Abstract
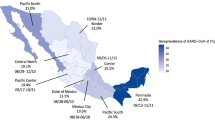
A national point seroprevalence study of SARS-CoV-2 was conducted in Sweden in April–May 2021. In total, 2860 individuals 3 to 90 years old from a probability-based web panel were included. Results showed that an estimated 32.6% of the population in Sweden had detectable levels of antibodies, and among non-vaccinated 20.1% had detectable levels of antibodies. We tested for differences in seroprevalence between age groups and by sex and estimated seroprevalence among previously infected participants by time since reporting.
Similar content being viewed by others
Introduction
At the beginning of April 2021, Sweden experienced a third wave of COVID-19, SARS-CoV-2 lineage B.1.1.7 (Alpha) variant dominating1. Vaccination against COVID-19 started in December 2020 with the age group 70 years and older being one of the prioritized groups, extending to include the 65 years and older in beginning of March 2021. By April 2021, 23% of individuals over 65 years and 8% of the Swedish population as a whole were fully vaccinated by either of Comirnaty, Spikevax or Vaxzevria vaccines2. In this report, we present the results of a point seroprevalence study of SARS-CoV-2 in April/May 2021 that was conducted in order to estimate seroprevalence at a national level at this time point.
Study participants and serological analyses
Participants were recruited from a national probability-based web panel regularly used for health-related questionnaires at the Public Health Agency of Sweden3. Among the 4477 registered individuals in the panel, informed consent for participation in the study was received from 3283 individuals. Participation by minors less than 16 years of age was by informed consent of the respective caretaker and minors 16–17 years of age by informed consent by both themselves and respective caretaker. At-home sampling on dried blood spot (DBS) cassettes was used to collect capillary blood (Capitainer®, Solna, Sweden)4. Sampling was conducted between April 26 and May 9, 2021. Capillary blood in DBS cassettes was returned to the laboratory and processed for analysis of anti-Spike IgG in an in-house SARS-CoV-2 ELISA5. Results of the serological analysis were communicated to the participants electronically and by mail. Of the 3283 at-home sampling kits sent out for the study, 423 were either not returned to the laboratory, did not have sufficient amount of capillary blood in them, or were taken outside of the sampling period (Fig. 1). Information about COVID-19 vaccination status and previous confirmed SARS-CoV-2 infection of the participants was collected from the national vaccination registry and the national mandatory notifications system of communicable diseases (SmiNet). Demographics of the participants are summarised in Table 1.
Seroprevalence in the population of Sweden
The seroprevalence was calculated as the weighted proportion and was corrected for the laboratory method with the Rogan–Gladen method6,7. The weights were based on the sampling weights which were adjusted for the survey non-response with logistic regression and calibrated with the GREG estimator to the population of Sweden with regards to age, sex, education, and geographical region8. Estimations are shown with the 95% confidence interval (CI) using the Clopper–Pearson method. Calculations were made in R v.4.0.2. with survey package v.4.0. The seroprevalence of the study population (n = 2860) can be seen in Table 2.
Differences between age groups and sex were tested by a weighted logistic regression where a p-value < 0.05 was considered significant. There was a significantly higher proportion of individuals with SARS-CoV-2 antibodies in the age group 65 years and older compared to the other age groups (p < 0.001). No statistical difference in the antibody prevalence by sex was found.
In total, 141 participants of the study population were vaccinated with two doses at least two weeks prior to sampling, and all of these had detectable levels of antibodies against SARS-CoV-2.
Seroprevalence among non-vaccinated individuals
Table 2 also shows seroprevalence among non-vaccinated individuals (n = 1876) where the highest proportion of individuals with antibodies was detected in the age group 11–19 years (30.5%; 95% CI 23.6–38.2). Seroprevalence in the other age groups ranged from 18.0 to 20.5%. The seroprevalence levels were similar between sexes.
Among non-vaccinated participants with previous laboratory-confirmed SARS-CoV-2 exposure (n = 245), the proportion that had detectable levels of antibodies varied with the time since infection (Table 3). These estimations were adjusted with the Rogan–Gladen method but unweighted7,9. The highest proportion was seen among individuals most recently infected (15–90 days before sampling) and was 97.8% (95% CI 92.1–100). In the group that had been infected over 6 months before sampling, 84.4% (95% CI 68.3–94.5) still had detectable levels of antibodies.
Ethics approval
Approval (registration number 2020-07029) was obtained from the Swedish Ethical Review Authority, Uppsala, Sweden. All experiments were performed in accordance with relevant guidelines and regulations (The Act concerning the Ethical Review of Research Involving Humans (Law 2003:460) and ISO/IEC 17025).
Consent to participate
Informed consent was obtained from all individual participants included in the study. Participation by minors was by informed consent of the respective caretaker.
Discussion
The results of this point prevalence study suggest that in total 32.6% (95% CI 30.3–34.9) of the population in Sweden had detectable levels of antibodies against SARS-CoV-2 between April 26 and May 9, 2021. This seroprevalence is between previous estimates at national level from March 2021 where 22.4% of blood-donors and 20.7% of outpatients had detectable levels of antibodies and later estimates from the end of May and beginning of June where 51.9% of blood-donors and 52.2% of outpatients had detectable levels10. A limitation of the study was that the used method could not distinguish between antibody response induced by a SARS-CoV-2 infection and that induced by vaccination. Due to the higher vaccination coverage in those 65 years and older, the proportion of this group with positive antibody response was higher (62.2%; 95% CI 58.6–65.8, p < 0.001) compared to the other age groups. Among non-vaccinated individuals, those 65+ years had similar antibody levels compared to those 20–64 years and 0–10 years, demonstrating that the higher seroprevalence in the age group 65 + years in the total study population was probably due to vaccination. However, when compared to previous seroprevalence data from Sweden, the proportion of non-vaccinated participants over 65 years with antibodies was still higher in this study10,11,12,13. It should be noted, though, that there were few participants in this group and thus there is large uncertainty in this estimate. Therefore, in this study the seroprevalence results due to previous infection in the age group 65+ should be interpreted with caution. Another limitation of the study was that it is not possible to verify that the correct individual was sampled as the participants conducted the sampling at home without supervision. However, we consider the risk of receiving sample from the wrong person to be very low as the participants are recruited from a panel that are regularly used for responding to health-related questionnaires and are known for being compliant to instructions given.
Although a correlation between protective immunity and antibody levels has not been determined, waning antibody levels in the group with previous laboratory-confirmed SARS-CoV-2 exposure was indicated over time which is in line with what has been described in other studies and could indicate waning immunity14,15,16. In the group with laboratory-confirmed SARS-CoV-2 exposure within 15–90 days before blood sampling, only 2.2% did not have detectable antibody levels as compared to 15.6% in the group with previous laboratory-confirmed SARS-CoV-2 exposure more than 181 days earlier.
Conclusions
The study showed that, in total, 32.6% of the population had detectable levels of antibodies, and among non-vaccinated, 20.1% had detectable levels of antibodies in late April and the beginning of May 2021. The antibody response among those having had COVID-19 seems to wane over time with a decreasing proportion of cases with persisting antibody levels with time passed since infection.
Data availability
The datasets generated and analysed during the current study are not publicly available due to existing general data protection rules but are available from the corresponding author on reasonable request.
References
Public Health Agency of Sweden. Bekräftade fall i Sverige–daglig uppdatering. https://www.folkhalsomyndigheten.se/smittskydd-beredskap/utbrott/aktuella-utbrott/covid-19/statistik-och-analyser/bekraftade-fall-i-sverige/ (Accessed 12 Sept 2021).
Public Health Agency of Sweden. Statistik för vaccination mot covid-19. https://www.folkhalsomyndigheten.se/smittskydd-beredskap/utbrott/aktuella-utbrott/covid-19/statistik-och-analyser/statistik-over-registrerade-vaccinationer-covid-19/ (Accesses 12 Sept 2021).
Public Health Agency of Sweden. Hälsorapport–en webbpanel https://www.folkhalsomyndigheten.se/folkhalsorapportering-statistik/om-vara-datainsamlingar/halsorapport/ (Accessed 12 Sept 2021).
Capitainer https://capitainer.se/kit/.
Rosendal, E. et al. Detection of asymptomatic SARS-CoV-2 exposed individuals by a sensitive S-based ELISA. medRxiv. https://doi.org/10.1101/2020.06.02.20120477 (2020).
Lagerqvist, N. et al. Evaluation of 11 SARS-CoV-2 antibody tests by using samples from patients with defined IgG antibody titers. Sci. Rep. 11(1), 7614 (2021).
Rogan, W. J. & Gladen, B. Estimating prevalence from the results of a screening test. Am. J. Epidemiol. 107(1), 71–76 (1978).
Särndal, C. & Lundström, S. Estimation in surveys with nonresponse 33–42 (Wiley, 2005).
Clopper, C. J. & Pearson, E. S. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika 26, 404–413 (1934).
Public Health Agency of Sweden. Var fjärde blodgivare i Stockholm och Västra Götaland har antikroppar https://www.folkhalsomyndigheten.se/nyheter-och-press/nyhetsarkiv/2021/maj/var-fjarde-blodgivare-i-stockholm-och-vastra-gotaland-har-antikroppar/.
Public Heath Agency of Sweden. Påvisning av antikroppar mot SARS-CoV-2 i blodprov från öppenvården https://www.folkhalsomyndigheten.se/publicerat-material/publikationsarkiv/p/pavisning-av-antikroppar-efter-genomgangen-covid-19-i-blodprov-fran-oppenvarden-delrapport-1/.
Public Health Agency of Sweden. Påvisning av antikroppar mot SARS-CoV-2 hos blodgivare https://www.folkhalsomyndigheten.se/publicerat-material/publikationsarkiv/p/pavisning-av-antikroppar-efter-genomgangen-covid-19-hos-blodgivare-delrapport-2/.
Public Health Agency of Sweden. Förekomsten av antikroppar mot SARS-CoV-2 bland äldre i Göteborg, 1–16 mars 2021 https://www.folkhalsomyndigheten.se/publicerat-material/publikationsarkiv/f/forekomsten-av-antikroppar-mot-sars-cov-2-bland-aldre-i-goteborg-1-16-mars-2021/.
Havervall, S. et al. Neutralization of VOCs including Delta one year post COVID-19 or vaccine. medRxiv 2021.08.12.21261951 (2021).
Kucinskaite-Kodze, I. et al. Persistence of SARS-CoV-2-specific antibodies for 13 months after infection. Viruses 13(11), 2313 (2021).
Self, W. H. et al. Decline in SARS-CoV-2 antibodies after mild infection among frontline health care personnel in a multistate hospital network—12 states, April–August 2020. MMWR Morb. Mortal. Wkly. Rep. 69(47), 1762–1766 (2020).
Acknowledgements
We are grateful to the participants for taking part in the study, including performing self-sampling. We also thank Moa Rehn and Marie Jansson Mörk for providing epidemiological data.
Funding
This work was funded by governmental grants provided to the Public Health Agency of Sweden for assignments S2020/0281/FS and S2020/08532 FS.
Author information
Authors and Affiliations
Contributions
J.B., K.T.W., and A.B. designed and planned the study. J.B., A.B., T.E., E.v.S., J.D., M.R., M.M., K.Z., J.W.B., M.F., R.G. contributed to the data collection and analysis. I.G. and S.K.B. contributed to the statistical analysis. J.B., T.E., and A.B. drafted the manuscript. All authors contributed to the interpretation of data and revised the manuscript. All authors approved the final version of the manuscript and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
JWB and MF are shareholders of Xerum AB that performed the serological analyses. All other authors declare that there is no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Beser, J., Galanis, I., Enkirch, T. et al. Seroprevalence of SARS-CoV-2 in Sweden, April 26 to May 9, 2021. Sci Rep 12, 10816 (2022). https://doi.org/10.1038/s41598-022-15183-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-15183-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.