increase in NO2 concentration.
SO2
There was an association between exposure to SO2 and incidence of PTB, pooled adjusted RR = 1.08 (95% CI: 1.04–1.12), p < 0.001, N = 9, I2 = 94.4% (Begg’s test, p = 0.517 and Egger’s test, p = 0.356) (Fig. 4)7,35,39,40,43,44,47,48,49. Hwang et al.27 (male RR = 1.07, 95% CI: 1.03–1.12 and female RR = 1.02, 95% CI: 0.98–1.07) reported similar findings in males. Likewise, Xiong et al.46 reported an association (RR = 1.62, 95% CI: 1.12–2.33) for a 5 µg/m3 increase in SO2 concentration.
Forest plot showing the individual and pooled risk ratios and odds ratios for pulmonary tuberculosis incidence for SO2 and O3. The dashed line on the Forest plot represents the overall pooled estimate. The grey squares and horizontal lines represent the odds ratios of each study and their 95% confidence intervals. The size of the grey square represents the weight contributed by each study in the meta-analysis. The diamond represents the pooled odds ratio and its 95% confidence intervals.
O3
There was no significant association between O3 exposure and incidence of PTB, pooled adjusted RR = 1.01 (95% CI: 0.97–1.06), p = 0.560, N = 4, I2 = 75.6% (Begg’s test, p = 0.734 and Egger’s test, p = 0.734) (Fig. 4)39,43,47,49. While Hwang et al.27 had similar findings (male RR = 0.99, 95% CI: 0.94–1.03 and female RR = 1.01, 95% CI: 0.97–1.05), Lai et al.32 rather found a protective effect (HR = 0.69, 95% CI: 0.49–0.98). Xiong et al.46 reported an association (RR = 0.96, 95% CI: 0.93–1.0) for a 5 µg/m3 increase in O3 concentration.
Table 3 summarises the percentage change in the number of PTB cases for the respective changes in air pollutant concentrations.
Association between air pollutants and hospital admissions and mortality due to pulmonary tuberculosis
Two studies reported a significant association between PM2.5 and PTB mortality; OR = 1.46 (95% CI: 1.15–1.85)33 and percentage change in cases of 0.08% (95% CI: 0.06–0.09)45. There was no significant association between CO, SO2, and O3 and PTB mortality47 (Table 4). Likewise, there was no significant association between PM10, CO, SO2, O3 and hospital admission30,47. NO2 was associated with hospital admission due to PTB, OR: 1.21 (95% CI: 1.10–1.33) (Table 4).
Subgroup analysis and meta-regression
Studies were categorised according to their duration (less than 5 years and 5 years or more), location (Asia and others), number of PTB cases (less than 5000 and 5000 or more) and study quality (good and fair/poor). None of these study characteristics could explain the observed heterogeneity across studies, except for study location with regards to exposure to PM2.5 air pollutant. There was a higher risk of PTB incidence with PM2.5 exposure in studies conducted out of Asia (Additional file 5).
Discussion
Exposure to PM2.5, PM10 and SO2 air pollutants was found to be associated with an increased incidence of PTB, while exposure to CO, NO2 and O3 was not. There was no observed association between exposure to these air pollutants and hospital admission or mortality due to PTB. The findings of this review are particularly relevant given the increasing global concentrations and exposure to some air pollutants such as SO2 and PM2.5 over the past decades50,51. Public health strategies aimed at ending the tuberculosis epidemic would therefore have to work alongside interventions aimed at improving overall air quality and addressing air pollution51.
Air pollutants including O3 and NO2 mainly originate from volatile organic compounds, combustion processes including heating, power generation, the engines of vehicles and ships and also from industry emissions52. SO2 originates from the burning of fossil fuels for power generation and the smelting of sulfur-containing mineral ores52. PM2.5 and PM10 which consist of particles of organic and inorganic substances are typically suspended in the air52. Air pollutants have been previously associated with the development of cardio-respiratory diseases in both children and adults8,9,11. Traffic-related pollution and several air pollutants such as O3, NO2, PM2.5 and PM10, have not only been associated with exacerbations of asthma and chronic obstructive pulmonary disease, but have also been implicated in the development of these conditions especially in childhood11,53,54. Air pollutants are known to increase the risk of infection when inhaled as they dampen the natural defence barriers of the respiratory tract, inhibit muco-ciliary clearance, inhibit macrophages and initiate a chronic inflammatory response with the release of pro-inflammatory mediators55,56. In a similar way, exposure to particulate matter for example has immunomodulatory effects on antimycobacterial activity through impaired expression of important cytokines and chemokines which are important in controlling mycobacterial infections57,58. This reduced antimycobacterial host immune response predisposes to tuberculosis infection.
Measures and policies in various sectors such as the transport, housing and industry sectors are known to reduce air pollutions, including; prioritising walking and cycling in cities, using low-emission vehicles; using clean technologies that reduce industrial emissions; improving access to clean household energy for heating, lighting and cooking; making cities more green; using low-emission fuels and combustion-free power sources, among others52. In 2015, the WHO member states adopted a resolution for enhanced global response to the adverse health effects of air pollution, and the WHO has been overseeing this response through; the production of air quality guidelines and exposure limits to these air pollutants52.
Even though the studies by Ge et al.59 and Xu et al.60 reported a possible association between short-term exposure to SO2, our review did not assess outpatient PTB visits as an outcome. This is therefore a subject amenable to further exploration.
The studies in this review were conducted over a 24-year period and we did not observe a particular change or variation in the trend of the reported associations between exposure to the air pollutants and PTB incidence over time across the older and newer studies. Close to four fifth of the studies in our review were conducted in Asia and up to half of the studies were in China, which could affect the generalisability of the findings of this review, however, China is still a high-burden country for tuberculosis61,62. The observed between-study heterogeneity highlights the need for more uniform study designs and methods in future studies aiming to assess this association.
Interpretation of the findings from this review should take into consideration some limitations. This review did not assess the contribution of indoor air pollution and other comorbidities to the increased risk of PTB incidence, hospital admission and mortality. The different study designs and methodologies affected the types of confounders that could be adjusted for in the different studies and therefore introducing inconsistency in the adjustment of confounders across studies. This review, therefore, focused on the strongest reported associations between air pollutant exposure and PTB incidence rather than on the duration of exposure to the air pollutants.
Conclusion
Exposure to PM2.5, PM10, NO2 and SO2 air pollutants was found to be associated with an increased incidence of PTB, while exposure to CO and O3 was not. These findings of this study and the overall quality of the evidence highlight the need for more rigorous exploration of this association.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
World Health Organization. Global Tuberculosis Report 2019 (World Health Organization, 2019).
GBD Tuberculosis Collaborators. Global, regional, and national burden of tuberculosis, 1990–2016: Results from the global burden of diseases, injuries, and risk factors 2016 study. Lancet Infect Dis. 18(12), 1329–1349 (2018).
United Nations. Sustainable Development Goals. Goal 3: Ensure healthy lives and promote well-being for all at all ages [Internet]. https://www.un.org/sustainabledevelopment/health/ [cited 2020 Jan 17].
Hargreaves, J. R. et al. The social determinants of tuberculosis: From evidence to action. Am. J. Public Health 101(4), 654–662 (2011).
Grobusch, M. P. & Kapata, N. Global burden of tuberculosis: Where we are and what to do. Lancet Infect. Dis. 18(12), 1291–1293 (2018).
Rajaei, E. et al. Outdoor air pollution affects tuberculosis development based on geographical information system modeling. Biomed. Biotechnol. Res. J. BBRJ 2(1), 39 (2018).
Li, Z. et al. Long-term effect of exposure to ambient air pollution on the risk of active tuberculosis. Int. J. Infect. Dis. 1(87), 177–184 (2019).
Requia, W. J. et al. Global association of air pollution and cardiorespiratory diseases: A systematic review, meta-analysis, and investigation of modifier variables. Am. J. Public Health 108(S2), S123–S130 (2017).
Atkinson, R. W., Kang, S., Anderson, H. R., Mills, I. C. & Walton, H. A. Epidemiological time series studies of PM2.5 and daily mortality and hospital admissions: A systematic review and meta-analysis. Thorax 69(7), 660–665 (2014).
Ab Manan, N., Noor Aizuddin, A. & Hod, R. Effect of air pollution and hospital admission: A systematic review. Ann. Glob. Health 84(4), 670–678 (2018).
Kelly, F. J. & Fussell, J. C. Air pollution and airway disease. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 41(8), 1059–1071 (2011).
Moore, E. et al. Global associations between air pollutants and chronic obstructive pulmonary disease hospitalizations. A systematic review. Ann. Am. Thorac. Soc. 13(10), 1814–1827 (2016).
Song, Q., Christiani, D. C., Wang, X. & Ren, J. The global contribution of outdoor air pollution to the incidence, prevalence, mortality and hospital admission for chronic obstructive pulmonary disease: A systematic review and meta-analysis. Int. J. Environ. Res. Public Health 11(11), 11822–11832 (2014).
Schwartz, J. PM10 ozone, and hospital admissions for the elderly in Minneapolis-St. Paul, Minnesota. Arch. Environ. Health Int. J. 49(5), 366–374 (1994).
Dockery, D. W. et al. Effects of inhalable particles on respiratory health of children. Am. Rev. Respir. Dis. 139(3), 587–594 (1989).
Sumpter, C. & Chandramohan, D. Systematic review and meta-analysis of the associations between indoor air pollution and tuberculosis. Trop. Med. Int. Health 18(1), 101–108 (2013).
Kolappan, C. & Subramani, R. Association between biomass fuel and pulmonary tuberculosis: A nested case–control study. Thorax 64(8), 705–708 (2009).
Kurmi, O. P., Sadhra, C. S., Ayres, J. G. & Sadhra, S. S. Tuberculosis risk from exposure to solid fuel smoke: A systematic review and meta-analysis. J. Epidemiol. Community Health 68(12), 1112–1118 (2014).
Popovic, I. et al. A systematic literature review and critical appraisal of epidemiological studies on outdoor air pollution and tuberculosis outcomes. Environ. Res. 170, 33–45 (2019).
Dimala, C. A., Kadia, B. M. & Hansell, A. The association between ambient air pollution and pulmonary tuberculosis: A systematic review protocol. Environ. Evid. 9(1), 29 (2020).
Haddaway, N. R., Macura, B., Whaley, P. & Pullin, A. S. ROSES RepOrting standards for Systematic Evidence Syntheses: Pro forma, flow-diagram and descriptive summary of the plan and conduct of environmental systematic reviews and systematic maps. Environ. Evid. 7(1), 7 (2018).
Study Quality Assessment Tools—NHLBI, NIH [Internet]. https://www.nhlbi.nih.gov/health-pro/guidelines/in-develop/cardiovascular-risk-reduction/tools [cited 2017 Nov 2].
What is GRADE?—BMJ Best Practice [Internet]. https://bestpractice.bmj.com/info/toolkit/learn-ebm/what-is-grade/ [cited 2020 Jan 17].
Unit Conversion | Air Pollution Information System [Internet]. http://www.apis.ac.uk/unit-conversion [cited 2020 Feb 10].
Begg, C. B. & Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 50(4), 1088–1101 (1994).
Egger, M., Smith, G. D., Schneider, M. & Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 315(7109), 629–634 (1997).
Hwang, S. et al. Impact of outdoor air pollution on the incidence of tuberculosis in the Seoul metropolitan area, South Korea. Korean J. Intern. Med. 29(2), 183–190 (2014).
Jassal, M. S., Bakman, I. & Jones, B. Correlation of ambient pollution levels and heavily-trafficked roadway proximity on the prevalence of smear-positive tuberculosis. Public Health 127(3), 268–274 (2013).
Smith, G. S., Schoenbach, V. J., Richardson, D. B. & Gammon, M. D. Particulate air pollution and susceptibility to the development of pulmonary tuberculosis disease in North Carolina: An ecological study. Int. J. Environ. Health Res. 24(2), 103–112 (2014).
Álvaro-Meca, A., Díaz, A., de Miguel, D. J., Resino, R. & Resino, S. Environmental factors related to pulmonary tuberculosis in HIV-infected patients in the combined antiretroviral therapy (cART) era. PLoS One 11(11), e0165944 (2016).
Chen, K. Y. et al. Particulate matter is associated with sputum culture conversion in patients with culture-positive tuberculosis. Ther. Clin. Risk Manag. 12, 41 (2016).
Lai, T. C. et al. Ambient air pollution and risk of tuberculosis: A cohort study. Occup. Environ. Med. 73(1), 56–61 (2016).
Peng, Z., Liu, C., Xu, B., Kan, H. & Wang, W. Long-term exposure to ambient air pollution and mortality in a Chinese tuberculosis cohort. Sci. Total Environ. 15(580), 1483–1488 (2017).
Smith Geneé, S. et al. Air pollution and pulmonary tuberculosis: A nested case-control study among members of a Northern California Health Plan. Environ. Health Perspect. 124(6), 761–768 (2016).
Zhu, S. et al. Ambient air pollutants are associated with newly diagnosed tuberculosis: A time-series study in Chengdu, China. Sci Total Environ. 631–632, 47–55 (2018).
Joob, B. & Wiwanitkit, V. Incidence of pulmonary tuberculosis and particulate matter 2.5 pollutant level: The association analysis for 2019 air pollution Crisis, Bangkok, Thailand. Biomed. Biotechnol. Res. J. 3(2), 126–126 (2019).
Yao, L. et al. Ambient air pollution exposures and risk of drug-resistant tuberculosis. Environ. Int. 124, 161–169 (2019).
Carrasco-Escobar, G., Schwalb, A., Tello-Lizarraga, K., Vega-Guerovich, P. & Ugarte-Gil, C. Spatio-temporal co-occurrence of hotspots of tuberculosis, poverty and air pollution in Lima, Peru. Infect. Dis. Poverty 9(1), 32 (2020).
Huang, K. et al. Association between short-term exposure to ambient air pollutants and the risk of tuberculosis outpatient visits: A time-series study in Hefei, China. Environ. Res. 184, 109343 (2020).
Wang, W. et al. Epidemiological characteristics of tuberculosis and effects of meteorological factors and air pollutants on tuberculosis in Shijiazhuang, China: A distribution lag non-linear analysis. Environ. Res. 22, 110310 (2020).
Yang, J. et al. A study on the relationship between air pollution and pulmonary tuberculosis based on the general additive model in Wulumuqi, China. Int. J. Infect. Dis. 1(96), 42–47 (2020).
You, S., Tong, Y. W., Neoh, K. G., Dai, Y. & Wang, C. H. On the association between outdoor PM2.5 concentration and the seasonality of tuberculosis for Beijing and Hong Kong. Environ. Pollut. Barking Essex 2016(218), 1170–1179 (1987).
Liu, Y., Cui, L. L., Hou, L. J., Yu, C. B., Tao, N. N., Liu, J. Y. et al. Ambient air pollution exposures and newly diagnosed pulmonary tuberculosis in Jinan, China: A time series study. Sci. Rep. [Internet] 8(1). https://www.scopus.com/inward/record.uri?eid=2-s2.0-85057222564&doi=10.1038%2fs41598-018-35411-6&partnerID=40&md5=688c7ce37da23c36a007e125ddddadbc (2018).
Kim, H., Yu, S. & Choi, H. Effects of particulate air pollution on tuberculosis development in seven major cities of Korea from 2010 to 2016: Methodological considerations involving long-term exposure and time lag. Epidemiol. Health 42, e2020012 (2020).
Liu, Y. et al. Effect of ambient air pollution on tuberculosis risks and mortality in Shandong, China: A multi-city modeling study of the short- and long-term effects of pollutants. Environ. Sci. Pollut. Res. Int. 28(22), 27757–27768 (2021).
Xiong, Y. et al. Association of daily exposure to air pollutants with the risk of tuberculosis in Xuhui District of Shanghai, China. Int. J. Environ. Res. Public Health 19(10), 6085 (2022).
Sohn, M. et al. Association of social deprivation and outdoor air pollution with pulmonary tuberculosis in spatiotemporal analysis. Int. J. Environ. Health Res. 29(6), 657–667 (2019).
Liu, F., Zhang, Z., Chen, H. & Nie, S. Associations of ambient air pollutants with regional pulmonary tuberculosis incidence in the central Chinese province of Hubei: A Bayesian spatial-temporal analysis. Environ. Health 19(1), 51 (2020).
Wang, H., Tian, C., Wang, W. & Luo, X. Temporal cross-correlations between ambient air pollutants and seasonality of tuberculosis: A time-series analysis. Int. J. Environ. Res. Public Health [Internet] 16(9). https://www.scopus.com/inward/record.uri?eid=2-s2.0-85065795216&doi=10.3390%2fijerph16091585&partnerID=40&md5=6b4fa1102eabb1f409e8770c89f3a35f (2019).
Butt, E. W. et al. Global and regional trends in particulate air pollution and attributable health burden over the past 50 years. Environ. Res. Lett. 12(10), 104017 (2017).
Shaddick, G., Thomas, M. L., Mudu, P., Ruggeri, G. & Gumy, S. Half the world’s population are exposed to increasing air pollution. Npj Clim. Atmos. Sci. 3(1), 1–5 (2020).
Ambient (outdoor) air pollution [Internet]. https://www.who.int/news-room/fact-sheets/detail/ambient-(outdoor)-air-quality-and-health [cited 2022 Jun 11].
McConnell, R. et al. Asthma in exercising children exposed to ozone: A cohort study. Lancet Lond. Engl. 359(9304), 386–391 (2002).
Gauderman, W. J. et al. The effect of air pollution on lung development from 10 to 18 years of age. N. Engl. J. Med. 351(11), 1057–1067 (2004).
Gasser, M. et al. Toxic effects of brake wear particles on epithelial lung cells in vitro. Part Fibre Toxicol. 6(1), 30 (2009).
Behndig, A. F. et al. Airway antioxidant and inflammatory responses to diesel exhaust exposure in healthy humans. Eur. Respir. J. 27(2), 359–365 (2006).
Rivas, C. E., Cantarella, P., Sarkar, S., Rockafellow, M., Osornio Vargas, A. R., Torres, M. et al. Particulate air pollution matter (PM) modifies innate immunity of type II pneumocytes (A549) against mycobacterium tuberculosis. Am. J. Respir. Crit. Care Med. [Internet]. 189, Meeting Abstracts. https://doi.org/10.1164/ajrccm-conference.2014.189.1_MeetingAbstracts.A2489 (2014).
Sarkar, S. et al. Suppression of the NF-κB pathway by diesel exhaust particles impairs human antimycobacterial immunity. J. Immunol. 188(6), 2778–2793 (2012).
Ge, E. et al. Ambient sulfur dioxide levels associated with reduced risk of initial outpatient visits for tuberculosis: A population based time series analysis. Environ. Pollut. 228, 408–415 (2017).
Xu, M. et al. Association of air pollution with the risk of initial outpatient visits for tuberculosis in Wuhan, China. Occup. Environ. Med. 76(8), 560–566 (2019).
Tuberculosis (TB) [Internet]. https://www.who.int/news-room/fact-sheets/detail/tuberculosis [cited 2021 Apr 3].
Tuberculosis China [Internet]. https://www.who.int/westernpacific/health-topics/tuberculosis [cited 2021 Apr 3].
Author information
Authors and Affiliations
Contributions
C.A.D. conceived and designed the experiments. C.A.D. and B.M.K. conducted the experiments. C.A.D. produced the manuscript. B.M.K. reviewed the manuscript. All authors approved the final copy of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dimala, C.A., Kadia, B.M. A systematic review and meta-analysis on the association between ambient air pollution and pulmonary tuberculosis. Sci Rep 12, 11282 (2022). https://doi.org/10.1038/s41598-022-15443-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-15443-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.