Abstract
The aim of the study was to evaluate the prevalence, distribution and correlates of dyslipidaemia among people (15–69 years) in Mongolia. National data were analyzed from 4,895 individuals (15–69 years, median age = 35 years) that took part in the Mongolia cross-sectional STEPS survey in 2019, and had complete lipid measurements. Dyslipidaemia was defined using the guidelines of the Adult Treatment Panel III. The prevalence of dyslipidaemia was 58.6%, 31.7% high triglycerides (TG), 26.9% high low-density lipoprotein cholesterol (LDL-C), 26.9% high total cholesterol (TC) and 14.6% low high-density lipoprotein cholesterol (HDL-C). Among those with dyslipidaemia, 6.2% were aware. Among those who were aware, the proportion of lipid-lowering drug treatment was 18.9% and among those who took lipid-lowering drugs, 21.5% had their dyslipidaemia controlled. In adjusted logistic regression, older age (40–69 years) (AOR: 1.19, 95% CI 1.02–1.40), urban residence (AOR: 1.24, 95% CI 1.04–1.48), obesity call II (AOR: 2.89, 95% CI 2.29–3.66), hypertension (AOR: 1.33, 95% CI 1.11–1.59), and diabetes (AOR: 1.62, 95% CI 1.20–2.18) were positively, and male sex (AOR: 0.84, 95% CI 0.72–1.00) was negatively associated with dyslipidaemia prevalence. Six in ten Mongolians 15 years and older had dyslipidaemia. Several factors associated with dyslipidaemia that can be used to target public health interventions were identified.
Similar content being viewed by others

Introduction
Dyslipidaemia constitutes one or a combination of elevated triglycerides (TG), high low-density lipoprotein cholesterol (LDL-C), low high-density lipoprotein cholesterol (HDL-C), and elevated total cholesterol (TC)1,2. Dyslipidaemias are associated with changes in the plasma lipid profile leading to major clinical conditions, such as cardiovascular disease (CVD), and the global burden has increased significantly over the past 30 years1. CVDs contribute to 40% of mortality in 2016 in Mongolia, which is a lower resource country located in East Asia3. Ischemic heart disease (IHD) is high and increasing in Mongolia, due to risk factors, such as dyslipidaemia, obesity, diabetes, smoking, and hypertension4. “Dyslipidaemias can be genetically determined (primary or familial dyslipidaemias) or secondary to other conditions (such as diabetes mellitus, obesity, or an unhealthy lifestyle), the latter being more common1.” Based on our review, we could not find any national data on dyslipidaemia in Mongolia.
In 35 low- and middle-income countries (LMIC) (≥ 15 years), the prevalence of high TC was 7.1% and high LDL-C 7.5%, ≥ 31% of them were aware of their diagnosis, ≥ 29% were treated, and ≥ 7% were controlled5. In China (> 18 years), the prevalence of dyslipidaemia was 34.0%6, and 31.0% were aware, 19.5% in treatment and 8.9% were controlled6. In northern Ethiopia (≥ 20 years), the prevalence of dyslipidaemia was 66.7%, elevated TC 30.8%, elevated TG 40.2%, low HDL-C 16.5%, and high LDL-C 49.5%2, in Jordan (≥ 25 years) the prevalence of elevated TC was 44.3%, high TC 41.9%, and low HDL-C 59.5%7, in Turkey (≥ 20 years), high LDL-C was 36.2%, high TG 35.7%, low HDL-C 41.5%, and high TC was 43%, and dyslipidaemia (at least one abnormal lipid value) ≥ 79%8, and in Pakistan (≥ 20 years) high TC 39.3%, high TG 48.9%, high LDL-C 39.7%9.
Sociodemographic factors associated with dyslipidaemia include increased age2,6, male sex6,10, ethnicity10, and living in urban areas6,10. Health risk factors associated with dyslipidaemia include obesity2,6,8,10,11, central obesity10, cardiovascular disease6. diabetes6,8,10,11,12, hypertension6,8,10,11,12. Health behaviour risk factors associated with dyslipidaemia, include smoking10, physical inactivity2, exposure to secondary smoke13, and alcohol use had lower odds of dyslipidaemia10.
Factors associated with high TC include older age14, female sex14, lower education9,14,15, hypertension7,9,12, diabetes7,9,12,14, obesity7,9, and smoking9. Factors associated with high LDL-C include increasing age14, female sex, hypertension, diabetes, and obesity9. Factors associated with low HDL-C include, decreasing age14, female sex9, male sex14, higher education14, hypertension7, diabetes7,9,14, and obesity7,9,14 cigarette smoking14,16. Factors associated with high TG include male sex12,14, higher education14, hypertension7,12,14, diabetes7,9,12,14, obesity7,9,12, physical inactivity14, and smoking or current tobacco use7,12,14.
Factors associated with awareness of dyslipidaemia diagnosis include older age5, living in urban areas11, higher education11, higher BMI5,11,17, comorbid diagnosis of diabetes5,11, hypertension5,11, and CVD11. Factors associated with the treatment of dyslipidaemia or hypercholesterolemia include women7, comorbid hypertension, diabetes5, and CVD11. Factors positively associated with control of dyslipidaemia include women7, urban residence7, obesity7, comorbid diabetes5, and CVD11, and overweight/obesity and physical inactivity were negatively associated with control of dyslipidaemia11. The aim of the study was to assess the prevalence, distribution, and correlates of dyslipidaemia among people aged 15 to 69 years in Mongolia.
Methods
Participants and procedures
Cross-sectional national data with complete lipid measurements from the 2019 Mongolia STEPS survey18 were analyzed; the study response rate was 98.1%19. According to STEPS survey procedures,
“Socio-demographic and behavioural information was collected in Step 1. Physical measurements such as height, weight, and blood pressure were collected in Step 2. Biochemical measurements were collected to assess blood glucose and cholesterol levels in Step 3. A multi-stage stratified sampling process (377 sampling units or clusters selected from 21 provinces and 9 districts of Ulaanbaatar) was carried out to randomly select participants from the target population. One individual within the age range of the survey (15–69 years) was selected per household. If the randomly selected individual had temporarily been out of the range of the survey clusters (soum/khoroo) during the whole period of the field work, he or she was excluded and re-sampling was conducted. Ethics approval was provided by the Ministry of Health Medical Ethical Committee, Mongolia, and written informed consent was obtained from all participants, including from a parent and/or legal guardian18,19.”
All methods were performed in accordance with the relevant guidelines and regulations.
Data collection followed the “WHO three STEPS methodology: step 1 included administration of a structured questionnaire (sociodemographics, medical history, medication use, and health risk behaviour) step 2 consisted of blood pressure and anthropometric measurements, and step 3 included biochemical tests (blood glucose and blood lipids)18.” Anthropometric measurements were taken using the “Somatometre-Stanley 04-116 device and electronic scales GIMA”)19. Of the three blood pressure measurements using “OMRON Model M5 automatic blood pressure monitor19,” the last two readings were averaged18. “Blood glucose, total cholesterol and triglycerides were measured in peripheral (capillary) blood at the data collection site using dry chemical methods using multi-functional ‘Prima home test’ diagnostic device, biochemical analysis and automated analyzer19.” Serum samples were taken to analyze LDL and HDL cholesterol19. Laboratory analysis included blood glucose, TC, TG, HDL, and LDL. Laboratory tests for LDL and HDL in blood, were performed and analyzed in “Gyals” LLC’s laboratory using biochemical automated analyzer. HDL and LDL content was measured in serum using a direct or two-point linear method19.
Measures
Dyslipidaemia was classified20 as:
“being on antilipidemic medication or having one or more of the following: elevated total cholesterol (TC): ≥ 5.17 mmol/l (200 mg/dl), high triglycerides (TG): ≥ 1.70 mmol/l (150 mg/dl), low HDL-C: female ≤ 1.29 mmol/l; male ≤ 1.03 mmol/l (50 mg/dl in women, 40 mg/dl in men) and high LDL-C: ≥3.36 mmol/l (130 mg/dl).”
The awareness rate of dyslipidaemia was defined as:
“having been diagnosed by a health care provider as having high cholesterol among those with dyslipidaemia. The rate of dyslipidaemia treatment was defined as the self-reported use of lipid lowering drugs among participants who were aware of dyslipidaemia. The control rate of dyslipidaemia was classified as the proportion among those treated for dyslipidaemia who reach the lipid standard: TG < 1.70 mmol/L, TC < 5.18 mmol/L, HDL-C ≥ 1.04 mmol/L and LDL-C < 3.37 mmol/L20.”
Other biological measures included measured central obesity (“waist circumference ≥ 90 cm in men, ≥ 80 cm in women”)21; measured Body Mass Index (BMI) (kg/m2): “normal weight 18.5–22.9 kg/m2, underweight < 18.5 kg/m2, overweight 23.0–24.9 kg/m2, obesity class I 24.9 kg/m2, − 29.9 kg/m2, and obesity class II ≥ 30.0 kg/m222”, Hypertension/raised blood pressure (BP): “systolic BP ≥ 140 mm Hg and/or diastolic BP ≥ 90 mm Hg or where the participant is currently on antihypertensive medication23.” Diabetes: “fasting plasma glucose levels ≥ 7.0 mmol/L (126 mg/dl); or using insulin or oral hypoglycaemic drugs; or having a history of diagnosis of diabetes24.”
Behavioural measures included current tobacco use, current heavy episodic drinking (“six or more standard drinks in a single drinking occasion”), exposure to secondary smoke, daily consumption of fruits and vegetables, and low, moderate and high physical activity and sedentary behaviour (≥ 8 h/day) based on the “Global Physical Activity Questionnaire25.” Sociodemographic variables included age (years), sex (male, female), education in years, region, residence status, and ethnic group19.
History of CVDs included self-reported “Have you ever had a heart attack or chest pain from heart disease (angina) or a stroke (cerebrovascular accident or incident)? (Yes, No)19.”
Data analysis
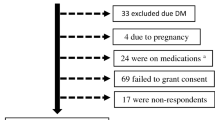
All statistical analyses were conducted with “STATA software version 14.0 (Stata Corporation, College Station, TX, USA).” “Analysis weights were calculated by taking the inverse of the probability of selection of each participant. These weights were adjusted for differences in the age-sex composition of the sample population as compared to the target population19.” Descriptive statistics are used to describe lipid profiles. Multivariable logistic regressions were used to assess the associations between sociodemographic (age, gender, education, and residence status) and health factors (BMI, hypertension, diabetes, cardiovascular disease, physical activity, sedentary behaviour, fruit/vegetable intake, current tobacco use, passive smoking, and heavy episodic drinking) and dyslipidaemia profiles as well as awareness and treatment. Afterward, significant variables in unadjusted analyses were included in the multivariate model. Covariates were selected based on previous literature review2,6,8,10,11,12,13,14,15,16,17. To account for the multi-stage sample design, Taylor linearization methods were utilized. P-values < 0.05 were considered significant. Statistical analyses were computed with complete cases of lipid profile measurement. Of the 6654 total study sample, 4895 had complete measures of lipid profile. The 1759 excluded individuals with an incomplete lipid profile were more likely to be younger, but did not differ in terms of residence status, sex, educational level, and ethnicity. Furthermore, sensitivity testing was done using multiple imputation by chained equations to fully impute the dataset. The logistic regression with dyslipidaemia as outcome was conducted with complete imputed data and results were compared with complete case analyses results.
Results
Sample characteristics
The sample with complete lipid measurements included 4,895 persons (15–69 years), with a median age of 35 years (IQR: 25–47 years) in 2019.
The prevalence of dyslipidaemia was 58.6% (54.5% using the restricted lipid profile, including high TC, high TG, and low HDL-C), 26.9% high TC, 31.7% high TG, 26.9% high LDL-C and 14.6% low HDL-C. Among the five regions in Mongolia, the highest proportion of dyslipidaemia was found in the central region (63.4%) and Ulaanbaatar (61.8%) and the lowest in the western region (48.8%). Further sample characteristics are described in Table 1 (see Table 1).
Distribution of dyslipidaemia awareness, treatment, and control
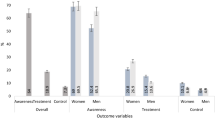
Among those with dyslipidaemia, 6.2% were aware. Among those who knew, the proportion of lipid-lowering drug treatment was 18.9%, and among those taking lipid-lowering drugs, 21.5% had their dyslipidaemia controlled (see Table 2).
Associations with dyslipidaemia prevalence
In adjusted logistic regression, older age (40–69 years) (AOR: 1.19, 95% CI 1.02–1.40), urban residence (AOR: 1.24, 95% CI 1.04–1.48), obesity call II (AOR: 2.89, 95% CI 2.29–3.66), hypertension (AOR: 1.33, 95% CI 1.11–1.59), and diabetes (AOR: 1.62, 95% CI 1.20–2.18) were positively and male sex (AOR: 0.84, 95% CI 0.72–1.00) was negatively associated with dyslipidaemia prevalence. Furthermore, in the unadjusted analysis, episodic heavy drinking was positively associated and increased physical activity was negatively associated with the prevalence of dyslipidaemia (see Table 3).
Furthermore, testing the consistency of these results using multiple imputation of missing data in the dependent variable were explored. The direction and significance of the relationship between covariates and dyslipidaemia in the two models (complete cases: Table 3 and imputed model: Supplementary Table 1) were similar to except in sex, education, and heavy episodic drinking where the level of significance differed).
Associations with prevalence of dyslipidaemia subcategories
In the adjusted logistic regression analysis, older age was positively associated with high TC, high TG, and low HDL-C, and inversely associated with high LDL-C. Male sex was positively associated with high TC and low HDL-C, and negatively associated with high LDL-C. Higher education was associated with high TC, high TG, and low LDL-C. Belonging to the Khalkh ethnic group was positively associated with high LDL-C. Obesity was associated with the four dyslipidaemia subcategories. Hypertension increased the odds of high TC and low LDL-C, and diabetes increased the odds of high TG and high LDL-C. High physical activity reduced the odds high TC, high TG, and low LDL-C. Current tobacco use was positively associated with high TC, high TG, and high LDL-C, while heavy alcohol use was positively associated with high TG (see Table 4).
Associations with dyslipidaemia awareness and treatment
In the adjusted logistic regression analysis, older age, higher education, urban residence, higher general body weight, hypertension, and cardiovascular disease were positively associated with dyslipidaemia awareness. Among those who were aware of their state of dyslipidaemia, belonging to the Khalkh ethnic group was positively associated and overweight or obesity class I was negatively associated with the treatment of dyslipidaemia (see Table 5).
Discussion
The study presents novel national data on the prevalence and distribution of dyslipidaemia in people 15 years and older in Mongolia in 2019. The proportion of dyslipidaemia in Mongolia (58.6%) was higher than in China (> 18 years, 34.0%)6, lower than in Northern Ethiopia (≥ 20 years, 66.7%)2, lower than in Turkey (≥ 20 years, ≥ 79%)8, and Pakistan (≥ 20 years, 96%)9. The prevalence of high TC (26.9%) in this study was higher than in the 35 LMIC study (≥ 15 years, 7.1%)5, lower than in Northern Ethiopia (≥ 20 years, 30.8%)2, in Jordan (≥ 25 years, 44.3%)7, in Turkey (≥ 20 years, 43%) and Pakistan (≥ 20 years, 39.3%)9. Some of these prevalence differences may be attributed to the use of different cut-off points of lipid abnormality. The high proportion of dyslipidaemia in Mongolia may be attributed to change in dietary behaviour, increased sedentary lifestyle, rapid urbanization, change in labour intensity of work and improved economic status2,19,26,28.
The most prevalent dyslipidaemia component was high TG (31.7%), followed by high TC (26.9%), high LDL-C (26.9%) and low HDL-C (14.6%). A similar rank order of prevalence of components of dyslipidaemia was found in Ethiopia, with low HDL-C having the lowest prevalence2, and in northeast China with the highest being high TG, followed by high TC11, while in national China the highest component was high low HDL-C, followed by high TG, high LDL-C and high TC6. The prevalence of high TG (31.7%) in this study was lower than in Northern Ethiopia (40.2%)2, in Turkey (35.7%)8, and Pakistan (48.9%)9. The prevalence of high LDL-C (26.9%) in this study was higher than in 35 LMIC (7.5%)5, lower than in northern Ethiopia (49.5%)2, Turkey (36.2%)8, and in Pakistan (39.7%)9. The prevalence of low HDL-C (14.6%) in this survey was lower than in Jordan (59.5%)7, in Turkey (41.5%)8, and Pakistan (> 80%)9.
Consistent with previous research2,6,8,10,11,12, older age, urban residence, obesity, hypertension and diabetes increased the odds of dyslipidaemia. Dyslipidaemia may increase with age because physical activity levels decrease leading to more fat accumulation2. Lifestyle changes, such as change in dietary and sedentary behaviour, may explain that with urbanization higher rates of dyslipidaemia were found9,26. The tendency to have a higher prevalence dyslipidaemia with higher levels of BMI can be attributed to “global metabolic effects of insulin resistance and an excess of visceral fat27.” A nutritional transition in Mongolia may have led to the found high prevalence of central obesity (56.7%) and high levels of body fat28, increasing the risk of dyslipidaemia.
Some studies in China6,10, found that male sex was positively associated with dyslipidaemia, while our study found a weak negative association between male sex and dyslipidaemia, mainly due to higher high TC and higher low HDL-C in men than women. We did not find a significant association between ethnicity and dyslipidaemia, contrary to some previous studies10. While some research6 showed a positive association between cardiovascular diseasen] and dyslipidaemia, we did not find this association. In unadjusted analyzes we found that increased physical activity was negatively associated with the prevalence of dyslipidaemia, which is consistent with some previous research2,9. Contrary to previous findings2,10,13, we did not find significant associations between tobacco use, passive smoking, alcohol use, and dyslipidaemia. Furthermore, we found regional differences in the prevalence and awareness of dyslipidaemia, such that prevalence and awareness was the lowest in the Western region (48.8%, and 3.3%, respectively) and among the highest in Ulaanbaatar (61.8%, and 8.5%, respectively). The role of geographic determinants of dyslipidaemia and awareness is important in designing effective intervention strategies17.
Factors associated with high TC in this study included older age, male sex, higher education, obesity, hypertension, physical inactivity, and current tobacco use. In previous studies7,9,12,14,16, older age, female sex, lower education, hypertension, diabetes, and obesity were associated with high TC. Factors associated with high TG in this study included older age, higher education, obesity, diabetes, physical inactivity, heavy episodic drinking, and current tobacco use. In previous studies7,9,12,14, male sex, higher education, hypertension, diabetes, obesity, and smoking or current tobacco use were associated with high TG. Factors associated with low HDL-C in this study included older age, male sex, higher education, obesity, hypertension, and physical inactivity. In previous studies, 7,14,15 decreased age, male sex, higher education, hypertension, diabetes, obesity, and smoking were associated with low HDL-C. Factors associated with high LDL-C in this study included lower age, female sex, being Khalkh, obesity, diabetes, and current tobacco use. In previous studies7,14, hypertension, diabetes, and obesity were associated with high LDL-C.
The prevalence of awareness of dyslipidaemia (6.2%), treatment (18.9%), and control (21.5%) were in terms of awareness and treatment lower than in the 35 LMIC study (31/36% and 29/33%, respectively) but higher in terms of control (7/19%)5. Awareness was also much lower than in China (31.0%) but treatment was similar to China (19.5%) and control was higher than in China (8.9%)6. In particular, the low awareness of dyslipidaemia emphasizes the need for opportunistic screening in Mongolia. Consistent with previous results5,11,17, older age, higher education, urban residence, obesity, hypertension, and cardiovascular disease were associated with increased awareness of dyslipidaemia in this study. The association between higher education, urban residence and higher awareness of dyslipidaemia may be explained by higher health literacy in urban educated Mongolians and better access to health care in urban settings17. The association between general obesity and awareness of dyslipidaemia may be attributed to lipids being part of weight management, yet overweight and obesity were negatively associated with dyslipidaemia treatment. Similar results were found for dyslipidaemia control in China, highlighting the greater difficulty of dyslipidaemia control among obese compared to people of normal weight17.
The strengths of the study include the use of a large nationally representative sample and standardized STEPS methodology and measures. Some variables were evaluated by self-report, which may have biased responses, and the cross-sectional design precludes causative conclusions between the evaluated variables. The sample was restricted to those with complete lipid measurements. The excluded participants were largely similar to those included in the analysis. We conducted additional analyses with imputed data, which provided similar results. Familial hyperlipidaemia and more detailed dietary behaviour were not assessed and should be included in future research. Another limitation was that the 2019 Mongolia STEPS survey only included people aged 15–69 years, while the pattern of dyslipidaemia in people aged ≥ 70 years is unknown.
Conclusion
Six in ten Mongolians 15 years and older had dyslipidaemia. Several factors, including sociodemographic and health factors, were identified for dyslipidaemia. The high prevalence of dyslipidaemia in Mongolia warrants enhanced public health interventions, including screening, better diagnose, treat, and control dyslipidaemia.
Data availability
The data source is publicly available at the World Health Organization NCD Microdata Repository (URL: https://extranet.who.int/ncdsmicrodata/index.php/catalog).
References
Pirillo, A., Casula, M., Olmastroni, E., Norata, G. D. & Catapano, A. L. Global epidemiology of dyslipidaemias. Nat. Rev. Cardiol. 18(10), 689–700. https://doi.org/10.1038/s41569-021-00541-4 (2021).
Gebreegziabiher, G., Belachew, T., Mehari, K. & Tamiru, D. Prevalence of dyslipidemia and associated risk factors among adult residents of Mekelle City, Northern Ethiopia. PLoS ONE 16(2), e0243103. https://doi.org/10.1371/journal.pone.0243103 (2021).
World Health Organization (WHO) Mongolia: Noncommunicable Diseases (NCD) Country Profiles, 2018. https://cdn.who.int/media/docs/default-source/country-profiles/ncds/mng_en.pdf?sfvrsn=1cc0022d_39&download=true. (Accessed 2 Aug 2021).
Enkh-Oyun, T., Kotani, K. & Swanson, E. Ischemic heart disease among the general Mongolian population: A review of epidemiological studies. Int. Health. 8(1), 13–17. https://doi.org/10.1093/inthealth/ihv064 (2016).
Marcus, M. E. et al. Unmet need for hypercholesterolemia care in 35 low- and middle-income countries: A cross-sectional study of nationally representative surveys. PLoS Med. 18(10), e1003841. https://doi.org/10.1371/journal.pmed.1003841 (2021).
Pan, L. et al. The prevalence, awareness, treatment and control of dyslipidemia among adults in China. Atherosclerosis 248, 2–9. https://doi.org/10.1016/j.atherosclerosis.2016.02.006 (2016).
Abujbara, M. et al. The prevalence of dyslipidemia among Jordanians. J. Lipids. 28(2018), 6298739. https://doi.org/10.1155/2018/6298739 (2018).
Bayram, F. et al. Prevalence of dyslipidemia and associated risk factors in Turkish adults. J. Clin. Lipidol. 8(2), 206–216. https://doi.org/10.1016/j.jacl.2013.12.011 (2014).
Basit, A., Sabir, S., Riaz, M., Fawwad, A., NDSP members. NDSP 05: Prevalence and pattern of dyslipidemia in urban and rural areas of Pakistan; a sub analysis from second National Diabetes Survey of Pakistan (NDSP) 2016–2017. J. Diabetes Metab. Disord. 19(2), 1215–1225. https://doi.org/10.1007/s40200-020-00631-z (2020).
Xi, Y. et al. Prevalence of dyslipidemia and associated risk factors among adults aged ≥35 years in northern China: A cross-sectional study. BMC Public Health 20(1), 1068. https://doi.org/10.1186/s12889-020-09172-9 (2020).
Zhang, F. L. et al. The prevalence, awareness, treatment, and control of dyslipidemia in northeast China: A population-based cross-sectional survey. Lipids Health Dis. 16(1), 61. https://doi.org/10.1186/s12944-017-0453-2 (2017).
Tripathy, J. P. et al. Burden and risk factors of dyslipidemia-results from a STEPS survey in Punjab India. Diabetes Metab. Syndr. 11(Suppl 1), S21–S27. https://doi.org/10.1016/j.dsx.2016.08.015 (2017).
Okekunle, A. P., Asowata, J. O., Adedokun, B. & Akpa, O. M. Secondhand smoke exposure and dyslipidemia among non-smoking adults in the United States. Indoor Air 32(1), e12914. https://doi.org/10.1111/ina.12914 (2022).
Huang, C. et al. Prevalence and related factors of dyslipidemia among urban adults aged 35 to 79 years in Southwestern China. Sci. Rep. 11(1), 17579. https://doi.org/10.1038/s41598-021-96864-w (2021).
Erem, C., Hacihasanoglu, A., Deger, O., Kocak, M. & Topbas, M. Prevalence of dyslipidemia and associated risk factors among Turkish adults: Trabzon lipid study. Endocrine 34(1–3), 36–51. https://doi.org/10.1007/s12020-008-9100-z (2008).
Malta, D. C. et al. Prevalence of altered total cholesterol and fractions in the Brazilian adult population: National Health Survey. Rev. Bras. Epidemiol. https://doi.org/10.1590/1980-549720190005.supl.2 (2019).
Opoku, S. et al. Awareness, treatment, control, and determinants of dyslipidemia among adults in China. Sci. Rep. 11(1), 10056. https://doi.org/10.1038/s41598-021-89401-2 (2021).
World Health Organization (WHO) STEPwise approach to surveillance (STEPS). https://www.who.int/ncds/surveillance/steps/en/ (Accessed 22 Aug 2021) (2018).
National Centre for Public Health. Fourth national STEPS survey on prevalence of non-communicable disease and injury risk factors-2019. Ulaanbaatar: National Centre for Public Health. https://extranet.who.int/ncdsmicrodata/index.php/catalog/836/related-materials (Accessed 5 Aug 2021) (2020).
Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of The National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA 285(19), 2486–2497. https://doi.org/10.1001/jama.285.19.2486 (2001).
Harvard School of Public Health. Abdominal obesity measurement guidelines for different ethnic groups, 2020. https://www.hsph.harvard.edu/obesity-prevention-source/waist-circumference-guidelines-for-different-ethnic-groups/ (Accessed 4 Feb 2022).
Wen, C. P. et al. Are Asians at greater mortality risks for being overweight than Caucasians? Redefining obesity for Asians. Public Health Nutr. 12(4), 497–506. https://doi.org/10.1017/S1368980008002802 (2009).
Chobanian, A. V. et al. Seventh report of the Joint National Committee of Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 42(6), 1206–1252. https://doi.org/10.1161/01.HYP.0000107251.49515.c2 (2003).
World Health Organization (WHO). WHO STEPS Surveillance Manual. https://www.who.int/ncds/surveillance/steps/STEPS_Manual.pdf (Accessed 10 Oct 2020).
Armstrong, T. & Bull, F. Development of the World Health Organization Global Physical Activity Questionnaire (GPAQ). J. Public Health 14, 66–70 (2006).
Bromage, S. et al. Diet and nutrition status of Mongolian adults. Nutrients 12(5), 1514. https://doi.org/10.3390/nu12051514 (2020).
Chan, D. C., Pang, J. & Watt, G. F. Dyslipidemia in obesity. In Metabolic Syndrome (ed. Ahima, R. S.) 526–540 (Springer International Publishing, 2016). https://doi.org/10.1007/978-3-319-11251-0_30.
Otgontuya, D., Khor, G. L., Lye, M. S. & Norhaizan, M. E. Obesity among Mongolian adults from urban and rural areas. Mal. J. Nutr. 15(2), 185–194 (2009).
Acknowledgements
This paper uses data from the 2019 Mongolia STEPS survey, implemented by the Ministry of Health and Public Health Institute with the support of the World Health Organization.
Author information
Authors and Affiliations
Contributions
All authors fulfil the criteria for authorship. S.P. and K.P. conceived and designed the research, performed statistical analysis, drafted the manuscript and made critical revision of the manuscript for key intellectual content. All authors read and approved the final version of the manuscript and have agreed to authorship and order of authorship for this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pengpid, S., Peltzer, K. National high prevalence, and low awareness, treatment and control of dyslipidaemia among people aged 15–69 years in Mongolia in 2019. Sci Rep 12, 10478 (2022). https://doi.org/10.1038/s41598-022-14729-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-14729-2
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.