Abstract
This study aims to improve the quality and quantity of winter wheat by using the potential of combining the use of cold plasma and waste biorefinery products for improving wheat yield. Plasma was applied by a radio frequency (RF) plasma reactor operated with air for 180 s and 50 W. The waste biorefinery products, including pyroligneous acid, biochar, and azolla compost, were used as plant nutrition. The effects of cold plasma treatment and waste biorefinery products were determined by measuring plant photosynthesis, grain yield, and content of chlorophyll, carotenoids, anthocyanin, protein, and starch. The experiment was conducted during the cropping seasons 2016−18 in a randomized complete block design with four replications. The combination of cold plasma and pyroligneous acid increased the grain yield up to 40.0%. The photosynthesis rate was improved up to 39.3%, and total chlorophyll content up to 48.3% in both years. Seed plasma treatment combined with biochar application increased the starch content by 36.8%. Adding azolla compost increased the protein content by 35.4%. Using seed plasma treatment with biochar increased the microbial biomass carbon by 16.0%. The application of plasma and azolla compost increased the microbial biomass nitrogen by 29.0%.
Similar content being viewed by others
Introduction
Climate change and population growth require an increase in agricultural production per unit area, and healthy crops and food safety play an essential role in the human health community1. Although the excessive application of chemicals in agriculture may increase the yield per unit area, it could cause problems concerning the quality and safety of agricultural products2. According to Balfour (2006), “the human physiological well-being and spiritual has roots in the soil,” and also, “you are what you eat”, indeed refers to the relationship between dietary composition and human physiology3. Therefore, in a sustainable agricultural system, besides yield, proper nutrient cycling management and long-term soil fertility should be taken into consideration4.
Recently, atmospheric-pressure cold plasma has been considered as a novel technique to improve grain yield and seed germination5,6. Plasma is one of the four fundamental states of matter and was first described by chemist Irving Langmuir as an ionized gas containing different oxygen radicals, charged particles, ions, and UV light7,8. Hempedu Bumi (Andrographis paniculata (Burm. f.) Wall. ex Nees) seeds, treated with a Dielectric Barrier Discharge (DBD) at 5950 V for 10 s, had faster germination and seedling emergence because of water uptake improvement9. Treatment of tomato seeds with a DBD reactor increased the tomato yield. The bloom times, the height, the caulis, the extent of the plants, and the average weight, length, and diameter of each fruit in seven treatment groups from 4760 to 6800 V were increased distinctly10.
Exposing safflower seeds to low-pressure radio frequency (RF) 20 W argon gas discharge under two different constant pressures (1.6 and 16 Pa) improved the seed germination by 50.0%6. Treating soybean seeds with 80 W of low-pressure RF plasma at 15 s increased the germination and vigor indices by 14.7% and 63.3%, respectively11. Arabidopsis thaliana seeds treated with a plasma produced by DBD at 7.96 kV improved germination by 56.00%12. Cold plasma treatment affects physiological processes in plants, resulting in the promotion of seed germination and seedling growth11,13, increasing photosynthesis rate14,15,16, carbon and nitrogen metabolism8,17.
Waste biorefinery is an eco-friendly solution to produce fertilizers, fuels, and value-added products18. Waste biorefinery fertilizers have shown to increase the number of microorganisms in the soil2, which are essential in the carbon (C) and nitrogen (N) cycles and the bio-degradation of environmental contaminants19,20,21. Also, soil microbial biomass is essential for soil fertility22.
The microbial biomass in the soil is affected by several factors, particularly by the content of soil organic matter23,24. Pyroligneous acid (PA) is a waste bio-refinery product. It is a brown-colored liquid produced through gas condensation from burning waste plants and wood under limited oxygen conditions25. Pyroligneous acid contains over 200 chemical components, including acetic acid, hydroxy aldehydes, hydroxyl ketones, sugars, carboxylic acid, and phenolic acid26,27. Pyroligneous acid is an excellent source of organic ingredients28, which is non-toxic for humans, animals, plants, and the environment29,30. Pyroligneous acid has been used in traditional Japanese agriculture for more than 400 years31 to improve the quantity and quality of some agricultural products such as Oryza sativa L. (rice), Ipomoea batatas L. (sweet potato), Saccharum officinarum L. (sugarcane), and Cucumis melo L. (melon)32,33,34.
Biochar is a substance produced by a pyrolysis process at a low carbonization temperature (350–700 °C) under conditions of complete or partial anoxia. The carbon content of biochar is high, and its chemical properties are stable35. Adding biochar to the soil can counteract global climate change by sequestering carbon into the soil36. Many studies have investigated the positive influence of biochar. Biochar improves soil fertility, soil structure, texture, particle size distribution, and ultimately, plant growth37. Biochar application at a rate of 15 and 20 t ha−1 has significantly increased wheat and maize grain yield 38,39.
Azolla (Azolla filiculoides Lam.) is considered an invasive plant in wetlands, freshwater lakes, and ditches. It has the possibility to fix atmospheric nitrogen with asymbiotic cyanobacteria (i.e., Anabaena azollae)40. Azolla is suitable as compost due to its high nitrogen content that can enhance crop productivity41,42,43. Azolla compost is a natural fertilizer and can be mixed with rice straw44. It is a nitrogen source for plant nutrition, increasing plant yield45.
We aimed to increase the quality and quantity of winter wheat by treating the seeds with cold plasma before sowing and sowing the seeds in soil with added biorefinery waste products and azolla compost. Furthermore, we studied how these waste products affected the chemical and biological properties of the soil.
Methods
Experimental design and general methodology
The research was carried out in a research field at the Agricultural Research Station of Tarbiat Modares University, Tehran, Iran (35°41' N, 51°10' E, 1265 m above sea level) on sandy-loam soil during October 2016 and 2017. The area is semi-arid (according to the Köppen climate classification)46 characterized by warm and dry summers, long-term (30 years) mean annual rainfall of 232.6 mm, and a mean temperature of 17.6 °C, respectively. The experiment was conducted in a randomized complete block design, with four replications in two cropping seasons. The wheat was a landrace (Pishgam) from the central plateau of Iran. On 9 October 2016 and 9 October 2017, 140 kg ha−1 seeds were sown. Textures and soil elements were determined before sowing. The field was fertilized with nitrogen (150 kg ha−1), phosphorous (180 kg ha−1), and potassium (120 kg ha−1). Wheat seeds were sown manually at a depth of two inches in each plot. The area of the field was 1114 m2. Each plot was five meter wide and six meter long, including 20 rows. Row distance was 25 cm (400 plants ha−1).
Treatments included 1) seed priming by cold plasma, 2) soil application with pyroligneous acid, 3) a combination of cold plasma treatment and pyroligneous acid application, 4) biochar application, 5) a combination of cold plasma treatment and biochar application, 6) azolla compost application, and 7) a combination of cold plasma treatment and azolla compost application. Cold plasma treatment of seeds lasted 180 s. Pyroligneous acid (0.04 L m−2), biochar (0.5 kg m−2), and azolla compost (0.7 kg m−2) were incorporated into the soil before planting. Untreated seeds and soil were used as control.
Plasma treatment
Seed priming with plasma was applied using a radio frequency (RF) plasma reactor (manufactured by H.G and M.S, Shahid Beheshti University, Tehran, Iran) operated with air adjusted to 13.56 MHz, 50 W for 180 s. The vacuum chamber was made of a cylindrical Pyrex tube with an inner diameter of 80 mm and 300 mm. A metallic mesh grounded the outside of the Pyrex tube. The aluminum power electrode was fixed at the center of the cylinder (50 mm in width and 100 mm in length). The sample was placed under the Pyrex tube. The gap between the power electrode and the sample was 40 mm (Fig. 1). The seeds were exposed to a plasma flow for 180 s before sowing. The optical spectra was measured with an Optical Emission Spectroscopy (OES) Model HR2000 + ES with a wavelength range of 190 nm‒1.1 μm and an optical resolution of 0.9 nm (Ocean Insight Company, 3500 Quadrangle Blvd, Orlando, FL 32,817, USA).
Figure 2 shows the optical spectra as a result of the RF plasma treatment. The main peaks are monitored in a range of 450−650 nm in the spectra, consisting of CO and hydrogen species. CO emission lines occur at 451.0, 483.5, 519.5, 561.0, and 607.9 nm, and an H emission line at 656.3 nm may represent products of the seed surface erosion process.
Pyroligneous acid, biochar, and azolla compost production
pyroligneous acid and biochar were produced by the carbonization process of lemon waste wood collected from the subtropical Mazandaran Province. At the beginning of the process, dry woods were placed in the closed furnace avoiding oxygen supply and heated to 350−700 °C. During the burning, the smoke from the wood went through a condenser and condensed to liquid (as pyroligneous acid). The solid part was obtained at 400 °C as biochar.
Measurements
Samples were taken from the middle of the plots to avoid border effects influencing the estimation of the grain yield and yield components. Photosynthesis (µmol m−2 s−1), intercellular CO2 concentration, and stomatal conductance (µmol m−2 s−1) were measured on the flag leaf 14 days after pollination by using a photosynthesis meter (model LI-COR 6400XT Version 6, Company, Lincoln, Nebraska, USA). Samples from green leaves in the middle of the canopy were taken fourteen days after the flowering stage to analyze the photosynthetic pigments.
Determination of chlorophyll and carotenoids
The content of chlorophyll and carotenoids was measured according to the method described by Krizek et al.47,48. Fourteen days after the flowering stage, four fresh leaf samples (0.2 g) were prepared. The samples were extracted in 10 ml 80% acetone solution and centrifuged for 10 min at 1600 rpm. The absorption spectra were measured at 663, 645, and 470 nm with a spectrophotometer (Model Perkin Elmer 3110, Granite Quarry, NC, USA).
Determination of anthocyanin concentration
We extracted 0.2 g fresh leaves in 15 ml glass centrifuge tubes containing 10 ml of acidified methanol (methanol: HCl, 99:1, vol: vol) and kept the tubes in the dark overnight. The absorbance was measured at 550 nm. The anthocyanin concentration was calculated using an extinction coefficient of 33,000 mol−1 cm−148.
Determination of protein content in wheat grain
Kjeldahl method was used to measure protein content49. Approximately 1 g of raw material was hydrolyzed with 15 ml concentrated sulfuric acid (H2SO4) containing two copper catalyst tablets in a heat block (Kjeltec system 2020 digestor, Tecator Inc. Herndon, VA, USA) at 420℃ for two hours. After cooling, H2O was added before neutralization and titration. The amount of total nitrogen in the raw materials was multiplied with both the traditional conversion factor of 6.25 and the species-specific conversion factors to determine the total protein content.
Determination of starch content in wheat grains
The starch content was estimated as described by McCleary et al. (1994)50 using the megazyme total starch analysis (AA/AMG) procedure. First, 100 mg grain was milled in an UDY cyclone mill to pass through a 0.5 mm screen, wetted with 0.2 ml ethanol, and treated with thermostable α-amylase (AA) to partially hydrolyze the starch. After completely dissolving the starch, dextrin was quantitatively hydrolyzed to glucose by amyloglucosidase (AMG). The starch content was estimated based on the glucose measurements.
Determination of the microbial biomass carbon (Cmic) and nitrogen (Nmic) in the soil
Microbial biomass carbon (Cmic) and nitrogen (Nmic) were determined by the chloroform fumigation–extraction method51. Cmic was measured by using a 15 g oven-dry equivalent of the field-moist soil sample, and the C content was extracted using the chloroform-fumigation-extraction method (FE) described by Vance et al. (1987)52. The concentration of Corg in the extractant was determined by a carbon analyzer (SHI-MADZU Model TOC-5050, Milton Freewater, Oregon, USA) after acidification with one drop of 2 M HCl to remove any dissolved carbonate. Carbon biomass was calculated as follows: Cmic = EC/KEC where EC = (organic C extracted from fumigated soil)—(organic C extracted from non-fumigated soil) and KEC = 0.45, which is the proportionality factor to convert EC to Cmic53.
Microbial biomass nitrogen (Nmic) was determined from the total nitrogen (Ntotal) released during fumigation–extraction54. Soil Nmic was determined by the chloroform-fumigation-incubation method (FI) as described by Horwath and Paul (1994)51 using a 15 g oven-dry equivalent of the field-moist soil sample after adjusting the moisture content to 55% of the water-holding capacity (WHC). Samples were conditioned for seven days at 25℃. The NH4+-N in the extracts was determined in 20 ml aliquots by steam distillation55. Biomass N was calculated using the equation: Nmic = EN/kIN Where EN = (flush of NH4+-N due to fumigation)–(NH4+-N produced in the non-fumigated soil during ten days incubation) and kIN = 0.57, which is the proportionality factor to convert EN to Nmic56. The Cmic and Nmic values were determined on the < 2 mm mesh field-moist samples. All results reported are averages of duplicated analyses and are expressed on a moisture-free basis. Moisture was determined after drying at 105 °C for 48 h.
Statistical analysis
Years were analyzed separately because Bartlett’s test was significant for most traits measured. The effect of treatments was determined by analysis of variance (ANOVA) using SAS (Version 9.2) (SAS, 2002) software57. The assumptions of variance analysis were tested by ensuring that the residuals were random, homogenous, with a normal distribution about/above a mean of zero. LSD test at the 0.01 probability level was used to check significant differences between means.
Results
Effect of combination of cold plasma and azolla compost on wheat treats
By examining plasma light emission spectroscopy with Scanning Electron Microscopy (SEM) and Atomic Force Microscopy (AFM) images of the sample surface, it has been shown how plasma treatment can increase seed growth components. Atomic Force Microscopy image was taken from two samples of wheat treated with air plasma at 50 W for 180 s. Atomic force microscope images showed the roughness and morphology of the seed surface of the two wheat seed samples (Fig. 3). The figure on the left shows the control sample. No carvings were observed on the seed surface of the control sample (Fig. 3). Figure 4 shows Scanning Electron Microscope (SEM) images of two wheat seed samples. The figure on the left shows a sample of a 50 W 180 s air plasma treatment. The bombardment of ions and free radicals in plasma is well seen on the sample surface, which has resulted in seed hydrophilicity. However, an excessive bombardment of the seed and rising temperature may cause the sample to die, which was reflected in the seed germination percentage.
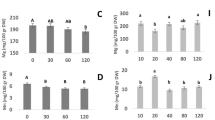
The plasma treatment effectively removed a thin layer of the seed lipid coat by reducing the bio-polymeric chains' length and the average molecular weight on the seed surface (Figs. 3 and 4). Table 1 shows that years (Y) and treatments (T) significantly affected the biochemical content in the wheat grains at a 1% level. There was also a significant interaction effect (Y*T). In both years, the combination of plasma treatment with azolla compost and pyroligneous acid treatments significantly increased the anthocyanin content in the leaves compared with the control. The highest anthocyanin content was obtained when the plasma treatment was combined with azolla compost. This treatment increased the anthocyanin by 39.9% compared with the control (Table 2).
In the second year, the combination of plasma treatment and azolla compost, biochar, and pyroligneous acid significantly increased the carotenoid content compared with control. In both years, the combination of plasma treatment and azolla compost increased the carotenoid content most (Table 2).
In both years, cold plasma treatment combined with azolla compost application significantly increased the grain protein by 35.0% compared with the control (Fig. 5). In the second year, plasma treatment combined with azolla compost, and plasma treatment combined with pyroligneous acid significantly increased the amount of grain protein.
The effect of combining cold plasma and pyroligneous acid treatment
The combination of plasma and pyroligneous acid significantly increased the total chlorophyll content and net photosynthesis rate in both years (Table 2). There was a significant difference between years at a 1% level in intercellular CO2 concentration and stomatal conductance. In addition, there was a significant difference between treatments and interaction between treatments and years on mesophyll conductance and stomatal conductance of wheat at a 1% level (Table 3).
The combination of plasma and pyroligneous acid and azolla compost increased the intercellular CO2 concentration in the first year (Table 4). The highest intercellular CO2 concentration was observed in both years when plasma and pyroligneous acid treatments were combined (Table 4).
There was a significant difference between years and interaction between years and treatments at a 1% level according to LSD test for wheat grain yield (Table 5). In both years, however, their combination of plasma and pyroligneous acid and azolla compost increased the grain yield (Fig. 6). The highest grain yield was observed when plasma and pyroligneous acid were combined.
Effect of pyroligneous acid on wheat treats
In 2017, cold plasma combined with pyroligneous acid and cold plasma combined with azolla compost and pyroligneous acid significantly increased stomatal conductance (Table 4). In both years, the maximum stomatal conductance (38.0% increase) was obtained in the pyroligneous acid application (Table 4).
The plasma treatment and plasma treatment combined with pyroligneous acid significantly increased the mesophyll conductance in the first year. In the second year, biochar and pyroligneous acid treatments significantly increased the mesophyll conductance (Table 4).
Effect of biochar on wheat treats
There was a significant difference between years and treatments at a 1% level according to LSD test for wheat grain starch (Table 5).
Plasma seed priming combined with azolla compost and biochar significantly increased the starch content in both years. The highest amount of starch (36.0% increase) was achieved by combining plasma seed priming with biochar (Fig. 7).
Effect of different treatments on the content of chemicals in the soil
The application of pyroligneous acid and the combination of pyroligneous acid and plasma seed priming significantly increased available phosphor, zinc, iron, and calcium in the soil (Table 6). Application of Azolla compost to the soil and the combination of plasma seed priming with azolla compost also significantly increased the amount of nitrogen in the soil. When biochar and plasma seed priming were combined, the amount of organic carbon significantly increased compared with the controls (Table 6).
All treatments where Azolla compost was included significantly increased the microbial biomass carbon content (up to 29.0%) compared with the control (Table 7). Also, the combination of biochar and plasma seed priming significantly increased the microbial biomass carbon (16.0%) (Table 7).
Discussion
Cold plasma treatment positively affected the physiological and biochemical measured parameters and yield (Tables 2 and 4). A positive effect of cold plasma on photosynthesis rate has been reported previosly58,59,60. The increasing germination caused by the plasma treatment is probably due to 1) changes in the surface of the seed coat resulting in increasing water and nutrient uptake, 2) changes that occur inside the cell that increases the biochemical activities14,61. When the plasma treatment enhances water and nutrient uptake, it results in greater seed weight62,63. This effect of plasma treatment has also been shown for beans (Phaseolus vulgaris L.)64.
Several OH and nitrogen species peaks can also be observed in the 250–400 nm spectral range after plasma treatment. Spectral emissions at 283.4, 309.0, and 312.7 nm indicate the presence of OH species, and a peak at 357.0 nm also corresponds to molecular nitrogen in the RF plasma. Plasma interaction with material surfaces leads to chemical changes on the surface, as a result increasing seed surface hydrophilicity characteristics.
The oxygen gas spectrum, showed two basic peaks, 777.0 nm, and 844.0 nm, which correspond to the first excited state of the oxygen atom (Fig. 2). In low-pressure cold plasma, free electrons' activation and excitation of molecules occur. For example, in air plasma, which is a combination of nitrogen and oxygen, the following processes also happen:
In other words, chain processes lead to the formation of unstable molecules such as ozone and nitrous oxide. The last reaction results from the quasi-stable-neutral collision of the nitrogen and oxygen molecules. This reaction, together with active compounds such as O, O2, O3, and the collision of ions and free radicals with the seed coat, probably has the most significant effect on surface carving and activation64.
The chemical interaction of active compounds leads to the formation of hydrophilic chemical bonds on its surface, changing the surface properties from a non-polar state (hydrocarbons and fats) to polar bonds, including oxygen, thus increasing the hydrophilicity of the seed surface. In the 600−4000 cm−1, peaks related to O–H and C−O bands belonging to the functional group of phenols, C−O carbonyl esters, and C−N and N–H functional groups of amines were observed. Therefore, seed hydrophilicity increases on the seed surface by breaking carbon bonds (often due to the presence of lipid on the seed surface) and the penetration of polar agents, including oxygen and nitrogen atoms that are actively present in the plasma.
The combination of pyroligneous acid and cold plasma treatment significantly affected the total chlorophyll, intercellular CO2 concentration, and net photosynthesis rate of winter wheat compared to the control (Tables 2 and 4). Previous studies have shown the positive effect of pyroligneous acid on accelerating somatic embryogenesis65, enhancing seed germination66, increasing flowering rate, and root biomass67,68,69. It has also been shown that pyroligneous acid can increase the growth of roots, stems, tubers, leaves, flowers, and fruits and improves soil fertility70.
Pyroligneous acid comprises ester compounds70. The esters can increase the chlorophyll content and stimulate the photosynthesis in several plants, increasing the synthesis of carbohydrates and amino acids in plants and, consequently, increasing plant yield and making plants more resistant to pests and diseases71,72. The biophysical properties of esters include lipid solubility. Transmembrane permeability may be a critical factor in the bioactivity of esters in the plant cells' physiological processes. The esters provoke stomatal closure in several plant species belonging to Solanaceae, Leguminosae, Brassicaceae, and Gramineae due to increasing CO2 uptake in plant leaves73. The stomatal aperture regulates CO2 uptake and water transpiration and influences the plant's resilience under drought stress condition74. Also, the accumulation of various metabolites, such as intermediates of the Calvin cycle, paramylon precursors, and pyruvate, as a substrate for pyruvate: NADP + oxidoreductase, by enhancements in photosynthetic capacity, is one of the key metabolic factors for the role of esters75.
Increase in stomatal conductance results in enhancement of the CO2 uptake, which increases the photosynthesis rate, and the flow of supply materials76 for grain performance77,78. The highest grain yield was achieved by combining cold plasma treatment with the application of pyroligneous acid in both years (Fig. 6).
Pyroligneous acid contains 15 macro and microelements, including calcium, cadmium, chromium, copper, iron, potassium, manganese, aluminum, sodium, and zinc72. It contains more than 200 substances, including organic acid (e.g., formic acid, acetic acid, acetic acid, propionic acid, butyric acid, phenol group, carbonyl group, formaldehyde, acetaldehyde, ethanol, methanol, acetyl)79,80. The simultaneous presence of acetic acid and iron and calcium cations creates a complex where the ionic bond replaces the covalent bonds, preventing iron deposition in the soil and leaching other elements72. Besides, pyroligneous acid helps enrich the phosphorus, calcium, iron, and potassium contents in the soil (Table 6).
The plasma treatment combined with azolla compost application increased the protein content (Fig. 5). Organic fertilizers such as azolla compost are known as a source of nitrogen. Also, it has been shown that compost increases nitrogen absorption in the plant81. High nitrogen absorption leads to an increased amount of protein content in the grain of crop72. The maximum concentration of anthocyanin in cold plasma treatment and azolla compost seems to be conditioned by the availability of assimilates82,83. In this way, nitrogen enters the microbial parts to help extend their activity. Compost feedstock can affect the carbon and nitrogen cycling dynamics of the soil84. Azolla compost can effectively increase soil organic matter content, resulting in a higher carbon and nitrogen mineralization85 and increased microbial biomass86. Compost affects the soil fertility gradients by C and N availability and, subsequently, improves soil microbial activity87.
Biochar application increased the starch content significantly (Fig. 7). Biochar was obtained by decomposing the forest, residual plants, and manure residues containing essential nutrients elements such as carbon, nitrogen, and sulfur88. Numerous studies have shown that biochar can significantly increase nutrient uptake, increasing starch content89,90,91. In addition, biochar also retains soil nutrients and soils cation exchange capacity due to less leaching92,93,94.
Azolla compost and biochar increased the microbial biomass nitrogen (MBN) and microbial biomass carbon (MBC). The flexibility of MBC is probably due to microbial death or the release of intracellular substrates95. Increasing carbon input (through organic amendment application) can increase the soil organic matter degradation rate96 as microbes use labile C to decompose recalcitrant soil organic matter97.
Biochar in soil has several direct and indirect influences on soil biota because of changes in several abiotic factors, including soil pH or altered substrate quality as a source of energy98. In addition, biochar increases positive changes to soil characteristics of water contents, EC, and pH status; all of these factors, directly and indirectly, affect the activity of life in the soil and, as a result, increase soil microbial activity99. Hence, soil microbial activities are related to soil fertility and agricultural productivity100.
Conclusion
A combination of plasma seed priming and pyroligneous acid application increased the grain yield of the winter wheat. Furthermore, combining seed plasma treatment with biochar and azolla compost application increased the starch and protein content in the harvested grain. The biorefinery products also improved the quality of the soil significantly. The amendments enhanced the soil microbial biomass and improved essential soil factors affecting the root system's ability to uptake soil water and nutrients. Cold plasma seed priming seems to be a promising technique to improve seed germination performance, plant establishment, growth, and grain yield. This research showed ways to improve crop yield and contribute to preserving the environment by reducing the effects of climate change side-effects by reducing the need for agrochemicals (fertilizers) during the growth cycle and improving soil microbial biomass. By combining cold plasma treatment with pyroligneous acid, biochar, and azolla compost, we improved the soil as a growth medium and the performance of winter wheat significantly, resulting in a higher yield.
References
FAO. World Food Situation (FAO, Rome, 2019).
Kafi, M., Borzoee, A., Salehi, M. & Kamandi, A. Physiology of environmental stresses in plants (University of Mashhad Mashhad., Iran, 2014).
Balfour, E. The Living Soils (Faber., London, 1943)
Tuomisto, H. L. et al. Effects of environmental change on agriculture, nutrition and health: A framework with a focus on fruits and vegetables. Wellcome Open Res. 2, 21 (2017).
Goldston, R. J. & Rutherford, P. H. Introduction to Plasma Physics (Taylor and Francis, 1995).
Dhayal, M., Lee, S. Y. & Park, S. U. Using low-pressure plasma for Carthamus tinctorium L. seed surface modification. Vacuum 80, 499–506. https://doi.org/10.1016/j.vacuum.2005.06.008 (2006).
Langmuir, I. & Tonks, L. Ion oscillations in a warm plasma. Phys. Rev. 33, 195–210 (1929).
Zhou, Z.W., Huang, Y. F., Yang, S. Z. & Xiong, D.Y. Progress in electromagnetics research symposium proceedings Kuala Lumpur, Malaysia (2012).
Jiayun, T. et al. Effects of atmospheric pressure air plasma pre-treatment on the seed germination and early growth of Andrographis paniculata. Plasma Sci. Technol. 16(3), 260–268. https://doi.org/10.1088/1009-0630/16/3/16 (2014).
Zhou, Z., Huang, Y., Yang, S. & Chen, Y. Introduction of a new atmospheric pressure plasma device and application on tomato seeds. Agri. Sci. 02(01), 23–27. https://doi.org/10.4236/as.2011.21004 (2011).
Ling, L. et al. Effects of cold plasma treatment on seed germination and seedling growth of soybean. Sci. Rep. 4(1), 1–7 (2014).
Kazunori, K. et al. Simple method of improving harvest by nonthermal air plasma irradiation of seeds of Arabidopsis thaliana (L). Appl. Phys. Express. 9, 016201 (2016).
Sera, B., Stranak, V., Sery, M., Tichy, M. & Spatenka, P. Germination of Chenopodium Album in response to microwave plasma treatment. Plasma Sci. Technol. 10, 506–511. https://doi.org/10.1088/1009-0630/10/4/22 (2008).
Sera, B., Spatenka, P., Sery, M., Vrchotova, N. & Hruskova, I. Influence of plasma treatment on wheat and oat germination and early growth. IEEE Trans Plasma Sci. 38, 2963–2968. https://doi.org/10.1109/TPS.2010.2060728 (2010).
Chen, H. H., Chen, Y. K. & Chang, H. C. Evaluation of physicochemical properties of plasma treated brown rice. Food Chem. 135, 74–79. https://doi.org/10.1016/j.foodchem.2012.04.092 (2012).
Saberi, M., Modarres-Sanavy, S. A. M., Zare, R. & Ghomi, H. Amelioration of photosynthesis and quality of wheat under non-thermal radio frequency plasma treatment. Sci. Rep. 8, 11655. https://doi.org/10.3860/irrn.v31i2.1132 (2018).
Henselová, M., Slováková, L., Martinka, M. & Zahoranová, A. Growth, anatomy and enzyme activity changes in maize roots induced by treatment of seeds with low-temperature plasma. Biologia 67, 490–497. https://doi.org/10.2478/s11756-012-0046-5 (2012).
Palmeros Parada, M., Osseweijer, P. & Posada Duque, J. A. Sustainable biorefineries, an analysis of practices for incorporating sustainability in biorefinery design. Ind. Crops Prod. 106, 105–123 (2017).
Fierer, N. & Jackson, R. B. The diversity and biogeography of soil bacterial communities. Proc. Natl. Acad. Sci. 103, 626–631. https://doi.org/10.1073/pnas.0507535103 (2006).
Green, J. L., Bohannan, B. J. M. & Whitaker, R. J. Microbial biogeography: from taxonomy to traits. Sci. 320, 1039–1043. https://doi.org/10.1126/science.1153475 (2008).
He, Z., Nostrnd, J., Deng, Y. & Zhou, J. Development and applications of functional gene microarrays in the analysis of the functional diversity, composition, and structure of microbial communities. Front. Environ. Sci. Engin. 5, 1–20. https://doi.org/10.1007/s11783-011-0301-y (2011).
Insam, H. Developments in soil microbiology since the mid1960s. Geoderma 100, 389–402. https://doi.org/10.1016/S0016-7061(01)00029-5 (2001).
Bauhus, J., Pare, D. & Cote, L. Effects of tree species, stand age and soil type on soil microbial biomass and its activity in a southern boreal forest. Soil Biol. Biochem. 30, 1077–1089. https://doi.org/10.1016/s0038-0717(97)00213-7 (1998).
Liu, X. M., Li, Q., Liang, W. J. & Jiang, Y. Distribution of soil enzyme activities and microbial biomass along a latitudinal gradient in farmlands of Songliao plain, northeast China. Pedosphere 18, 431–440. https://doi.org/10.1016/S1002-0160(08)60034-X (2008).
Nurhayati, T., Roliadi, H. & Bermawie, N. Production of Mangium (Acacia mangium) wood vinegar and its utilization. J. For. Res. 2(1), 13–25 (2005).
Fengel, D. & Wegener, G. Wood-Chemistry, Ultrastructure, Reactions (Walter de Gruyter., Berlin, 1983).
Guillen, M. D. & Manzanos, M. J. Study of the volatile composition of an aqueous oak smoke preparation. Food Chem. 79, 283–292. https://doi.org/10.1016/S0308-8146(02)00141-3 (2002).
Yatagai, M., Nishimoto, M., Hori, K., Ohira, T. & Shibata, A. Termiticidal activity of wood vinegar, its components and their homologues. J. Wood Sci. 48, 338–342. https://doi.org/10.1007/BF00831357 (2002).
Tongdeethare, S. Wood vinegar the new organic compound for agriculture. Kehakaset J. 26(9), 96–101 (2002).
Pangnakorn, U. Utilization of wood vinegar by- product from Iwate kiln for organic agricultural system. Technology and Innovation for Sustainable Development Conf. (TISD2008), Faculty of Engineering, Khon Kaen University, Thailand (2008).
Mu, J., Uehara, T. & Furuno, T. Effect of bamboo vinegar on regulation of germination and radicle growth of seed plants. J. Wood Sci. 49, 262–270. https://doi.org/10.1007/s10086-002-0472-z (2003).
Tsuzuki, E., Wakiyama, Y., Eto, H. & Handa, H. Effect of pyroligneous acid and mixture of charcoal with pyroligneous acid on the growth and yield of rice plant. Jpn. J. Crop Sci. 58, 592–597 (1989).
Du, H. G., Mori, E., Terao, H. & Tsuzui, E. Effect of the mixture of charcoal with pyroligneous acid on shoot and root growth of sweet potato. Jpn. J. Crop Sci. 67, 149–152 (1989).
Uddin, S. M. M., Murayama, S., Ishmine, Y. & Tsuzuki, E. Japanese J Crop Sci. 38, 281–285 (1995).
Song, Y. J. & Gong, J. Effects of biochar application on soil ecosystem functions. J. Ludong. Univ. 26, 361–365 (2010).
Abit, S. M., Bolster, C. H., Cai, P. & Walker, S. L. Influence of feedstock and pyrolysis temperature of biochar amendments on transport of Escherichia coli in saturated and unsaturated soil. Environ. Sci. Tech. 46, 8097–8105. https://doi.org/10.1021/es300797z (2012).
Xu, G. et al. Recent advances in biochar application in agricultural soils: Benefits and environmental implications. Clean-Soil Water Air. https://doi.org/10.1002/clen.201100738 (2012).
Solaiman, M. Z. et al. Biochar influence seed germination and early growth of seedlings. Intern. J. Plant-Soil Relation. 353, 273–287. https://doi.org/10.1007/s11104-011-1031-4 (2011).
Uzoma, K. C. et al. Effect of cow manure biochar on maize productivity under sandy soil condition. Soil Use Manage. 27(2), 205–212. https://doi.org/10.1111/j.1475-2743.2011.00340.x (2011).
Singh, P. Use of Azolla in Asian agriculture. Appl. Agric. Res. 4(3), 149–161 (1990).
Zbytniewski, R. & Buszewski, B. Characterization of natural organic matter (NOM) derived from sewage sludge compost. Part 2: Multivariate techniques in the study of compost maturation. Bioresour. Technol. 96, 479–484 (2005).
Jumadi, O., Hiola, S. F., Hala, Y., Norton, J. & Inubushi, K. Influence of Azolla (Azolla microphylla Kaulf.) compost on biogenic gas production, inorganic nitrogen and growth of upland kangkong (Ipomoea aquatica Forsk.) in a silt loam soil. Soil Sci. Plant Nutr. 60, 722–730 (2014).
Braun-Howland, E. B. & Nierzwicki-Bauer, S. A. Azolla-Anabaena symbiosis: Biochemistry, physiology, ultrastructure, and molecular biology. In CRC Handbook of Symbiotic Cyanobacteria (CRC Press: Boca Raton, FL, USA, 2018)
Farahdahr, F., Valad-Abadi, S. A., Daneshiyan, J., Razavipoor, T. & Amiri, A. Effect of irrigation intervals and Azo compost on Agronomic traits in rice (Oryza sativa). J. Biol Sci. 4(1), 99–111 (2011).
Yazdani Biouki, R., Bannayan Aval, M., Khazaei, H. R. & Sodaeeizadeh, H. Investigating the effects of urea, azocompost and cutting on quantitative and qualitative characteristics of oregano (Origanum Vulgare Virid). Inter. J. Adv. Biol. Biomed. Res. 2(4), 993–1010 (2014).
Köppen, W. "C". In Köppen, Wladimir; Geiger (publisher), Rudolf (eds.). Handbuch der Klimatologie. Vol. 1. (Berlin, Borntraeger, 1936).
Arnon, A. N. Method of extraction of chlorophyll in the plants. Agro. J. 23, 112–121 (1967).
Krizek, D. T., Kramer, G. F., Upadhyaya, A. & Mirecki, R. M. UV-B response of cucumber seedling grown under metal halid and high pressure sodium/deluxe lamps. Physiol. Plant. 88, 350–358. https://doi.org/10.1111/j.1399-3054.1993.tb05509.x (1993).
Krotz, L., Cicerci, E. & Giazzi, G. Protein determination in cereals and seeds. J. Food Qual. 15(4), 37–39 (2008).
Mc Leary, B. V., Solah, V. & Gibson, T. S. Quantitative measurement of total starch in cereal flours and products. J. Cereal Sci. 20, 51–58 (1994).
Horwath, W. R. & Paul, E. A. Microbial biomass. In: Weaver RW, Angel GS, Bottomley PS (eds) Methods of soil analysis, part 2. Book Series No.5. (SSSA, Madison, (1994).
Vance, E. D., Brookes, P. C. & Jenkinson, D. S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 19, 703–707 (1987).
Wu, J., Joergensen, R. G., Pommerening, B., Chaussod, R. & Brookes, P. C. Measurement of soil microbial biomass C by fumigation extraction – an autoclaved procedure. Soil Biol. Biochem. 22, 1167–1169 (1990).
Bremner, J. M. & Mulvaney, C. S. Nitrogen – total methods of soil analysis (ASA, Madison, 1982).
Keeney, D. R. & Nelson, D.W. Nitrogen—inorganic forms. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis, part 2. (ASA, Madison, 1982).
Jenkinson, D. S. Determination of microbial biomass carbon and nitrogen in soil. In Advances in nitrogen cycling in agricultural ecosystems (ed. Wilson, J. R.) 368–386 (CAB International, 1988).
SAS Institute Inc. SAS/STAT user’s guide. Version 9.1. (SAS Institute Inc., North Carolina, 2016).
Volin, J. C., Denes, F. S., Young, R. A. & Park, S. M. T. Modification of seed germination performance through cold plasma chemistry technology. Crop Sci. 40, 1706–1718. https://doi.org/10.2135/cropsci2000.4061706x (2000).
Meiqiang, Y., Mingjing, H., Buzhou, M. & Tengcai, M. Stimulating effects of seed treatment by magnetized plasma on tomato growth and yield. Plasma Sci. Tech. 7, 3143–3147. https://doi.org/10.1088/1009-0630/7/6/017 (2005).
Jiang, J. et al. Effect of Cold Plasma Treatment on Seed Germination and Growth of Wheat. Plasma Sci. Technol. 16(1), 54 (2014).
Filatova, I. et al. Plasma seeds treatment as a promising technique for seed germination improvement 31st ICPIG (Granada, 2013).
Bremner, P. & Rawson, H. The weights of individual grains of the wheat ear in relation to their growth potential, the supply of assimilate and interaction between grains. Funct. Plant Biol. 5, 61–72. https://doi.org/10.1071/PP9780061 (1978).
Calderini, D. F. & Reynolds, M. P. Changes in grain weight as a consequence of de-graining treatments at pre- and post-anthesis in synthetic hexaploid lines of wheat (Triticum durum–T. tauschii). Aust. J. Plant Physiol. 27, 183–191 (2000).
Bormashenko, E., Grynyov, R., Bormashenko, Y. & Drori, E. Cold radiofrequency plasma treatment modifies wettability and germination speed of plant seeds. Sci. Rep. 2, 741. https://doi.org/10.1038/srep00741 (2012).
Van Staden, J., Jager, A. K., Light, M. E. & Burger, B. V. Isolation of the major germination cue from plant derived smoke. S. Afr. J. Bot. 70, 654–657 (2004).
Nelson, D. C., Flematti, G. R., Ghisalberti, E. L., Dixon, K. W. & Smith, S. M. Regulation of seed germination and seedling growth by chemical signals from burning vegetation. Annu. Rev. Plant. Biol. 63, 107–130 (2012).
Keeley, J. E. Smoke- induced flowering in the fire-lily Cyrtanthus ventricosus. S. Afr. J. Bot. 59, 638–639 (1993).
Hwang, Y., Matsushita, Y., Sugamoto, K. & Matsui, T. Antimicrobial effect of the wood vinegar from Crytomenia japonica sapwood on plant pathogenic microorganisms. J. Microbiol. Biotecnol. 15(5), 1106–1109 (2005).
Narwal, S. S. Allelopathic interactions in multiple cropping systems (Kluwer Academic Publishers, 2000).
Zulkarami, B., Ashrafuzzaman, M., Husni, M. O. & Ismail, M. R. Effect of pyroligneous acid on growth, yield and quality improvement of rockmelon in soilless culture. Aust. J. Crop Sci. 5(12), 1508–1514 (2011).
Saberi, M., Askary, H., Sarpeleh, A. & Hosseini, A. Wood vinegar as a biological product for managing Fusarium oxysporum f. sp. radisis-cucumerinum. Canadian J. Plant Pro. 1(4), 129–133 (2013).
Taiz, L. & Zeiger, E. Plant physiology (Sinauer Associates Inc., 2006).
Lisón, P., Lopez-Gresa, M. P., Rodrigo, I., and Belles, J. M. National Patent. Compuesto Para la Protección de Plantas Mediante Cierre Estomático, Uso, Composición y Método Relacionados. P201730685. Spain patent application (2017).
Cominelli, E., Galbiati, M. & Tonelli, C. Transcription factors controlling stomatal movements and drought tolerance. Transcription. 1, 41–45. https://doi.org/10.4161/trns.1.1.12064 (2010).
Takahisa, O. et al. Enhancement of photosynthetic capacity in Euglena gracilis by expression of cyanobacterial fructose-1,6-/sedoheptulose-1,7-bisphosphatase leads to increases in biomass and wax ester production. Biotechnol. Biofuels. 8, 80 (2015).
Ferguson, B. J. et al. Molecular analysis of legume nodule development and autoregulation. J. Integr. Plant Biol. 52(1), 61–76 (2010).
Caba, J. M., Centeno, M. L., Fernández, B., Gresshof, P. M. & Ligero, F. Inoculation and nitrate alter phytohormone levels in soybean roots: differences between a super nodulating mutant and the wild type. Planta 211(1), 98–104 (2000).
Gresshof, P. M. Plant Function in Nodulation and Nitrogen Fixation in Legumes in New Horizons in Nitrogen Fixation. Current Plant Science and Biotechnology in Agriculture (eds. Palacios R., Mora J., Newton W. E.) 31–42 (Springer, Dordrecht, 1993).
Jackson, S. D. Multiple signaling pathways control tuber induction in potato. Plant Physiol. 119, 1–8 (1999).
Xinxi, J. Wood charcoal and pyroligneous liquor technology. Focus on form: Retrieved 2009, from http://www.cn/dz/en/charcoal-tech.htm (2004).
Erhart, E. & Hartl, W. Mulching with compost improves growth of blue spruce in Christmas tree plantations. Eur. J. Soil Biol. 39(3), 149–156. https://doi.org/10.1016/S1164-5563(03)00030-X (2003).
Hussain, I., Khan, M. A. & Khan, E. A. Bread wheat varieties as influenced by different nitrogen levels. J. Zhejiang Univ. Sci. B 7(1), 70–78. https://doi.org/10.1631/jzus.2006.B0070 (2006).
Bustos, D. V., Riegel, R. & Calderini, D. F. Anthocyanin content of grains in purple wheat is affected by grain position, assimilate availability and agronomic management. J. Cereal Sci. 55, 257–264. https://doi.org/10.1016/j.jcs.2011.12.001 (2012).
Cambardella, C. A., Richard, T. & Russell, A. Compost mineralization in soil as a function of composting process conditions. Eur. J. Soil Biol. 39, 117–127. https://doi.org/10.1016/s1164-5563(03)00027-x (2003).
Benito, M., Masaguer, A., De Antonio, R. & Moliner, A. Use of pruning waste compost as a component in soilless growing media. Bioresour. Technol. 96, 597–603. https://doi.org/10.1016/j.biortech.2004.06.006 (2005).
Thiessen, S., Gleixner, G., Wutzler, T. & Reichstein, M. Both priming and temperature sensitivity of soil organic matter decomposition depend on microbial biomass-an incubation study. Soil Biol. Biochem. 57, 739–748. https://doi.org/10.1016/j.soilbio.2012.10.029 (2013).
Hobbie, S. E. Plant species effects on nutrient cycling: revisiting litter feedbacks. Trends Ecol. Evol. 30, 357–363 (2015).
Sánchez-Reinoso, A. D., Pedraza, E. A. A. & Restrepo-Diaz, H. USE OF BIOCHAR IN AGRICULTURE. Acta Biologica Colombiana. 25(2), 327–338 (2019).
Biedermann, L. A. & Harpole, W. S. Biochar and its effects on plant productivity and nutrient cycling: A meta-analysis. GCB Bioenergy. 5, 202–214. https://doi.org/10.1111/gcbb.12037 (2013).
Dietrich, C. C., Arifur Rahaman, M. D. A., Robles-Aguilar, A., Latif, L. & Intani, K. Nutrient loaded biochar doubled biomass production in Juvenile Maize Plants (Zea mays L.). Agron. 10, 567 (2020).
Hafez, Y. et al. Beneficial effects of biochar and chitosan on antioxidative capacity, osmolytes accumulation, and anatomical characters of water-stressed barley plants. Agron. 10(5), 630. https://doi.org/10.3390/agronomy10050630 (2020).
Laird, D., Fleming, P., Wang, B., Horton, R. & Karlen, D. Biochar impact on nutrient leaching from a Midwestern agricultural soil. Geoderma 158, 436–442. https://doi.org/10.1016/j.geoderma.2010.05.012 (2010).
Liang, B. et al. Black carbon increases cation exchange capacity in soils. Soil Sci. Soc. Am. J. 70, 1719–1730. https://doi.org/10.2136/sssaj2005.0383 (2006).
Major, J. A., Rondon, M., Molina, D., Riha, S. J. & Lehmann, J. Nutrient leaching in a Columbian savanna oxisol amended with biochar. J. Environ. Qual. 41, 1076–1086. https://doi.org/10.2134/jeq2011.0128 (2011).
Zornoza, R. et al. Assessing the effects of air-drying and rewetting pre-treatment on soil microbial biomass, basal respiration, metabolic quotient and soluble carbon under Mediterranean conditions. Eur. J. Soil Biol. 43, 120–129 (2007).
Wild, B., Li, J., Pihlblad, J., Bengtson, P. & Rütting, T. Decoupling of priming and microbial N mining during a short-term soil incubation. Soil Biol. Biochem. 129, 71–79. https://doi.org/10.1016/j.soilbio.2018.11.014 (2019).
Zhao, Z. et al. Carbon and nitrogen availability in paddy soil affects rice photosynthate allocation, microbial community composition, and priming: combining continuous 13 C labeling with PLFA analysis. Plant Soil. 445, 137–152. https://doi.org/10.1007/s11104-018-3873-5 (2019).
Thies, J. E., Rillig, M. C. & Graber, E. R. Biochar effects on the abundance, activity and diversity of the soil biota. Edited by Johannes Lehmann, Stephen Joseph. Sci., Technol. Implement. 13, 327–389 (2015).
Lehmann, J. et al. Biochar effects on soil biota. Soil Biol. Biochem. 43, 1812–1836. https://doi.org/10.1016/j.soilbio.2011.04.022 (2011).
Dempster, N., Gleeson, B., Solaiman, M., Jones, L. & Murphy, V. Decreased soil microbial biomass and nitrogen mineralization with eucalyptus biochar addition to a coarse textured soil. Plant Soil. 354, 311–324. https://doi.org/10.1007/s11104-011-1067-5 (2012).
Author information
Authors and Affiliations
Contributions
M.S. performed experiments. M.S. wrote the first draft of the manuscript. H.G. supervised the work. M.S, H.G., and C.A. improved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Saberi, M., Ghomi, H. & Andreasen, C. Eco-friendly approach to improve traits of winter wheat by combining cold plasma treatments and carbonization of subtropical biomass waste. Sci Rep 12, 11218 (2022). https://doi.org/10.1038/s41598-022-15286-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-15286-4
This article is cited by
-
Automated resolution of the spiral torsion spring inverse design problem
Scientific Reports (2024)
-
Cold Plasma and Foliar-Applied Selenium Nanoparticles Modulated Cadmium Toxicity Through Changes in Physio-biochemical Properties and Essential Oil Profile of Sage (Salvia officinalis L.)
Journal of Soil Science and Plant Nutrition (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.