Abstract
Retinal drusen are deposits of inflammatory proteins that are found in macular degeneration and glomerulonephritis and result, in part, from complement activation. This was a cross-sectional observational study of individuals with inflammatory bowel disease (IBD) recruited from a Gastroenterology clinic who underwent non-mydriatic retinal photography. Deidentified images were examined for drusen, and drusen counts and size were compared with matched controls, and examined for clinical associations. The cohort with IBD comprised 19 individuals with ulcerative colitis, 41 with Crohn’s disease and three with indeterminate colitis, including 34 males (54%) and an overall median age of 48 (IQR 23) years. Their median IBD duration was 7 (IQR 10) years, median CRP level was 7 (IQR 14) mg/L, and 28 (44%) had complications (fistula, stricture, bowel resection etc.), while 28 with Crohn’s disease (68%) had colonic involvement. Drusen counts were higher in IBD than controls (12 ± 34, 3 ± 8 respectively, p = 0.04). Counts ≥ 10 were also more common (14, 22%, and 4, 6%, p = 0.02, OR 4.21, 95%CI 1.30 to 13.63), and associated with longer disease duration (p = 0.01, OR 1.06, 95%CI 1.00 to 1.13), an increased likelihood of complications (p = 0.003, OR 6.90, 95%CI 1.69 to 28.15) and higher CRP levels at recruitment (p = 0.008, OR1.02, 95%CI 1.00 to 1.05). Increased retinal drusen were found in all four individuals with Crohn’s disease and IgA glomerulonephritis. IBD and drusen may share pathogenetic mechanisms and underlying risk factors such as complement activation.
Similar content being viewed by others
Introduction
Inflammatory bowel disease (IBD) appears to result from an inappropriate inflammatory response to intestinal microbes in a genetically-susceptible host1. It affects at least 1 in 300 individuals2, and includes both ulcerative colitis and Crohn’s disease. Ulcerative colitis and Crohn’s disease share some clinical and pathological features but also have important differences1. Thus, ulcerative colitis affects mainly the large bowel mucosa and Crohn’s disease includes transmural discontinuous lesions in the terminal ileum; Crohn’s disease is more likely to have complications, such as strictures, fistulae, and perianal disease; both have extra-intestinal manifestations including arthritis, skin features and ocular abnormalities (episcleritis, retinal vasculitis, vasoocclusive disease, choroidal infiltrates, optic neuritis3; and Crohn’s disease is more likely to require immunosuppression and biologicals, as well as maintenance therapy to prevent relapses.
Genetic and environmental factors are important in the pathogenesis of both ulcerative colitis and Crohn’s disease. The intestinal microbiota, smoking status1 and diet all contribute. Genome-wide association studies (GWAS) have, to date, implicated more than 200 genes or risk alleles4 affecting proteins often in the innate (NOD2, TLR4, CARD9, IL23R, STAT3) and adaptive (HLA-C, TNFSF15, IRF5, PTPN22)5 arms of intestinal immunity6,7. Thirty % of genetic variants are common to both ulcerative colitis and Crohn’s disease, and some are shared with other immune- mediated diseases such as type I diabetes and rheumatoid arthritis8. Most of these variants are rare with a small effect size (OR < 1.3).
In the bowel, specialised epithelial cells and the lymphoid tissue are critical in the balance between tolerance of the microbiota and the recognition of pathogens and preventing pathogen invasion. Complement contributes to intestinal immunity through opsonisation of pathogens, the recruitment of inflammatory cells, lysis of bacterial cells by the membrane attack complex, promoting cell death and clearing apoptotic and necrotic cells9.
Aberrant complement activity contributes to IBD pathogenesis10,11,12. The normal intestinal epithelium when exposed to bacterial wall lipopolysaccharide produces more C313, which prevents bacterial overgrowth and invasion. Mice deficient in complement proteins do not respond appropriately to intestinal infections14. In IBD, the mucous layer overlying the epithelium is defective allowing persistent contact with bacteria and activation of the inflammatory defences, including complement15. Components of the alternative complement pathway, in particular, are upregulated in IBD. GWAS have confirmed the role of complement in IBD pathogenesis16.
Drusen are retinal deposits that are characteristic of age-related macular degeneration, and are often found where there is alternative complement pathway activation17. They comprise cell debris, immune material including complement, and extracellular matrix components18.
Drusen also occur in some forms of glomerulonephritis that have an autoimmune or genetic basis such as systemic lupus erythematosus (SLE), IgA glomerulonephritis, C3 glomerulopathy and atypical haemolytic syndrome19,20,21, especially where the alternative complement pathway is affected. IgA glomerulonephritis sometimes complicates IBD too22.
Drusen develop from the accumulation of cell debris including cell wall lipid from the highly metabolically-active photoreceptor cells23. Complement is activated when the drusen lipid is altered by oxidative stress with increasing age, smoking, poor diet or prolonged ultraviolet light exposure.
There is also a strong genetic predisposition to drusen formation especially with variants in the genes encoding alternate complement pathway components. More than half the susceptibility24 in European populations is from variants in the genes for CFH17 and ARMS2/HTRA125. CFH is the major inhibitory regulator of the alternative complement pathway, that normally reduces the inflammation from oxidation-modified lipids. The common CFH variants lessen complement inactivation, and increase membrane attack complex activity and cell damage26. ARMS2/HTRA1 has serine protease activity that weakens the extracellular matrix27, allowing retinal deposits to increase in size and promoting local inflammation. It is also directly linked to the complement system and the opsonisation of apoptotic and necrotic cells28.
This study examined individuals with IBD for retinal drusen and whether the presence of drusen correlated with any clinical features.
Methods
Study design
This was a single centre, cross-sectional observational study of consecutive individuals with IBD recruited from the Gastroenterology clinic of a metropolitan teaching hospital over a 6-month period. Recruitment, data capture, and retinal photography were coordinated in a single episode. Deidentified retinal images were graded for drusen and correlated with clinical features. Results were compared with those from age- and gender- matched hospital controls.
The primary outcome was to determine if drusen were more common in individuals with IBD, and the secondary outcomes to identify if drusen were associated with disease extent, complications or duration. There were no changes to the study design after its commencement and no interim analyses.
This project was approved by the Northern Health Human Research Ethics Committee and written, informed consent was obtained from participants according to the principles of the Declaration of Helsinki. All procedures were performed in accordance with the relevant guidelines and regulations.
Participants
Consecutive individuals attending a routine clinic review for management of their IBD were invited to participate. They had been diagnosed previously with ulcerative colitis or Crohn’s disease by a specialist gastroenterologist using conventional clinical, radiological, colonoscopic and histopathological criteria29. All had been treated according to current international guidelines30 and immunosuppression treatment (azathioprine, methotrexate) and the use of biologicals were noted. Disease activity (active or inactive disease) was assessed by the treating clinician and recorded from the clinic charts.
Control participants were age- and gender- matched hospital patients without systemic inflammatory disease who were recruited contemporaneously from general medical and preoperative surgical clinics.
Exclusion criteria were where retinal images were ungradable bilaterally.
Measurements
Clinical features
Participants were assisted to complete a structured questionnaire for demographic details (age, gender), clinical features (diagnosis, large bowel involvement in Crohn’s disease, disease duration), and complications (fistula, stricture, perianal disease, small bowel obstruction, bowel resection, carcinoma, extraintestinal manifestations). Data were confirmed from the participants’ electronic medical records. Laboratory data (C-reactive protein (CRP), serum albumin, estimated glomerular filtration rate (eGFR)) were obtained at recruitment. Drusen risk factors (age, hypertension, smoking history, diabetes) were noted.
Retinal imaging and grading
Participants underwent digital retinal imaging centred on the macula and disc of both optic fundi using a non-mydriatic retinal camera (CR5-45, Japan). Deidentified images were examined by two trained graders and a physician independently, using a grid overlay corresponding to the Wisconsin Age-Related Maculopathy Grading System31,32. Drusen were counted in the central zones of the inner ring, and in the inferior, superior, temporal and nasal zones of the intermediate and external rings of the grid. Peripheral drusen at least two disc diameters from the foveola were also counted. Numbers from the eye with the greater count were recorded. Drusen ≥ 10 were considered abnormal31,33. Counts were highly reproducible, with an intra-observer interassay coefficient of variation of 18%.
Drusen were also assessed as small (< 63 µm), medium (63–125 µm) or large (> 125 µm) by comparison with the width of the largest venule where it crossed the ipsilateral disc margin32. All images were examined by an ophthalmologist who excluded other causes of the retinal appearance.
Statistical analyses
This was a pilot study to determine if drusen were more common in IBD than controls and to ascertain all clinical associations so a power calculation was not possible and multiple analyses were undertaken without correction.
Continuous variables were described as mean and standard deviation (for normally distributed data) or median and interquartile range (IQR, where non-normally distributed). Categorical variables were compared using Fisher’s exact test and the one-way ANOVA test. Continuous variables were compared with the student’s t-test or the Mann–Whitney test (non-normally distributed). The relationship of drusen with more extensive disease, complications or disease duration were then calculated using Fisher’s exact test.
Drusen associations were examined with the calculation of odds ratios and 95% confidence intervals with univariate logistic regression analyses. Analyses were performed using the Statistics Package for the Social Sciences (IBM, US). A p-value of less than 0.05 was considered significant.
Results
Demographic and clinical features
Sixty-three individuals with IBD were examined, including 19 (30%) with ulcerative colitis, 41 (65%) with Crohn’s disease, and 3 (5%) with indeterminate colitis (Table 1). Five others (7%) had been excluded because of bilaterally ungradable retinal photographs.
The final cohort comprised 34 men (54%) and 29 women (46%) with a median age of 48 years (IQR 37–60). They were of Northern European (45, 71%), Southern European (16, 25%) or Asian (2, 3%) ancestry.
Eleven (17%) had treated hypertension, 8 (13%) were current or former smokers, and 36 (57%) had diabetes. They had a median IBD duration of 7 years (IQR 10), 28 (44%) had complications, and 28 with Crohn’s disease (68%) had colonic involvement. Their median CRP was 7 (IQR 14) mg/L and their median serum albumin was 37 (IQR 6) g/L with a median eGFR of 90 (IQR 15) ml/min/1.73m2.
Participants with IBD were not different from controls in age, sex, ethnicity or comorbidities except that they were less likely to have hypertension (p = 0.02, OR 0.33, 95%CI 0.15 to 0.79) (Table 1).
Ophthalmic examinations
None of the participants with IBD had any of the characteristic retinal features. Coincidental abnormalities included a choroidal naevus, macular scar, thrombosed vessel, and glaucomatous optic disc. Retinal haemorrhages were present in 5 individuals with IBD (8%) and 5 controls (8%), and exudates were found in 7 with IBD (11%) and 8 controls (12%).
Drusen
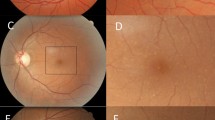
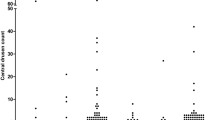
Participants with IBD had a mean drusen count of 12 ± 34 compared with 3 ± 8 in hospital controls (p = 0.04) (Table 2). Abnormal counts ≥ 10 were more common in IBD than controls (p = 0.02, OR = 4.21 (1.30 to 13.63). Drusen in IBD were more widely distributed than in controls (p = 0.01, OR 3.31, 95%CI 1.12 to 9.85) but were not larger. Drusen were mainly small, soft, and well-circumscribed (Fig. 1). Optical coherence tomography (OCT) demonstrated that they were located beneath the retinal pigment epithelium in the same site as the drusen found in SLE, IgA glomerulonephritis and dense deposit disease (Fig. 1)19,20,21.
(A) Right optic fundus from an individual with Crohn’s disease demonstrating clusters of small central drusen; (B) enlarged view of box in A demonstrating drusen more clearly (arrow); (C). right optic fundus from an individual with ulcerative colitis demonstrating drusen (arrow); (D). autofluorescence view of the fundus in (C). demonstrating hyperfluorescent drusen (arrow), hypofluorescent drusen (arrow) and areas of hypofluorescence (asterisks); and (E). focal drusen in (C) and (D) resulting in irregularity of outer retinal layers on optical coherence tomography (OCT, Heidelberg Engineering) (arrow).
Clinical associations
Participants with ulcerative colitis had a mean drusen count of 4 ± 6 (n = 19) compared with Crohn’s disease where the mean count was 16 ± 42 (n = 41) (p = 0.22). However counts ≥ 10, distribution and size were not different in ulcerative colitis and Crohn’s disease.
Participants with IBD and disease duration ≥ 10 years had a mean drusen count of 25 ± 53 (n = 24) and those with a duration < 10 years had a mean count of 2 ± 4 (n = 35) (p = 0.04).
Participants with IBD and complications had a mean drusen count of 22 ± 49 (n = 28) compared with 2 ± 3 in those with uncomplicated disease (n = 31) (p = 0.02).
Participants with Crohn’s disease and large bowel involvement had a mean drusen count of 22 ± 50 (n = 28) compared with 2 ± 4 in those with disease limited to the small bowel (n = 13) (p = 0.16).
The highest drusen counts (124, 122 and 108) were found in the periphery (Fig. 1). These occurred in three individuals with Crohn’s disease aged 39, 56 or 79 years, and all with disease duration of at least 10 years. All three had colonic involvement, and one had complications with a small bowel obstruction. None had any features of extraarticular Crohn’s disease.
Clinical associations of drusen counts ≥ 10
Fourteen individuals with IBD (22%) and 4 controls (6%) had drusen ≥ 10 (p = 0.02, OR 4.21, 95% CI 1.30 to 13.63). The participants with IBD included 3 with ulcerative colitis, 10 with Crohn’s disease and one with indeterminate colitis.
Drusen counts ≥ 10 were not more likely with Crohn’s disease than ulcerative colitis (p = 0.35, OR 0.55, 95%CI 0.05 to 6.60) (Table 3).
Participants with IBD and drusen ≥ 10 were older (p = 0.04, OR1.02, 95%CI 0.98 to 1.07) and less likely to be current or former smokers than those with < 10 (p = 0.01, OR 0.21, 95%CI 0.06 to 0.78) (Table 3). Drusen counts ≥ 10 were not associated with gender, ancestry, or the diagnosis of hypertension or diabetes (p all NS).
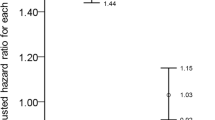
However drusen counts ≥ 10 were more common in individuals with IBD of longer disease duration (p = 0.01, OR 1.06, 95%CI 1.00 to 1.013), increased complications (p = 0.003, OR 6.90, 95%CI 1.69 to 28.15) or with higher CRP levels at recruitment (p = 0.008, OR1.02, 95%CI 1.00 to 1.05). Drusen counts ≥ 10 were not more common in patients assessed to have active disease clinically (p = 0.68, OR 0.60, 95% CI 0.13 to 2.74) nor were they affected by current immunosuppressive treatment (p = 1.00, OR 1.22, 95%CI 0.36 to 4.17). They were also not associated with extraintestinal manifestations (p = 0.11, OR2.85, 95%CI 0.75 to 10.77), reduced serum albumin (p = 0.36, OR 0.93, 95%CI 0.77 to 1.13) or lower eGFR levels (p = 0.47, OR 1.00, 95%CI 0.96 to 1.06).
Association with IgA glomerulonephritis
In addition, four individuals with Crohn’s disease had renal-biopsy-proven IgA glomerulonephritis. All had increased drusen counts of 22, 40, 49 or 222. These drusen occurred in two men and two women, aged 39, 45, 56 or 79 years, with disease of at least 10 years’ disease.
Discussion
Retinal drusen are more common in IBD than other hospital patients. Abnormal drusen counts were found in about 20% of individuals with IBD, and were associated with longer disease duration, more frequent complications and higher CRP levels at a clinic visit. These associations suggest that drusen were more abundant with more prolonged and possibly more active disease. However abnormal drusen counts did not correlate with clinical assessments of disease activity nor were counts lower with immunosuppression. Drusen were also increased in all individuals with IgA glomerulonephritis complicating Crohn’s disease.
The association of drusen with IBD suggests shared risk factors and pathogenetic mechanisms. Drusen in macular degeneration have both genetic and environmental risk factors. The major environmental risks are age, smoking, hypertension, ancestry, diabetes and renal impairment34. Most participants with IBD in this study were too young for the drusen to be age-related, and drusen were not associated with smoking despite it also being a risk factor for Crohn’s disease35. In addition, drusen were not associated with hypertension, ancestry, diabetes or renal impairment.
Genetic risk factors for drusen in macular degeneration affect pathways involved in complement activation, fatty acid oxidation, membrane integrity and apoptosis 23,36. Some of the genetic risk factors for macular degeneration are also risk factors for IBD such as alleles in the complement pathway genes, and sometimes, while there is no association with a risk allele, the corresponding protein has been implicated (VEGFA, ABCA1)37,38. The main difference in genetic risk factors is the lack of immunological processes in macular degeneration.
The major risk allele for drusen in macular degeneration affects the alternative complement pathway regulator, CFH. Complement activation and genetic risk alleles in complement pathway genes occur in both drusen and IBD development23,36. Drusen incorporate photoreceptor cell membrane lipid that is oxidised by smoking and sunlight, and activates complement, inducing inflammation and apoptosis. In IBD, exposure of microbial cell wall lipid to normal intestinal epithelium increases C3 production13. The defective intestinal mucosa in IBD allows ongoing bacterial contact and further activation of the alternative complement pathway15,16. CRP also binds bowel wall bacteria and triggers classical pathway complement activation39.
Complement involvement is different in Crohn’s disease and ulcerative colitis, and increased complement activation in Crohn’s disease may explain the greater drusen number. In Crohn’s disease the intestinal mucosa produces more C3 and C410, and crypt abscess formation induces further C3 expression13,14,40. In addition, more extensive Crohn’s disease may be associated with increased complement activation since colonic epithelial cells express more C3 than ileal cells13 and the major C3 stimulator, Toll-like receptor 4 (TLR-4) is up-regulated in the colon in mouse models of IBD13.
Drusen were present in all individuals with Crohn’s disease and biopsy proven IgA glomerulonephritis in this study. IgA glomerulonephritis has been reported previously in both ulcerative colitis and Crohn’s disease41,42 and was excluded in other study participants based on their normal urinary sediments. Drusen have been described previously in only occasional cases of IgA glomerulonephritis21,43 but drusen and glomerular immune deposits in other forms of glomerulonephritis share their composition of complement, immunoglobulins, glycoproteins, and extracellular matrix44,45. Complement activation and complement risk alleles are important in both the pathogenesis of macular degeneration and Crohn’s disease, but also IgA glomerulonephritis.
Overall the drusen found in IBD appeared to be smaller than those found in macular degeneration and dense deposit disease but of similar size to those in SLE20 and IgA glomerulonephritis21. Drusen were located mainly in the central retina, in the same location as for IgA glomerulonephritis, SLE and dense deposit disease 19,20,21.
Drusen were not present in all individuals with IBD or Crohn’s disease who were examined. Retinal imaging is relatively insensitive for drusen detection20 and smaller drusen may be even more abundant than found here. This means that drusen large enough to be visualised on retinal imaging are associated with longer disease duration, more complicated disease and higher CRP levels at a clinic visit. There was no correlation with clinically-assessed active disease nor fewer drusen with immunosuppression. Importantly, the drusen in IBD, unlike those in macular degeneration, do not affect vision.
The drusen in IBD are unlikely to be coincidental because counts were higher than in other hospital patients, and drusen were present in patients who also had IgA glomerulonephritis. Drusen or a drusen-like appearance secondary to other retinal disease was excluded by independent examination of all images by an ophthalmologist.
The strengths of this study were the clinical characterisation of study participants, and the standardisation and reproducibility of drusen counts. The study’s major limitations were the relatively small cohort and the lack of a more objective assessment of disease activity such as a Disease Activity or Harvey-Bradshaw index. Further studies are required to confirm the clinical associations of drusen in IBD, and the association of drusen with Crohn’s disease, especially with colonic involvement.
Retinal drusen are more common in IBD, and associated with longer disease duration, more complicated disease and higher CRP levels at a clinic visit. The identification of drusen in IBD suggest that complement activation is important in IBD pathogenesis. Treatments used for drusen including those targeting the complement pathway in macular degeneration may be relevant in IBD. The finding of drusen in both IBD and IgA glomerulonephritis further suggests a role for complement activation in these conditions.
Data availability
The datasets generated and/or analysed during the current study are not publicly available because the authors hope to undertake further analysis, but are available from the corresponding author on reasonable request.
References
Abraham, C. & Cho, J. H. Inflammatory bowel disease. N. Engl. J Med. 361(21), 2066–2078 (2009).
Ray, K. IBD: The changing epidemiology of IBD. Nat. Rev. Gastroenterol. Hepatol. 14(12), 690 (2017).
Calvo, P. & Pablo, L. Managing IBD outside the gut: Ocular manifestations. Dig. Dis. 31(2), 229–232 (2013).
Jostins, L. et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 491(7422), 119–124 (2012).
Van Limbergen, J., Wilson, D. C. & Satsangi, J. The genetics of Crohn’s disease. Annu. Rev. Genomics Hum. Genet. 10, 89–116 (2009).
Davies, J. M. & Abreu, M. T. The innate immune system and inflammatory bowel disease. Scand. J. Gastroenterol. 50(1), 24–33 (2015).
Mayer, L. Evolving paradigms in the pathogenesis of IBD. J. Gastroenterol. 45(1), 9–16 (2010).
Zhernakova, A., van Diemen, C. C. & Wijmenga, C. Detecting shared pathogenesis from the shared genetics of immune-related diseases. Nat. Rev. Genet. 10(1), 43–55 (2009).
Jain, U., Otley, A. R., Van Limbergen, J. & Stadnyk, A. W. The complement system in inflammatory bowel disease. Inflamm. Bowel Dis. 20(9), 1628–1637 (2014).
Ahrenstedt, O. et al. Enhanced local production of complement components in the small intestines of patients with Crohn’s disease. N. Engl. J. Med. 322(19), 1345–1349 (1990).
Hodgson, H. J., Potter, B. J. & Jewell, D. P. C3 metabolism in ulcerative colitis and Crohn’s disease. Clin. Exp. Immunol. 28(3), 490–495 (1977).
Feinstein, P. A., Kaplan, S. R. & Thayer, W. R. Jr. The alternate complement pathway in inflammatory bowel disease Quantitation of the C3 proactivator (factor B) protein. Gastroenterology 70(2), 181–185 (1976).
Sunderhauf, A. et al. Regulation of epithelial cell expressed C3 in the intestine—Relevance for the pathophysiology of inflammatory bowel disease?. Mol. Immunol. 90, 227–238 (2017).
Elvington, M., Schepp-Berglind, J. & Tomlinson, S. Regulation of the alternative pathway of complement modulates injury and immunity in a chronic model of dextran sulphate sodium-induced colitis. Clin. Exp. Immunol. 179(3), 500–508 (2015).
Johansson, M. E. et al. Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and patients with ulcerative colitis. Gut 63(2), 281–291 (2014).
Ostvik, A. E. et al. Mucosal toll-like receptor 3-dependent synthesis of complement factor B and systemic complement activation in inflammatory bowel disease. Inflamm. Bowel Dis. 20(6), 995–1003 (2014).
Hageman, G. S. et al. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc. Natl. Acad. Sci. USA. 102(20), 227–7232 (2005).
Anderson, D. H., Mullins, R. F., Hageman, G. S. & Johnson, L. V. A role for local inflammation in the formation of drusen in the aging eye. Am. J. Ophthalmol. 134(3), 411–431 (2002).
Savige, J. et al. Retinal disease in the C3 glomerulopathies and the risk of impaired vision. Ophthal. Genet. 37(4), 369–376 (2016).
Invernizzi, A. et al. Drusen-like deposits in young adults diagnosed with systemic lupus erythematosus. Am. J. Ophthalmol. 175, 68–76 (2017).
Lally, D. R. & Baumal, C. Subretinal drusenoid deposits associated with complement-mediated IgA nephropathy. JAMA Ophthalmol. 132(6), 775–777 (2014).
Trimarchi, H. M., Iotti, A., Iotti, R., Freixas, E. A. & Peters, R. Immunoglobulin A nephropathy and ulcerative colitis. A focus on their pathogenesis. Am J Nephrol. 21(5), 400–405 (2001).
Fritsche, L. G. et al. Age-related macular degeneration: Genetics and biology coming together. Annu. Rev. Genomics Hum. Genet. 15, 151–171 (2014).
Weger, M. et al. Association of the HTRA1 -625G>A promoter gene polymorphism with exudative age-related macular degeneration in a Central European population. Mol. Vis. 13, 1274–1279 (2007).
Fritsche, L. G. et al. Age-related macular degeneration is associated with an unstable ARMS2 (LOC387715) mRNA. Nat. Genet. 40(7), 892–896 (2008).
Shaw, P. X. et al. Complement factor H genotypes impact risk of age-related macular degeneration by interaction with oxidized phospholipids. Proc. Natl. Acad. Sci. USA. 109(34), 13757–13762 (2012).
Vierkotten, S., Muether, P. S. & Fauser, S. Overexpression of HTRA1 leads to ultrastructural changes in the elastic layer of Bruch’s membrane via cleavage of extracellular matrix components. PLoS ONE 6(8), e22959 (2011).
Micklisch, S. et al. Age-related macular degeneration associated polymorphism rs10490924 in ARMS2 results in deficiency of a complement activator. J. Neuroinflammat. 14(1), 4 (2017).
Silverberg, M. S. et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 19(Suppl 3), 5A-36A (2005).
Lamb, C. A. et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 68(Suppl 3), s1–s106 (2019).
Klein, R. et al. The Wisconsin age-related maculopathy grading system. Ophthalmology 98(7), 1128–1134 (1991).
Ferris FL, Davis MD, Clemons TE, Lee LY, Chew EY, Lindblad AS, et al. A simplified severity scale for age-related macular degeneration: AREDS Report No. 18. Arch Ophthalmol. 2005;123(11):1570–4.
Sarks, S. H., Arnold, J. J., Killingsworth, M. C. & Sarks, J. P. Early drusen formation in the normal and aging eye and their relation to age related maculopathy: a clinicopathological study. Br J Ophthalmol. 83(3), 358–368 (1999).
Smith, W. et al. Risk factors for age-related macular degeneration: Pooled findings from three continents. Ophthalmology 108(4), 697–704 (2001).
Cottone, M. et al. Smoking habits and recurrence in Crohn’s disease. Gastroenterology 106(3), 643–648 (1994).
Fritsche, L. G. et al. A large genome-wide association study of age-related macular degeneration highlights contributions of rare and common variants. Nat. Genet. 48(2), 134–143 (2016).
Scaldaferri, F., Vetrano, S., Sans, M., Arena, V., Straface, G., Stigliano, E. et al. VEGF-A links angiogenesis and inflammation in inflammatory bowel disease pathogenesis. Gastroenterology, 136(2):585–95 e5 (2009).
Verma, N., Ahuja, V. & Paul, J. Profiling of ABC transporters during active ulcerative colitis and in vitro effect of inflammatory modulators. Dig. Dis. Sci. 58(8), 2282–2292 (2013).
Mold, C., Gewurz, H. & Du Clos, T. W. Regulation of complement activation by C-reactive protein. Immunopharmacology 42(1–3), 23–30 (1999).
Laufer, J. et al. Cellular localization of complement C3 and C4 transcripts in intestinal specimens from patients with Crohn’s disease. Clin. Exp. Immunol. 120(1), 30–37 (2000).
Hirsch, D. J., Jindal, K. K., Trillo, A. & Cohen, A. D. Acute renal failure in Crohn’s disease due to IgA nephropathy. Am. J. Kidney Dis. 20(2), 189–190 (1992).
Joher, N., Gosset, C., Guerrot, D., Pillebout, E., Hummel, A., Boffa, J.J., et al. IgA nephropathy in association with inflammatory bowel diseases: results from a national study and systematic literature review. Nephrol. Dial. Transplant. (2021).
Cetin, N., Basmak, H., Gencler, A. & Acikalin, M. F. Perimacular drusenoid deposits in a child with IgA nephropathy. Can. J. Ophthalmol. 53(2), e71–e74 (2018).
Mullins, R. F., Aptsiauri, N. & Hageman, G. S. Structure and composition of drusen associated with glomerulonephritis: implications for the role of complement activation in drusen biogenesis. Eye (Lond). 15(Pt 3), 390–395 (2001).
Mullins, R. F., Russell, S. R., Anderson, D. H. & Hageman, G. S. Drusen associated with aging and age-related macular degeneration contain proteins common to extracellular deposits associated with atherosclerosis, elastosis, amyloidosis, and dense deposit disease. FASEB J. 14(7), 835–846 (2000).
Funding
There was no external funding for this project. EN, YH, DN and KA were medical students when they undertook this as a research project towards their degrees. PH is a research higher degree student.
Author information
Authors and Affiliations
Contributions
D.N., S.G. were responsible for recruiting study subjects; E.N., Y.H., D.N., K.A., P.H. were responsible for grading retinal images, and statistical analysis; H.M. and D.C. were responsible for confirming retinal grading; E.N. and Y.H. prepared first draft of manuscript; J.S. supervised the project, and was responsible for final version of the manuscript. The final version was approved by all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nicklason, E., Ham, Y., Ng, D. et al. Retinal drusen counts are increased in inflammatory bowel disease, and with longer disease duration, more complications and associated IgA glomerulonephritis. Sci Rep 12, 11744 (2022). https://doi.org/10.1038/s41598-022-15232-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-15232-4
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.