Abstract
Giant unilamellar vesicles (GUVs) are composed of lipophilic layers and are sensitive to the action of reactive oxygen species (ROS). The use of GUVs as microcarriers of biological macromolecules is particularly interesting since ROS produced by gametes or embryos during in vitro culture can induce the opening of pores in the membrane of these vesicles and cause the release of their content. This study investigated the behavior of GUVs [composed of 2-dioleoyl-sn-glycero-3-phosphocholine and 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(lissamine rhodamine B sulfonyl)] in co-culture with in vitro produced bovine embryos, as well as their embryotoxicity and effectiveness as cysteine carriers in culture medium. Embryonic developmental rates were unaffected, demonstrating the absence of toxicity of GUVs co-cultured with the embryos. No increase of intracellular ROS levels was observed in the embryos co-cultured with GUVs, indicating that the higher lipid content of the culture environment resulting from the lipid composition of the GUV membrane itself did not increase oxidative stress. Variations in the diameter and number of GUVs demonstrated their sensitivity to ROS produced by embryos cultured under conditions that generate oxidative stress. Encapsulation of cysteine in GUVs was found to be more effective in controlling the production of ROS in embryonic cells than direct dilution of this antioxidant in the medium. In conclusion, the use of GUVs in in vitro culture was found to be safe since these vesicles did not promote toxic effects nor did they increase intracellular ROS concentrations in the embryos. GUVs were sensitive to oxidative stress, which resulted in structural changes in response to the action of ROS. The possible slow release of cysteine into the culture medium by GUV rupture would therefore favor the gradual supply of cysteine, prolonging its presence in the medium. Thus, the main implication of the use of GUVs as cysteine microcarriers is the greater effectiveness in preventing the intracytoplasmic increase of ROS in in vitro produced bovine embryos.
Similar content being viewed by others
Introduction
In vitro production (IVP) of bovine embryos has grown significantly in recent decades. This biotechnology has become an important tool for accelerating the genetic improvement of cattle herds and has been applied on a large commercial scale1, 2.
The success of IVP is directly related to gamete quality3. However, the results are also influenced by extrinsic factors resulting from the excessive manipulation and exposure of gametes and embryos to the adverse conditions of the culture environment. The high concentrations of oxygen and the prolonged light exposure of these manipulated and in vitro cultured structures result in the exacerbated production of reactive oxygen species (ROS), which can reduce the developmental rates and quality of the produced embryos4, 5. Hydrogen peroxide (H2O2) is the most stable of all ROS and an increase in the concentration of this free radical has been previously demonstrated in in vitro cultured embryos compared to in vivo derived embryos6.
Reactive oxygen species are derived from various sources, including oxidative phosphorylation in the mitochondrial respiratory chain, enzymatic activation of cytochrome p450 and NADPH oxidases7, and these metabolites can be produced in multiple cellular compartments, including the cytosol, mitochondria, endoplasmic reticulum, peroxisomes and by plasma membrane-bound NADPH oxidase complex (NOX)8. The balance between the production and elimination of ROS maintains the homeostasis of the cellular reduction and oxidation (redox) processes9. At low concentrations, ROS participate in different physiological cell signaling pathways10, 11; however, the condition of oxidative stress is established when there is an imbalance between ROS production and antioxidant mechanisms. Excessive ROS production is extremely harmful to gametes and embryos since it culminates in lipid peroxidation, cellular DNA damage12, 13, and loss of membrane integrity as a result of oxidation, which promotes structural modifications and increases permeability of the lipid bilayer4, 13. In an attempt to reduce the deleterious effects caused by ROS, antioxidants are added to culture media in order to increase the antioxidant capacity of in vitro produced gametes and embryos14–16.
Low molecular weight thiol compounds such as cysteine have been added to oocyte maturation and embryo culture media15, 17–19 as precursors for the intracellular production of glutathione (GSH), a non-enzymatic antioxidant4 that is involved in the detoxification of lipid peroxides and removal of ROS, especially (H2O2)20. The biosynthesis of GSH depends on the presence of cysteine in the medium21, however, cysteine is unstable outside the cells and is rapidly oxidized to cystine; thus, cysteine concentrations are significantly reduced after preparation and equilibration of the medium in an incubator22. A previous study showed that only 40% and 3% of the initial cysteine concentration of 0.6 mM remained in the culture medium after 3 and 24 h of incubation, respectively23. To overcome this problem, it would be highly desirable to develop a system that permits the slow and gradual release of cysteine into the medium in order to ensure its availability for a longer period of time during culture.
Vesicles consisting of lipophilic layers were initially developed as models that mimic biological membranes24, 25. However, their use as potential nanotools designed to control the release of encapsulated drugs has been proposed recently26. These vesicles are distinguished according to the number of layers (unilamellar or multilamellar) and their size (small or liposomes, large and giant)27. Because of their large size (10 to 100 µm), giant unilamellar vesicles (GUVs) are particularly interesting as microcarriers of biological macromolecules28, 29. Additionally, GUVs are potentially interesting as oxidative stress markers during in vitro culture since they are extremely sensitive to the action of ROS30,31,32. Specifically for the use of the potential of GUVs as oxidative stress signalers in IVP cultures, it is assumed that the ROS with the greatest action would be the H2O2 produced by the cultured embryos because, unlike the other ROS, it can cross the cell membrane and diffuse for the culture medium8. In this context, the increased concentrations of H2O2 in the medium could act directly on the membrane of the GUVs, promoting changes in their structure with consequent release of molecules carried by them.
Despite the potential use of GUVs for molecule encapsulation29, 30, 33, 34, the application of these structures in reproductive biotechnologies, specifically IVP, has not yet been proposed. Therefore, this study was designed to test the hypothesis that GUVs co-cultured in vitro with bovine embryos can act as microcarriers for the delivery of the antioxidant cysteine in culture medium in the presence of oxidative stress. The aim of this study was to investigate the behavior of GUVs co-cultured with embryos, their embryotoxicity, and their effectiveness as cysteine carriers.
Results
GUVs cultured in vitro in the absence of cells are not affected by the oxidizing effect of menadione
Menadione (MD) is a compound used to induce oxidative stress due to its capacity to trigger the production of superoxide anion (O2−), H2O2 and other ROS in cells cultured in the presence of oxygen30. ROS can cause structural modifications in GUVs, especially alterations in their diameter33, 35,36,37,38. The variation in GUV diameter was therefore used as a parameter to evaluate menadione-induced oxidative stress in the in vitro culture system in the absence of cells. The diameter of GUVs cultured at different concentrations of menadione (Fig. 1) was similar between treatments at the beginning of culture (day 1—D1; P > 0.05), after 72 h (D3; P > 0.05) and at the end of culture (D7; P > 0.05). There was no difference between any of the concentrations tested and the control group (P > 0.05).
Diameter of giant unilamellar vesicles (GUVs) cultured in vitro with different concentrations of menadione (MD). Data are expressed as the mean ± standard error of three independent replicates (control, n = 147; MD 1.0 µM, n = 119; MD 2.5 µM, n = 134; MD 5.0 µM, n = 132; and MD 7.5 µM, n = 148). *D1 = day 1 (beginning of cultivation); D3 = day 3 (72-h cultivation); D7 = day 7 (168-h cultivation). No differences were observed between treatments within each assessment day (P > 0.05).
The morphometry of GUVs cultured in vitro in the absence of cells is affected by hydrogen peroxide
In this experiment we evaluated the alterations in the diameter of in vitro-cultured GUVs after stimulation with H2O2, one of the main and most potent free radicals produced by cells in response to oxidative stress4. The diameter of GUVs cultured with H2O2 in the absence of cells (Fig. 2a) was similar between treatments at the beginning of culture (D1; P > 0.05). However, on D3, a reduction (P < 0.05) in diameter was observed in GUVs cultured in the presence of the higher concentrations of H2O2 tested (0.1 mM: 17.71 ± 0.30 µm; 0.5 mM: 17.32 ± 0.29 µm; 1.0 mM: 16.63 ± 0.31 µm) when compared to control (18.96 ± 0.27 µm). Likewise, the diameter of GUVs cultured in the presence of the higher concentrations of H2O2 tested (0.5 mM: 16.14 ± 0.36 µm; 1.0 mM: 16.13 ± 0.31 µm) was reduced at the end of culture (D7) when compared to control (17.67 ± 0.30 µm). Representative photomicrographs of GUVs cultured for 7 days with or without H2O2 are shown in Fig. 2b.
Diameter of giant unilamellar vesicles (GUVs) cultured in vitro with different concentrations of hydrogen peroxide (H2O2). (a) Data are expressed as the mean ± standard error of three independent replicates (control, n = 1,031; H2O2 0.01 mM, n = 891; H2O2 0.05 mM, n = 882; H2O2 0.1 mM, n = 786; H2O2 0.5 mM, n = 907; and H2O2 1.0 mM, n = 698). *D1 = day 1 (beginning of cultivation); D3 = day 3 (72-h cultivation); D7 = day 7 (168-h cultivation). Different letters within each assessment day indicate significant differences (P < 0.05). (b) Representative photomicrographs of GUVs collected from culture wells on D7 of the different treatments. ×40 Magnification.
GUVs are not embryotoxic but are sensitive to oxidative stress induced by menadione in in vitro cultured embryos
In the first series of this experiment, we demonstrated the induction of oxidative stress in cultured embryos exposed to menadione. Next, we evaluated (1) the embryotoxicity of GUVs; (2) intracytoplasmic concentrations of ROS in embryos co-cultured with GUVs, and (3) the morphometry and quantity of GUVs co-cultured with embryos subjected to menadione-induced oxidative stress.
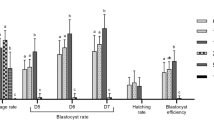
The effects of supplementation with menadione during embryo culture on embryo outputs and generation of oxidative stress are given in Fig. 3. No difference (P > 0.05) in the cleavage rate was observed between treatments, but blastocyst rate was decreased in the presence of menadione (control group: 37.24% ± 5.4 vs. MD 5.0 μM group: 15.75% ± 0.9; P < 0.05, Fig. 3a). Higher intracellular ROS concentrations (Fig. 3b) were observed in embryos of the MD 5.0 μM group (26.61 ± 3.29 AUF) when compared to the control (13.56 ± 0.99 AUF; P < 0.05).
Cleavage and development (a) and intracellular concentrations of reactive oxygen species (ROS) (b) of bovine embryos cultured in vitro and subjected to menadione (MD)-induced oxidative stress. Data are expressed as the mean ± standard error of four independent replicates. Embryos were cultured for 7 days, and menadione (5.0 μM) was included in the medium only on the sixth day of culture (from D6 to D7).
No difference (P > 0.05) in the cleavage rate was observed between treatments (Table 1). There was no difference (P > 0.05) in the embryonic developmental rate up to the blastocyst stage between control (26.70% ± 7.30) and GUV groups (25.18% ± 4.32), but both groups exhibited higher blastocyst rates (P < 0.05) than the groups treated with different concentrations of menadione (GUV + MD 5.0 μM: 6.95% ± 3.13 and GUV + MD 7.5 μM: 0.95% ± 0.95).
Higher intracellular ROS concentrations (Table 1) were observed in embryos of the GUV + MD 5.0 μM group (16.28 ± 4.61 AUF), which differed (P < 0.05) from the control (3.76 ± 0.46 AUF) and GUV (3.99 ± 0.33 AUF) groups. Embryos of the GUV + MD 7.5 μM group were not evaluated because of the lack of development of an adequate number of structures for this assessment.
There was no difference in the diameter of GUVs between the different treatments at the beginning of culture (D1; P > 0.05) (Table 2). However, an increase (P < 0.05) in GUV diameter was observed at the end of culture (D7) in the GUV + MD 7.5 μM group (17.79 ± 0.94 µm) compared to the GUV (14.63 ± 0.82 µm) and GUV + MD 5.0 μM (13.49 ± 0.59 µm) groups. There was also a variation overtime (D1 vs. D7) in the diameter of GUVs cultured in the presence of menadione (P > 0.05).
No difference between treatments (P > 0.05) was found for the variation in the number of GUVs (%) present in the wells at the end of culture (D7) compared to D1, with 13.6% ± 4.9 of remnant GUVs on D7 in the GUV group, 21.6% ± 4.7 in the GUV + MD 5.0 μM group, and 13.5% ± 5.3 in the GUV + MD 7.5 μM group (Fig. 4). However, the number of remnant GUVs in the wells was decreased overtime (D1 vs. D7) in all groups (P < 0.05).
Percentage of giant unilamellar vesicles (GUVs) present in droplets of in vitro cultured bovine embryos. The percentage of GUVs on the last day of culture (D7: black bars) was determined in relation to the total number of GUVs present on the first day (D1: light gray bars). On D6, oxidative stress was induced in the embryos by the addition of different concentrations of menadione (MD) for 24 h. There was no significant difference between treatments at each time point (P > 0.05). The asterisk indicates a significant difference within the same treatment when evaluated on different days (P < 0.05).
Supplementation of in vitro culture medium with antioxidant encapsulated in GUVs provides greater protection against embryonic oxidative stress than supplementation through direct dilution in the medium
The suitability of GUVs as antioxidant carriers in embryo culture medium was evaluated in this experiment. For this purpose, the effect of the antioxidant cysteine diluted in culture medium [concentration of 0.6 mM as recommended in previous studies14, 15 was compared to that of cysteine (Cyst) encapsulated in GUVs [GUV(Cyst 3 mM)]. These GUVs were obtained by electroformation in a solution containing 3 mM cysteine (5X solution) and subsequent dilution in the culture medium (20%, v/v) in order to obtain a maximum final concentration of cysteine in the medium of 0.6 mM after complete content release from the GUVs.
The cleavage rate was similar (P > 0.05) between treatments (Table 3). The rate of embryo development to the blastocyst stage was lower (P < 0.05) in the groups subjected to menadione-induced oxidative stress (MD: 9.46% ± 6.04; Cyst + MD: 1.19% ± 1.19; GUV(Cyst 3 mM) + MD: 1.15% ± 1.15) compared to the control (25.58% ± 4.53), Cyst (31.77% ± 0.37), and GUV(Cyst 3 mM) (30.16% ± 1.59) groups.
Intracellular concentrations of ROS were evaluated in embryos on D7 (Table 4), except for the Cyst + MD and GUV(Cyst) + MD groups because of the lack of development of an adequate number of structures for this assessment. Embryos of the GUV(Cyst 3 mM) group had lower (P < 0.05) ROS concentrations (0.09 ± 0.01 AUF) than those of the Cyst group (0.16 ± 0.02 AUF), but neither group differed from the control group (0.10 ± 0.01 AUF). The highest ROS concentrations were observed in the MD group (0.34 ± 0.05 AUF), which differed from the other treatments (P < 0.05). Representative photomicrographs of embryos from the different treatments stained for reactive oxygen species are shown in Fig. 5.
Discussion
This is the first study that proposes the use of GUVs as antioxidant carriers for supplementation of in vitro culture media of bovine embryos. Overall, the results showed that GUVs are not embryotoxic and are sensitive to ROS produced by embryos cultured under conditions that generate oxidative stress. Additionally, encapsulation of the antioxidant in GUVs was found to be more effective in controlling the production of ROS in embryonic cells than direct dilution of the antioxidant in the medium.
The present results revealed no changes in the diameter of GUVs cultured in vitro in the presence of menadione. This compound has been used in previous studies to induce oxidative stress in in vitro cultured cells39. It acts by disrupting the cellular pyridine nucleotide redox balance, compromising cellular ATP production and mitochondrial respiratory activity40. As a consequence, cells produce the main ROS such as O2− and H2O241. Although we did not investigate the presence of ROS in the culture medium, the results suggest that menadione did not induce the generation of ROS because of the absence of cells; thus, this drug did not directly trigger oxidative stress in GUVs. In a second step, we evaluated the direct effect of a free radical (H2O2) on GUVs. This radical promoted changes in the diameter of these structures even when cultured in vitro in the absence of cells. This variation in surface area and the concomitant morphological changes in GUVs have been previously observed in response to irradiation in the presence of photooxidative substances36.
After determination of the sensitivity of GUVs to H2O2, these structures were co-cultured with embryos under conditions that generate oxidative stress (menadione challenge) in order to evaluate the susceptibility of GUVs to ROS produced by cultured cells. Variations in the diameter and number of remnant GUVs were observed at the end of the culture period in the group treated with the highest menadione concentration tested (7.5 µM). This result corroborates the sensitivity of GUVs to H2O2 and might be explained by the fact that these vesicles, which are composed of unsaturated phospholipids, are extremely sensitive to lipid peroxidation, a chain reaction mediated by oxidative products42. Recent studies have shown that the exposure of GUVs to photooxidative substances leads to the cleavage of lipid chains and the consequent opening of pores in the membrane, causing changes in its structure33, 43, 44 such as surface area variation, associated or not with membrane fluctuations and the release of “buds” due to oxidative stress36, 37, 42. Indeed, it has previously been reported that ROS can react with the unsaturations of the lipid chains forming hydroperoxides. The hydrophilic nature of these groups favors migration towards the polar heads of the lipids leading to an increase of the mean molecular area per lipid and the overall surface area expansion of the GUVs33, 45. In addition, the propagation of the oxidation reactions may lead to the chain cleavage at the unsaturation sites, inducing the pore opening in the lipid membrane33, 46 and further decrease of the GUV dimension. Although small, the reduction in population of GUVs over time is indeed significant. This effect is inherent to the stability of the colloidal system formed by self-assembled lipid structures47, which can be attributed to a variety of environmental conditions, such as temperature and ionic strength. Thus, our results thus suggest that the variation in the diameter of GUVs may be the result of their sensitivity to oxidative stress. Although we did not evaluate the ultrastructure of GUVs, literature data indicate that the variation in GUV diameter may be a consequence of membrane damage caused by ROS, especially by the H2O2 radical33, 35–38, 43, 44. One may thus assume that this membrane damage promotes possible extravasation of the content of GUVs.
Since GUVs are considered useful tools for macromolecule encapsulation29 and free radicals can damage their membrane37, causing possible extravasation of their content, these structures are excellent candidate vehicles for the gradual release of antioxidants into the in vitro culture medium of embryos in situations that generate oxidative stress. Within this context, the supply of antioxidant to the medium via GUVs would permit a response to the specific needs of the culture system since the unnecessary or excessive inclusion of antioxidants can be harmful19. For example, at low concentrations, ROS play a physiological role in cells; more specifically, they regulate cell function by controlling the production and activation of substances with biological activity in the main signaling pathways48, 49. To our knowledge, there are no studies that have co-cultured GUVs with embryos. Thus, their embryotoxicity needs to be evaluated. The present results demonstrated that GUVs did not affect embryonic development and do therefore not exert any morphologically evident embryotoxic effect. Likewise, no increase of intracellular ROS levels was observed in embryos cultured in vitro in the presence of GUVs, indicating that the higher lipid content of the culture environment resulting from the lipid composition of the GUV membrane itself did not cause any increase in oxidative stress. Based on these satisfactory results, cysteine was encapsulated in GUVs by electroformation since the presence of cysteine in the culture medium is essential for the synthesis of GSH by the embryo14, favoring the quality of the quality of the embryos produced. However, supplementation of the culture medium with cysteine, either diluted or encapsulated in GUVs, did not promote an increase in blastocyst production rates when the cultures were not challenged with menadione, although an interesting numerical increase in embryo production was observed [25.58% in the control group vs. 31.77% and 30.16% in the Cyst and GUV(Cyst 3 mM) groups, respectively]. Similarly, in the absence of oxidative stress, neither cysteine supplementation system reduced intracytoplasmic ROS levels in the embryos compared to control. According to the literature, the addition of antioxidants during in vitro culture produces contradictory results in terms of embryo development, with studies reporting beneficial14, undesirable50, or no effects51. It is most likely that beneficial effects are only seen when the culture conditions are inadequate.52 Thus, the lack of effects of the antioxidant suggests that the culture conditions in this study were satisfactory and did not generate excessive oxidative stress.48, 49
In the present study, although supplementation of the medium with 0.6 mM cysteine did not compromise embryo developmental rates, the results showed that supplementation with this antioxidant encapsulated in GUVs was more effective than its direct dilution in the medium in preventing the increase of intracytoplasmic ROS levels in embryos caused by the inherent conditions of the culture system itself, i.e., in the absence of menadione [0.09 AUF in the GUV (Cyst 3 mM) vs. 0.16 AUF in the Cyst group]. One may speculate that the direct dilution of cysteine in the culture medium may have resulted in its rapid oxidation into cystine53, 54, consequently reducing the availability of the former as precursors of GSH synthesis17, 53. On the other hand, the possible slow release of cysteine into the culture medium by rupture of the GUVs would favor the gradual supply of cysteine, prolonging its presence in the medium. These results are promising and encourage the continuation of this study in order to implement this tool in reproductive biotechnology protocols.17, 53
In summary, we demonstrated that in vitro cultured GUVs had no toxic effect and did not increase intracellular ROS concentrations in the embryos. The use of these structures was safe for the IVP purposes described in this study. Since they are sensitive to oxidative stress, GUVs underwent structural changes in response to the action of ROS, indicating that these structures are suitable microcarriers for the on-demand release of antioxidants. Compared to the system in which cysteine was diluted directly in the medium (without encapsulation), GUVs containing cysteine were more effective in preventing the intracytoplasmic increase of ROS in in vitro produced bovine embryos. These results indicate that the use of GUVs as microcarriers is safe and beneficial for improving the quality of in vitro produced embryos. The findings should encourage new studies implementing the use of this support tool in order to improve the efficiency of reproductive biotechnologies.
Methods
Ethics approval
All experimental procedures were approved by the Ethics Committee on Animal Experimentation of UNESP (Protocol No. 009981/17) and were conducted in accordance with relevant guidelines and regulations of São Paulo State University, Campus of Araçatuba and Campus of Assis, Brazil. All methods used in this study are reported in accordance with ARRIVE guidelines for the reporting of animal experiments.
Chemical reagents and media
The reagents were purchased from Sigma-Aldrich (St Louis, MO, USA), unless otherwise stated. Plastic materials and tubes were obtained from Corning Inc. (Acton, MA, USA).
The in vitro maturation (IVM) medium consisted of TCM199 supplemented with 10% (v/v) fetal calf serum (FCS; Gibco BRL, Grand Island, NY, USA), 0.2 mM sodium pyruvate, 25 mM sodium bicarbonate, 50 μg/mL amikacin, 0.5 μg/mL FSH (Folltropin-V; Bioniche Animal Health, Ontario, Canada), and 100 IU/mL hCG (Vetecor; Hertape Calier, Juatuba, MG, Brazil). The fertilization medium (IVF-TALP) consisted of Tyrode’s albumin lactate pyruvate (TALP) containing 0.2 mM Na-pyruvate, 6 mg/mL fraction V fatty acid–free BSA, 25 mM sodium bicarbonate, 13 mM Na-lactate, 50 μg/mL amikacin, 10 μM hypotaurine, 20 μM penicillamine, 2 μM epinephrine, and 10 μg/mL heparin. The embryo culture medium contained modified synthetic oviductal fluid (mSOF) supplemented with 0.2 mM L-glutamine, 0.34 mM sodium citrate, 2.8 mM myo-inositol, 2% essential amino acids, 1% non-essential amino acids, 0.2 mM pyruvate, 50 μg/mL amikacin, 5 mg/mL BSA, and 2.5% (v/v) FCS.
Cysteine (Sigma C7352) was freshly diluted in mSOF daily during the experiment to obtain a final concentration of 0.6 mM14, 15.
Electroformation and evaluation of giant unilamellar vesicles
The GUVs were prepared by electroformation33, with some modifications55. Phospholipids 2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) and 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(lissamine rhodamine B sulfonyl) (Liss Rod PE) were purchased from Avanti Polar Lipids, Inc. (Alabaster, AL, USA). DOPC GUVs were diluted in chloroform at a concentration of 10−3 mol/L. Fluorescent GUVs were obtained by adding 0.5 mol% of Liss Rod PE. Next, 10 μL of the chloroform solution containing the lipid mixture was spread on the surfaces of two conductive Indium tin oxide-coated slides, which were then assembled to form a growing chamber separated by a 2-mm-thick Teflon frame. After drying under vacuum, the chamber was filled with 260 mOsm kg−1 sucrose solution that contained 3 × 10−3 mol/L cysteine [GUV (Cyst 3 mM)] or not [GUV]. The osmolarity of the sucrose solution was set to match the osmolarity of the mSOF medium (260 mOsm kg−1) measured with an osmometer (Osmomat 3000). The chamber was then connected to an alternating power generator (Gerador de Função Digital Minipa MFG-4202, Minipa do Brasil Ltda) operating at 1 V and frequency of 10 Hz for 3 h.
The GUVs were evaluated under an inverted fluorescence microscope (Nikon Eclipse Ti-S) equipped with a 40× objective using excitation (515 to 575 nm) and emission (50 to 80 nm) filters. The images obtained with the Nikon NIS-Elements AR Software (version 3.0) were subsequently evaluated using the Image J program.
Oocyte collection and maturation
Cumulus-oocyte complexes (COCs) were obtained from cattle ovaries collected at a local abattoir by aspirating antral follicles (3–8 mm in diameter) with an 18-gauge needle attached to a 10-mL syringe. COCs with at least four layers of cumulus cells with homogenous cytoplasm were selected for the experiments. The selected COCs were washed and cultured in 500 μL of IVM medium (30–40 oocytes per well) in a 4-well dish (Nunc, Nunclon Delta Treated 4-Well IVF Dish, Denmark), without mineral oil covering, for 22 h, at 38.5 °C under an atmosphere of 5% CO2 in air at maximum humidity.
In vitro fertilization and embryo culture
Following IVM, oocytes were cultured for 18 h in 500 μL IVF-TALP containing spermatozoa. Motile sperm were obtained by centrifuging frozen-thawed semen through a discontinuous Percoll (GE Healthcare, Uppsala, Sweden) density gradient (45% over 90%) at 2,500 g for 5 min at room temperature. Sperm were diluted in IVF-TALP to achieve a final concentration of 1 × 106/mL in the fertilization well. After removal of cumulus cells, the presumptive zygotes were transferred to 500 μL mSOF medium (25–30 zygotes per well) in a 4-well plate, without mineral oil covering. Embryos were cultured at 38.5 °C in a humidified atmosphere of 5% CO2 in air for up to 7 days. The day of in vitro fertilization (IVF) was defined as day 0 (D0). The cleavage rate was assessed at 72 h post-insemination (D3) and the blastocyst rate at 168 h post-insemination (D7). The D7 blastocyst rate was determined based on the number of inseminated oocytes.
Oxidative stress detection
Intracellular ROS levels in D7 blastocysts were quantified using a fluorescent probe (CellROX Green Reagent, Thermo Fisher Scientific, Waltham, MA, USA). The embryos were stained for 30 min in the dark at 38.5 °C in an atmosphere of 5% CO2 in air. Next, the embryos were washed twice in PBS, fixed with 4% paraformaldehyde for 15 min, and washed again with PBS. The embryos were permeabilized with 0.5% Triton X-100 in 0.1% sodium citrate for 10 min. The DNA of the embryos was stained with Hoechst 33342 (1 μg/mL) for 15 min. Stained embryos were washed twice with PBS and observed immediately with a 40× objective under an Olympus IX51 inverted microscope running the Q-Capture Pro Image software (Media Cybernetics, Inc., Version 5.0.1.26) at excitation and emission wavelengths, respectively, of 495 and 520 nm (green emission filter) and 404 and 526 nm (blue emission filter) to quantify the fluorescence signal intensities (pixels). The background signal intensity was subtracted from the measured values of the treated photomicrographs. For each embryo, the CellROX staining intensity was normalized to the DNA. The mean relative expression values were expressed as arbitrary units of fluorescence (AUF).
Experimental design
Experiment 1: effect of menadione on oxidative stress induction in in vitro cultured GUVs
GUVs were electroformed and diluted in mSOF (20%, v/v). From this solution, 500 μL was transferred to 4-well plates and increasing concentrations of menadione (1.0, 2.5, 5.0, and 7.5 µM) were added to each well. In the control group, GUVs were cultured in the absence of menadione. The plates were incubated for up to 168 h at 38.5 °C in a 5% CO2 atmosphere at maximum humidity. The diameter (µm) of GUVs was measured at the beginning of culture (D1) and after 72 (D3) and 168 h (D7) of culture.
Experiment 2: effect of hydrogen peroxide on oxidative stress induction in in vitro cultured GUVs
GUVs were electroformed and diluted in mSOF (20%, v/v), as described for Experiment 1. From this solution, 500 μL was transferred to 4-well plates and increasing concentrations of H2O2 (0.01, 0.05, 0.1, 0.5, and 1.0 mM) were added to each well. The plates were incubated for up to 168 h at 38.5 °C in a 5% CO2 atmosphere at maximum humidity. The diameter (µm) of GUVs was measured at the beginning of culture (D1) and after 72 (D3) and 168 h (D7) of culture.
Experiment 3: induction of oxidative stress by menadione in embryos cultured in vitro in the presence of GUVs
In the first series of this experiment, presumptive zygotes produced in vitro were removed from the fertilization droplets on D1, washed, and transferred in groups of 25–30 zygotes per well to 4-well plates in 500 μL mSOF without (control, n = 219) or with 5.0 μM menadione supplementation from D6 to D7 (MD 5.0 μM, n = 172). The embryos were cultured for 7 days and the expanded D7 blastocysts were stained for oxidative stress detection.
In another experiment, the presumptive zygotes were removed from the fertilization droplets on D1, washed, and transferred in groups of 25–30 zygotes per well to 4-well plates in 500 μL mSOF according to the following treatments: (1) control (n = 100): embryos cultured in mSOF; (2) GUV (n = 104): embryos cultured in mSOF plus 20% (v/v) GUVs; (3) GUV + MD 5.0 μM (n = 104): embryos cultured as described for the GUV group and supplemented with 5.0 μM menadione from D6 to D7, and 4) GUV + MD 7.5 μM (n = 100): embryos cultured as described for the GUV group and supplemented with 7.5 μM menadione from D6 to D739. The embryos were cultured for 7 days without medium changes (feeding) during culture since one of the objectives of this experiment was to evaluate the morphometry and proportion of remnant GUVs at the end of culture. The diameter and quantity (expressed as percentage) of GUVs were evaluated at the beginning (D1) and end of culture (D7). The percentage of GUVs on D7 was determined in relation to the total number of GUVs present on D1. Blastocysts obtained on D7 were evaluated regarding intracellular ROS levels. The culture system was defined as embryotoxic when there was a significant decrease in the blastocyst rate compared to control.
Experiment 4: evaluation of the effectiveness of GUVs as cysteine carriers in in vitro culture medium of bovine embryos
Presumptive zygotes were transferred in groups of 25–30 zygotes per well to 4-well plates in 500 μL mSOF and cultured according to the following treatments: (1) control (n = 111): culture in mSOF; (2) Cyst (n = 112): culture in mSOF supplemented with 0.6 mM cysteine from D1 to D7; (3) MD (n = 111): culture in mSOF supplemented with 5.0 μM menadione from D6 to D7; (4) MD + Cyst (n = 110): culture in mSOF supplemented with 0.6 mM cysteine from D1 to D7 and with 5.0 μM menadione from D6 to D7; (5) GUV(Cyst 3 mM) (n = 116): culture in mSOF plus 20% (v/v) GUVs electroformed with 3 mM cysteine (from D1 to D7); (6) GUV(Cyst 3 mM) + MD (n = 113): culture in mSOF plus 20% (v/v) GUVs electroformed with 3 mM cysteine (from D1 to D7) and supplemented with 5.0 μM menadione from D6 to D7. The embryos were cultured at 38.5 °C in a 5% CO2 atmosphere in air at maximum humidity for up to 7 days. Since one of the objectives of this experiment was to evaluate the morphometry and proportion of remnant GUVs at the end of culture, no medium changes (feeding) were performed during the culture period. Blastocysts obtained on D7 were evaluated regarding intracellular ROS levels.
Statistical analysis
The experiment was repeated at least three times for each proposed evaluation. The data were analyzed by ANOVA using the JMP 5.0.1a software (SAS Institute, Inc., Cary, NC, USA). Percentage data were arcsine transformed before being submitted to ANOVA when necessary. If a statistically significant effect was found, means were compared by Tukey’s multiple comparisons test. Differences with probabilities (P) less than 0.05 were considered to be significant. Values are reported as the mean ± standard error of the mean (SEM).
Data availability
All data generated or analyzed during this study are included in this published article.
References
Wu, B. & Zan, L. Enhance beef cattle improvement by embryo biotechnologies. Reprod. Domest. Anim. 4, 865–871 (2012).
Viana, J. 2019 Statistics of embryo production and transfer in domestic farm animals: Divergent trends for IVD and IVP embryos. Embryo Technol. Newsl. 38(4), 1–15 (2020).
Rizos, D., Ward, F., Duffy, P., Boland, M. P. & Lonergan, P. Consequences of bovine oocyte maturation, fertilization or early embryo development in vitro versus in vivo: Implications for blastocyst yield and blastocyst quality. Mol. Reprod. Dev. 61, 234–248 (2002).
Guérin, P., Mouatassim, S. E. L. & Ménézo, Y. Oxidative stress and protection against reative oxygen species in the pre-implantation embryo and its surroundings. Hum. Reprod. Update 7, 175–189 (2001).
Wang, F. et al. Beneficial effects of melatonin on in vitro bovine embryonic development are mediated by melatonin receptor 1. J. Pineal Res. 56, 333–342 (2014).
Goto, K., Noda, Y., Mori, T. & Nakano, M. Increased generation of reactive oxygen species in embryos cultured in vitro. Free Radic. Biol. Med. 15, 6975 (1993).
Bae, Y. S., Oh, H., Rhee, S. G. & Yoo, Y. D. Regulation of reactive oxygen species generation in cell signaling. Mol. Cells 32(6), 491–509 (2011).
Hebbar, S. & Knust, E. Reactive oxygen species (ROS) constitute an additional player in regulating epithelial development. BioEssays 43, 2100096 (2021).
Zhao, Y. et al. ROS signaling under metabolic stress: Cross-talk between AMPK and AKT pathway. Mol. Cancer 16, 1–12 (2017).
Basini, G., Simona, B., Sujen, E. S. & Grasselli, F. Reactive oxygen species and anti-oxidant defences in swine follicular fluids. Reprod. Fertil. Dev. 20, 269–274 (2008).
Dowling, D. K. & Simmons, L. W. Reactive oxygen species as universal constraints in life-history evolution. Proc. R. Soc. B Biol. Sci. 276, 1737–1745 (2009).
Feugang, J. M. et al. Effect of prooxidant agents added at the morula/blastocyst stage on bovine embryo development, cell death and glutathione content. Zygote 11, 107–125 (2003).
Tamura, H. et al. The role of melatonin as an antioxidant in the follicle. J. Ovarian Res. 5, 1–9 (2012).
Ali, A. A., Bilodeau, J. F. & Sirard, M. A. Antioxidant requirements for bovine oocytes varies during in vitro maturation, fertilization and development. Theriogenology 59, 939–949 (2003).
Rocha-Frigoni, N. A. S., Leão, B. C., Nogueira, É., Accorsi, M. F. & Mingoti, G. Z. Reduced levels of intracellular reactive oxygen species and apoptotic status are not correlated with increases in cryotolerance of bovine embryos produced in vitro in the presence of antioxidants. Reprod. Fertil. Dev. 26, 797–805 (2014).
Pioltine, E. M. et al. Treatment of in vitro-matured bovine oocytes with tauroursodeoxycholic acid modulates the oxidative stress signaling pathway. Front. Cell Dev. Biol. 9, 623852 (2021).
Takahashi, M. et al. Effect of thiol compounds on in vitro development and intracellular glutathione content of bovine embryos. Biol. Reprod. 49, 228–232 (1993).
De Matos, D. G., Furnus, C. C. & Moses, D. F. Glutathione synthesis during in vitro maturation of bovine oocytes: Role of cumulus cells. Biol. Reprod. 57, 1420–1425 (1997).
Rocha-Frigoni, N. A. S., Leão, B. C., Nogueira, É., Accorsi, M. F. & Mingoti, G. Z. Effects of gaseous atmosphere and antioxidants on the development and cryotolerance of bovine embryos at different periods of in vitro culture. Zygote 23, 159–168 (2013).
Johnson, M. H. & Nars-Esfahani, M. H. Radical solutions and cultural problems: Could free oxygen radicals be responsible for the impaired development of preimplantation mammalian embryos in vitro?. BioEssays 16, 31–39 (1994).
Rathbun, W. B. & Murray, D. L. Age-related cysteine uptake as rate-limiting in glutathione synthesis and glutathione half-life in the cultured human lens. Exp. Eye Res. 53, 205–212 (1991).
Sagara, J., Miura, K. & Bannai, S. Cystine uptake and glutathione level in fetal brain cells in primary culture and suspension. J. Neurochem. 61, 1667–1671 (1993).
De Matos, D. G., Furnus, C. C., Moses, D. F., Martinez, A. G. & Matkovic, M. Stimulation of glutathione synthesis of in vitro matured bovine oocytes and its effect on embryo development and freezability. Mol. Reprod. Dev. 45, 451–457 (1996).
Walde, P., Cosentino, K., Engel, H. & Stano, P. Giant vesicles: Preparations and applications. ChemBioChem 11, 848–865 (2010).
Sanderson, J. Resolving the kinetics of lipid, protein and peptide diffusion in membranes. Mol. Membr. Biol. 29, 118–143 (2012).
Watkins, R., Ling, W., Zhang, C., Davis, R. M. & Xu, B. Natural product-based nanomedicine: Recent advances and issues. Int. J. Nanomed. 10, 6055–6074 (2015).
Lasic, D. D. The mechanism of vesicle formation. Biochem. J. 256, 1–11 (1988).
Bhatia, T. et al. Preparing giant unilamellar vesicles (GUVs) of complex lipidmixtures on demand: Mixing small unilamellar vesicles of compositionally heterogeneous mixtures. Biochem. Biophys. Acta 1848, 3175–3180 (2015).
Jiménez, M., Martos, A., Cabré, E. J., Raso, A. & Rivas, G. Giant vesicles: A powerful tool to reconstruct bacterial division assemblies in cell-like compartments. Environ. Microbiol. 15, 3158–3168 (2013).
Itri, R., Junqueira, H. C., Mertins, O. & Baptista, M. S. Membrane changes under oxidative stress: The impact of oxidized lipids. Biophys. Rev. 6, 47–61 (2014).
Runas, K. A. & Malmstadt, N. Low levels of lipid oxidation radically increase the passive permeability of lipid bilayers. Soft Matter 11, 499–505 (2015).
Junqueira, H. et al. Molecular organization in hydroperoxidized POPC bilayers. BBA Biomembr. 1863(10), 183659 (2021).
Aoki, P. H. B., Schroder, A. P., Constantino, C. J. L. & Marques, C. M. Bioadhesive giant vesicles for monitoring hydroperoxidation in lipid membrane. Soft Matter 11, 5995–5998 (2015).
Aoki, P. H. B. et al. Molecular-level modifications induced by photo-oxidation of lipid monolayers interacting with erythrosin. Langmuir 32(15), 3766–3773 (2016).
Caetano, W. et al. Photo-induced destruction of giant vesicles in methylene blue solutions. Langmuir 23, 1307–1314 (2007).
Riske, K. A. et al. Giant vesicles under oxidative stress induced by a membrane-anchored photosensitizer. Biophys. J. 97, 1362–1370 (2009).
Mertins, O. et al. Physical damage on giant vesicles membrane as a result of methylene blue photoirradiation. Biophys. J. 106, 162–171 (2014).
Weber, G. et al. Lipid oxidation induces structural changes in biomimetic membranes. Soft Matter 10(24), 4241–4247 (2014).
Moss, J. I., Pontes, E. & Hansen, P. J. Insulin-like growth factor-1 protects preimplantation embryos from anti-developmental actions of menadione. Arch. Toxicol. 83, 1001–1007 (2009).
Circu, M. L., Maloney, R. E. & Aw, T. Y. Disruption of pyridine nucleotide redox status during oxidative challenge at normal and low-glucose states: Implications for cellular adenosine triphosphate, mitochondrial respiratory activity, and reducing capacity in colon epithelial cells. Antioxid. Redox Signal. 14, 2151–2162 (2011).
Monks, T. J., Hanzlik, R. P., Cohen, G. M., Ross, D. & Graham, D. G. Quinone chemistry and toxicity. Toxicol. Appl. Pharmacol. 112, 2–16 (1992).
Heuvingh, J. & Bonneau, S. Asymmetric oxidation of giant vesicles triggers curvature-associated shape transition and permeabilization. Biophys. J. 97, 2904–2912 (2009).
Gaschler, M. M. & Stockwell, B. R. Lipid peroxidation in cell death. Biochem. Biophys. Res. Commun. 482(3), 419–425 (2017).
Bacellar, I. O. L. et al. Permeability of DOPC bilayers under photoinduced oxidation: Sensitivity to photosensitizer. BBA Biomembr. 1860, 2366–2373 (2018).
Pereira, L. S. A. et al. Evidence of photoinduced lipid hydroperoxidation in Langmuir monolayers containing Eosin Y. Colloids Surf. B Biointerfaces 171, 682–689 (2018).
Moreira, L. G. et al. Chain cleavage of bioinspired bacterial membranes photoinduced by eosin decyl ester. Langmuir 36, 9578–9585 (2020).
Dimova, R. & Marques, C. M. The Giant Vesicle Book (CRC Press, 2020).
Buhimschi, I. A., Kramer, W. B., Buhimschi, C. S., Thompson, L. P. & Weiner, C. P. Reduction-oxidation (redox) state regulation of matriz metalloproteinase activity in human fetal membranes. Am. J. Obstet. Gynecol. 182, 458–464 (2000).
Marshall, H. E., Merchant, K. & Stamler, J. S. Nitrosation and oxidation in the regulation of gene expression. FASEB J. 14, 1889–1900 (2000).
Van Soom, A. et al. Prevalence of apoptosis and inner cell allocation in bovine embryos cultured under different oxygen tensions with or without cysteine addition. Theriogenology 57, 1453–1465 (2002).
Iwata, H., Akamatsu, S., Minami, N. & Yamada, M. Effect of antioxidants on development of bovine IVM/IVF embryos in various concentration of glucose. Theriogenology 50, 365–375 (1998).
Harvey, A. J. The role of oxygen in ruminant preimplantation embryo development and metabolism. Anim. Reprod. Sci. 98, 113–128 (2007).
Merton, J. S. et al. Cysteamine supplementation during in vitro maturation of slaughterhouse and opu-derived bovine oocytes improves embryonic development without affecting cryotolerance, pregnancy rate, and calf characteristics. Theriogenology 80, 365–371 (2013).
Zhou, Z. et al. Using cysteine/cystine to overcome oxidative stress in goat oocytes and embryos cultured in vitro. Mol. Med. Rep. 14, 1219–1226 (2016).
Mingoti, G. Z., Nogueira, M. F. G., Aoki, P. H. B. & Rossi, L. T. R. Método para encapsulamento de antioxidantes dentro de vesículas unilamelares gigantes como carregadoras de antioxidantes em sistemas de produção in vitro de embriões de mamíferos. BR 10 2020 025567 3 (2020).
Acknowledgements
This work was supported by São Paulo Research Foundation (FAPESP, Brazil; Grants #2019/11174-6, #2018/16713-0 and #2012/50533-2), National Council for Scientific and Technological Development (CNPq, Brasil; Grants #314136/2018-5 and #403713/2016-1) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil: Finance Code 001).
Author information
Authors and Affiliations
Contributions
L.T.R.R. carried out the experiment, participated in the data collection and drafted the manuscript. G.B.N. carried out the experiment. C.R.S. carried out the experiment. H.R. carried out the experiment. P.H.S. carried out the experiment. M.F.G.N. conceived and designed the experiments and contributed with reagents/materials/analysis tools, analysis and interpretation of data, acquisition of funding and preparation of the final version of the manuscript. P.H.B.A. conceived and designed the experiments and contributed with reagents/materials/analysis tools, analysis and interpretation of data, acquisition of funding and preparation of the final version of the manuscript. G.Z.M. conceived and designed the experiments and contributed with reagents/materials/analysis tools, analysis and interpretation of data, acquisition of funding and preparation of the final version of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors GZ Mingoti, MFG Nogueira, PHB Aoki and Rossi LTR declare that they hold a patent of the method developed for encapsulation of antioxidants within giant unilamellar vesicles as carriers of antioxidants in in vitro mammalian embryo production systems (Método para encapsulamento de antioxidantes dentro de vesículas unilamelares gigantes como carregadoras de antioxidantes em sistemas de produção in vitro de embriões de mamíferos. 2020; BR 10 2020 025567 3). The other authors have no conflict of interest to disclose.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rossi, L.T.R., Nunes, G.B., da Silva, C.R. et al. Use of giant unilamellar lipid vesicles as antioxidant carriers in in vitro culture medium of bovine embryos. Sci Rep 12, 11228 (2022). https://doi.org/10.1038/s41598-022-14688-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-14688-8
This article is cited by
-
Design of double functionalized carbon nanotube for amphotericin B and genetic material delivery
Scientific Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.