Abstract
Vertebrate growth can be phenotypically plastic in response to predator–prey and competitive interactions. It is unknown however, if it can be plastic in response to mutualistic interactions. Here we investigate plasticity of vertebrate growth in response to variation in mutualistic interactions, using clown anemonefish and their anemone hosts. In the wild, there is a positive correlation between the size of the fish and the size of the anemone, but the cause of this correlation is unknown. Plausible hypotheses are that fish exhibit growth plasticity in response to variation in food or space provided by the host. In the lab, we pair individuals with real anemones of various sizes and show that fish on larger anemones grow faster than fish on smaller anemones. By feeding the fish a constant food ration, we exclude variation in food availability as a cause. By pairing juveniles with artificial anemones of various sizes, we exclude variation in space availability as a single cause. We argue that variation in space availability in conjunction with host cues cause the variability in fish growth. By adjusting their growth, anemonefish likely maximize their reproductive value given their anemone context. More generally, we demonstrate vertebrate growth plasticity in response to variation in mutualistic interactions.
Similar content being viewed by others
Introduction
A fundamental question of evolutionary ecology is, what determines body size? While the growth and size of vertebrates is thought to be relatively fixed compared to that of invertebrates, plasticity of vertebrate growth and size has been known to occur in response to a wide variety of both abiotic and biotic conditions1. Abiotic conditions and ecological interactions (competition and predation) have all been known to elicit plasticity of vertebrate growth1,2,3,4,5. Understanding this plasticity of growth is essential for understanding relationships between organisms and their biotic and abiotic environments6. Changes in growth rate are not always a simple consequence of changing energetics (e.g., temperature or resource availability), but rather growth rate can also be adjusted in anticipation of changing circumstances. For example, shrews of the genus Sorax change their skull sizes in anticipation of seasonal changes7,8, crucian carp (Carassius carassius) increase their body depth when in the presence of their predator, piscivorous pike (Esox lucius)2; and, Kalahari meerkats (Suricata suricatta) increase their growth rates when the growth rate of reproductive competitors is artificially increased5. To our knowledge, there are no documented examples of vertebrate growth plasticity in response to variation in mutualistic interactions.
Mutualisms are species interactions where the inclusive fitness of each party is increased through actions of its partner9. Mutualisms are common, crossing all domains of life and playing a central role in ecosystem evolution10. Many mutualistic partners are size matched in the wild and often the apparent good fit between one partner and the other may be adaptive. For example, fig pollinator wasps cannot grow too large as to not fit through the fig’s ostiole, even though larger wasps are more successful disperses11. Similarly, extinct large-bodied frugivores were size matched to the large seeds they dispersed over long-distances12. While there are examples of invertebrates, vertebrates, and plants evolving to match each other’s size13,14, there are no examples of plasticity in response to mutualism.
A tractable system to test whether vertebrate growth can be plastic in response to a mutualistic partner is the well-known mutualism between anemonefishes of the genus Amphiprion and their cnidarian host15,16,17. Anemonefishes occupy anemones in groups of 2–13 individuals, organized in size-based dominance hierarchies18,19. The anemone provides the fish and their eggs with protection from predators20,21, and the fish provide the anemone with protection from predators too15. Nutrients are also transferred from the host anemone and symbiotic zooxanthellae to the anemonefish22, and from anemonefish to the host23. Across the Amphiprion genus, there is a widespread correlation between anemone size and fish size, both the size of the dominant individual and the summed standard lengths of the whole group15,19,24,25,26,27,28. It is possible that the correlation between fish and anemone size is a simple product of variation in food or space availability or that the fish can regulate their growth in an anticipatory fashion to maximize their reproductive value on a given anemone.
Here, we investigated whether vertebrate growth can be plastic in response to variation in the size of a mutualistic partner. We used the clown anemonefish, Amphiprion percula, which has been shown to exhibit growth plasticity in response to competition during social group formation and maintenance19,29. In A. percula, the correlation between host size and fish size is unlikely to be a result of variation in mortality since mortality rates of A. percula do not vary with anemone size30. Also, A. percula do not switch anemone hosts once they are settled31, excluding the possibility of larger fish usurping larger hosts. In this study we first tested the hypothesis that the standard length of dominant rank one A. percula is correlated with the size of their anemone host, using data from a wild population in Papua New Guinea. Second, we tested the hypothesis that anemonefish growth will be positively correlated with anemone size, using real anemones in the laboratory. Third, we tested alternative hypotheses for the cue of the observed effect (food and space availability), using fake anemones in the laboratory. We found that vertebrate growth can indeed be plastic in response to variation in a mutualistic interaction and we found that space availability together with a biological cue from the host are the most likely trigger for growth plasticity in the anemonefish-anemone mutualism.
Materials and methods
Field measurements
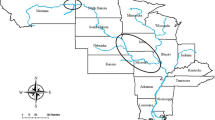
To test the correlation between anemone size and the size of A. percula (hypothesis 1), we conducted a field study in November and December 2019 on inshore reefs near Mahonia Na Dari Research and Conservation Centre in Kimbe Bay, Papua New Guinea. We located 202 groups of Amphiprion percula on Heteractis magnifica on 15 reefs. All individual fish in each group were caught using hand nets and their standard length was measured using calipers to the nearest 0.1 mm. Rank was determined based on size, as this species maintains a strict size ratio between ranks19. The fish were returned to their host following measurement. Anemone oral disks were measured using soft tailors’ tape, measuring to the nearest cm from tentacle tip to tentacle tip on the longest diameter and again from tentacle tip to tentacle tip on the diameter perpendicular to the longest diameter. To account for expansion behavior, anemones were measured three times on three different days and the measurements were averaged. Anemone area was calculated as the area of an ellipse (A = π*a*b, with A anemone area in cm2, a the major axis and b the minor axis). The study is reported according to ARRIVE guidelines.
Lab experiments
To test whether anemonefish growth is positively related to anemone size (hypothesis 2) and whether this is a response to space availability (hypothesis 3), experiments were conducted at Boston University (Boston, MA, USA) from January 2020 to June 2021 using the clown anemonefish, Amphiprion percula. Experimental protocols were approved by Boston University’s Institutional Animal Care and Use Committee (protocol number 17-001). All fish used in this experiment were reared from fish that were caught as non-breeders (less than 30 mm in standard length) in Papua New Guinea and supplied by Quality Marine and Sea Dwelling Creatures. All breeding pairs were paired in the lab and had been breeding for more than 5 years. A detailed description of broodstock housing conditions can be found elsewhere32,33. Fish used in the experiment were offspring of these breeding pairs and were reared for 28 days (±1 day), at which time they were moved into juvenile housing.
Juvenile housing
All experimental juvenile fish for each of the two experiments were housed in separate 113.5 L tanks with identical set up. Each tank had a 15 cm2 ceramic tile, with a reef rock, a few anemones (Entacmaea quadricolor), and a breeding pair of clownfish. At the opposite end of the tank, at a distance of approximately 20 cm, we placed a mesh cylinders (0.16 or 0.32 cm mesh), with a radius of 10.16 cm and a height of 30.5 cm, that exceeded the water level (~ 27 cm), creating a segregated space for our experimental juveniles and their anemones. Fish were introduced and covered by a 40 oz food storage container with drilled in holes covered by mesh until they settled into their anemone. Introduction chambers were removed after 24 h, leaving the fish free to move about the cylinders, though mostly they stayed nestled in their anemone tentacles.
Experiment 1
To test whether anemonefish growth is positively related to anemone size (hypothesis 2), we placed similar sized fish into anemones (Entacmaea quadricolor) of varying sizes. A single juvenile clownfish and one anemone were paired haphazardly, so that initial fish size was unrelated to initial anemone size. Experiment 1 was conducted over six months between January and June 2020 (ninitial = 44, nend = 25). Experimental juvenile fish were from two sets of parents.
To rule out the possibility that variation in growth is a response to variation in food availability in anemones of different sizes, as might be the case in the wild, the amount of food that the fish received was controlled: fish were fed to satiation using a rationed diet of 0.25 ml of granulated pellets dressed with Haematococcus, once a day, seven days a week. Subsequently the size of anemones and growth of fish was monitored each month for six months.
Experiment 2
To test whether anemonefish growth is simply a response to the variation in space provided by the anemone tentacles (hypothesis 3), we placed similar sized fish into artificial anemones of varying sizes. Experiment 2 was conducted over six months between September 2020 and June 2021 (ninitial = 87, nend = 24). Since the mortality of juvenile fish in artificial anemones was higher than in experiment 1 (see Supplemental Material and Results), juveniles from six clutches, i.e. six separate parent pairs, were used in experiment 2.
The artificial anemones were made of silicone with a weighted base (Tetra Blooming Collection Anemone Aquarium Decor, White). We manipulated the size of artificial anemones, to have 10 sizes by cutting the artificial tentacles to match the sizes of the real anemones at the start of experiment 1. The feeding regime was the same as in experiment 1 above. If juveniles died, they were removed from the tanks along with their anemones and housing units. Their data were only used until the previous month measured.
Anemone metrics
Anemone measurements for real anemones were made four times per month, at one-week intervals, over the course of the experiment. Artificial anemones were measured each month to account for possible wear of the silicone. Photographs were taken using an underwater camera (GoPro Hero7) from a position that maximized visible tentacle breadth including a 1 × 1 inch card for scale. ImageJ34 software was used to trace the perimeter of all live tissue (or equivalent in the case of artificial anemones) and estimate surface area of the anemone. Multiple measures of each anemone were taken to account for expansion behavior and growth across the month, and the average of these measures was used as an estimate of anemone size each month. Two real anemones fissioned during the study, and they were removed from the dataset for all subsequent months.
Fish metrics
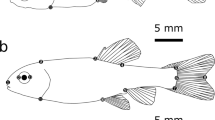
Fish measurements were made seven times, at four-week intervals, over the course of both experiments (t0, t1, t2, t3, t4, t5, t6). To measure them, fish were removed from their tank and placed in a small cylinder filled with tank water for transport. They were then picked up from the cylinder using a plastic spoon and briefly placed on a microscope slide with an engraved 1 mm gridline system as a scale. The fish were photographed laterally using a stereoscopic microscope (Nikon SMZ745: 7.5 × zoom and 115 mm working distance) with a camera (Canon EOS60D). ImageJ was used to determine the standard length (SL; length measured from the distance between the tip of the snout to the last vertebrae) to 0.01 mm from each photograph three times. The average of the three measurements for SL were used as the monthly measurement for each individual. After being placed back in their cylinders, all fish swam directly to their anemones. Fish growth per month was calculated as the difference in size between two time points.
Statistical analysis
All statistical analyses were conducted using R version 3.6.135. To test the correlation between anemone size and fish size in the wild we used Pearson’s correlation coefficient. To test the hypothesis that fish growth will be a plastic response to the size of the anemone we conducted Bayesian mixed model analyses on data from experiment 1 and 2 using the rstanarm package weakly informative default priors36. We used fish growth per month (change in SL) as the dependent variables and average anemone size for each month as the independent variable of primary interest. Average anemone size each month was ln (loge) transformed to improve model fit. We controlled for the effect of initial fish size at the beginning of each month, because fish growth is often negatively correlated with size37. Fish ID was used as a random effect to account for repeated measures of the same individuals and non-independence of data points. Fit of the models was checked using the shinystan package38. Bayesian R2 was calculated using the performance package39,40. Model plots were produced using the bayesplot package41,42. Diagnostic plots for each model can be found in the supplementary material (suppl. Figure 2–7). We also tested the interaction between the fixed factors by conducting a log likelihood test between the model with the interaction and the model without the interaction.
Results
Size correlation in the wild (hypothesis 1)
The standard length of rank 1 A. percula was positively correlated with the size of their anemone host in the wild (H. magnifica) (Fig. 1, Pearson’s r = 0.765, t = 16.816, p < 0.001).
Growth plasticity (Hypotheses 2 and 3)
At the start of experiment 1, the mean (±SE) standard length (SL) of juvenile A. percula was 10.86 ± 0.19 mm, the mean (± standard error (SE)) anemone size (E. quadricolor) was 12.32 ± 1.44 cm2 (range 0.96–46.32 cm2), and initial fish size was unrelated to initial anemone size (Pearson Correlation: r = −0.089, p = 0.563).
The size of real anemones in the laboratory had a significant positive effect on fish growth per month (beta = 0.81, standard deviation (SD) = 0.15, 95% credible interval (CI) [0.51, 1.11]; Fig. 2a, b). A fish on an anemone that was 50% larger grew 0.33 mm more per month on average. Initial standard length had a significant negative effect on fish growth, with larger fish growing slower (beta = −0.25, SD = 0.02, 95% CI [−0.29, −0.21]; Fig. 2a). The fixed effects explained 41% of variation in the data (Bayes R2 marginal: 0.41, 89% CI 0.339, 0.478).
Results for two Bayesian linear mixed models testing the effect of initial standard length (SL) and Ln anemone area on growth of Amphiprion percula: (a) Posterior distributions with medians and 95% intervals for experiment 1, where fish were paired with Entacmaea quadricolor; (b) Posterior distributions with medians and 95% intervals for experiment 2, where fish were paired with artificial anemones; (c) Predicted change in standard length (mm) dependent on Ln anemone area (cm2) for A. percula on E. quadricolor; (d) Predicted change in standard length (mm) dependent on Ln anemone area (cm2) for A. percula on artificial anemones.
At the start of the experiment 2 the mean (±SE) SL of the juveniles was 9.63 ± 0.21 mm, the mean (±SE) of artificial anemone size was 28.26 ± 2.02 cm2 (range 6.89–53.09) and initial fish size was unrelated to initial anemone size (Pearson Correlation: r = −0.022, p = 0.811).
The size of artificial anemones had no effect on fish growth (beta = −0.16, SD = 0.37, 95% CI [−0.87, −0.58]; Fig. 2b, d). Initial standard length had a slight positive effect on fish growth (beta = 0.04, SD = 0.04, 95% CI [−0.04, −0.12]; Fig. 2b). The fixed effects explained 4% of variation in the data (Bayes R2 marginal: 0.038, 89% CI 0.000016, 0.086). Not only did fish in artificial anemones not exhibit a plastic response to anemone size, but their demographic rates were different in other ways too. Survival was much lower for fish in artificial anemones compared to those paired with real anemones (suppl. Figure 1a). Growth was much lower and more variable for fish in artificial anemones compared to those paired with real anemones (suppl. Figure 1b).
Discussion
Our experiments showed that vertebrate growth can be plastic in response to variation in a mutualistic interaction. In one of the most well-studied marine mutualisms between fish of the genus Amphiprion and tropical sea anemones, there is a well-documented positive correlation between host anemone size and the size of the largest fish16,19,28,43. We confirmed this relationship for a wild population of Amphiprion percula in Kimbe Bay, Papua New Guinea. Amphiprion percula is the most site restricted member of the genus and rarely strays beyond the periphery of their anemone’s tentacles44,45, so the correlation could be a result of phenotypic plasticity in response to food or space availability. In our experiment, juvenile A. percula had growth rates that were positively related to the size of their anemone hosts. This growth plasticity was not a response to variation in food availability alone, since we fed fish the same amounts of food. Further, growth plasticity was likely not a response to variation in space availability alone, since it did not occur when juveniles were paired with artificial anemones. It is likely that another biological aspect of the mutualism, such as egesta produced by the anemone or cues necessary for the fish to assess that they are in suitable habitat, caused the observed phenotypic plasticity.
One potential explanation for the results is that the egesta produced by the anemone provided nutrients not provided by the pellet food and that larger anemones provided more nutrients leading to increased growth rates. In the wild and in laboratory settings where anemones are fed heterotrophic food, anemones expel undigested waste products in the form of egesta. It is known that anemonefishes compete aggressively for and consume anemone egesta in the wild (personal observations) and recent studies have shown that anemone egesta provide a substantial and predictable source of nutrients such as carbon and nitrogen for anemonefishes22. However, in our experiments we did not feed anemones, though some fish food might have been consumed by anemones, and we did not observe any anemones producing egesta. Other members of our lab group have not observed this laboratory population of A. percula consuming egesta or this population of E. quadricolor producing egesta in hundreds of hours of video analysis (Barbasch and Pacaro, pers. comm. 2021). Therefore, it is unlikely that the anemonefish in experiment 1 received different nutrients from the fish in experiment 2. Some other biological characteristic of the real anemones that was not present in the artificial anemones must provide the cue for growth plasticity in anemonefish.
Another, more likely, explanation for our results is that the anemonefish only respond to available space in conjunction with cues that indicate suitable habitat, i.e., real anemones. We found that A. percula juveniles paired with artificial anemones had lower survival compared to those paired with real anemones, and those that did survive grew slower and had more variable growth rates than those paired with real anemones. This raises the question of whether the pairing with real anemones is necessary for the fish to develop normally. All 28 species of anemonefish are obligate mutualists with one or more species of sea anemone, never found without their hosts in the wild [see reviews in15,20,45]. Changes in the host anemone, such as bleaching due to thermal stress, can have cascading effects on anemonefishes, including enhanced stress and changes to reproduction, behavior, and metabolism46,47. Mortality rates in juvenile anemonefishes are often high in aquaria [48 and references therein]. Though the reasons are not always known, it seems quite likely that anemones provide cues necessary for typical development of juvenile anemonefishes, and that stress levels and metabolism rates are different in anemonefish lacking anemones, influencing survival and growth patterns as observed in our second experiment. (With the emergence of anemonefishes as a model system for eco-evo-devo (Roux et al. 2020), this is something investigators should be aware of, since many anemonefishes used for research purposes are raised without host anemones and this seems likely to influence results and conclusions of investigations).
Growth plasticity in response to the size of their mutualistic hosts is likely adaptive for anemonefishes, though more research is necessary. On the one hand, selection will favor fish that grow large, since larger size is correlated with lower predation rate and higher reproductive output43,49,50. On the other hand, selection will favor fish that do not become too large for the available resources, since being too large may expose them to predators15 and/or mean that they cannot sustain themselves on available resources51. Amphiprion percula are plankton feeders and restricted in their foraging area by the space provided by the protective anemone tentacles. It would therefore be adaptive for dominant breeders to adjust their growth rate based on the size of their mutualistic host. However, since the current study presents an aquarium experiment and we found a relatively small effect size for the impact of anemone area on fish growth, we must ask ourselves, how relevant are our findings when thinking about wild populations?
To answer this question, we used our model estimates to predict the sizes of anemonefish starting at the same size (10.86 mm, the average size of juveniles in experiment 1) and growing in anemones of different sizes for 12 months (until they asymptote). Using the mean probability estimates of the Bayesian mixed model analyses (Fig. 2a), we calculated change in SL per month as a function of initial SL and ln anemone area: \({\Delta SL}_{t0-1} ={initial\, SL}_{t0}+6.42+\left(0.81*\mathrm{ln}\left(anemone\, area\right)\right)-\left(0.25*{initial\, SL}_{t0}\right).\) The anemone sizes were chosen to be representative of the sizes of anemones we find with A. percula in the wild. The result is that we predict to find fish of significantly different sizes in different anemone sizes (Fig. 3a). Even with our simple model, only taking initial size and anemone size into account, the predicted fish sizes are within the range of those found in wild fish paired with anemones of the sizes used in the calculation (Fig. 3b). Small as the effect of anemone size on growth may seem, over the lifetime of an anemonefish, which can live for several decades52, adapting to be the ideal size for the available host may make a large difference.
(a) Predicted sizes of rank 1 Amphiprion percula over 12 months in anemones of varying sizes (ln 5–ln 10 cm2). Using the mean probability estimates of the Bayesian mixed model analyses (Fig. 2a), change in SL per month was calculated as a function of initial SL and ln anemone area: \({\Delta SL}_{t0-1} ={initial\, SL}_{t0}+6.42+\left(0.81*\mathrm{ln}\left(anemone\,area\right)\right)-\left(0.25*{initial\, SL}_{t0}\right)\). (b) Distribution of rank 1 A. percula SL (mm) and the size of the anemone they reside in (binned, Ln cm2) from a population in Kimbe Bay, Papua New Guinea.
While we provide the first experimental study of the phenomenon, phenotypic plasticity of growth in response to a mutualistic interaction is likely widespread in both vertebrates and invertebrates. Many mutualistic partners are size matched in the wild and often, like we observed in the clown anemonefish, the size of one partner appears to be a compromise between maximizing reproduction and dispersal, and not outgrowing their partner, stabilizing the mutualism. For example, fig pollinator wasps are more successful at dispersal when larger but the fig’s ostiole limits maximum wasp size11. In ant-plant mutualisms, plant growth rates and ant colony growth, as well as plant size and ant body size are often synchronized, suggesting positive feedback loops53,54. Phenotypic plasticity commonly mediates ecological interactions [reviewed by55] and may facilitate evolutionary matching between mutualistic partners53,56,57,58. More studies on mutualistic interactions involving vertebrates may shed light on how common growth plasticity and other forms of developmental plasticity are in this type of species interaction.
Conclusion
In conclusion, we provide the first experimental evidence for vertebrate growth plasticity in response to a mutualistic interaction. We rule out food availability and space availability by itself as possible mechanisms. It is likely that space availability together with a biological cue from the mutualistic host are responsible for the pattern. The ability to anticipate the ideal body size in relation to the mutualistic partner is most likely present in other mutualistic relationships. More research is necessary to understand the intricate interplay of mutualistic partners and the effect on animal growth regulation and developmental phenotypic plasticity.
Data availability
Data will be made fully accessible on Dryad upon publication of the study.
References
Pfennig, D. The adaptive significance of an environmentally-cued developmental switch in an anuran tadpole. Oecologia 85, 101–107 (1990).
Brönmark, C. & Miner, J. G. Predator-induced phenotypical change in body morphology in Crucian carp. Science 258, 1348–1350 (1992).
Wikelski, M. & Thom, C. Marine iguanas shrink to survive El Niño. Nature 403, 37–38 (2000).
Agrawal, A. A. Phenotypic plasticity in the interactions and evolution of species. Science 294, 321–326 (2001).
Huchard, E., English, S., Bell, M. B. V., Thavarajah, N. & Clutton-Brock, T. Competitive growth in a cooperative mammal. Nature 533, 532–534 (2016).
Travis, J. Evaluating the adaptive role of morphological plasticity. In: Ecological morphology (pp. 99–122) (The University of Chicago Press, Chicago, 1994).
Lázaro, J., Dechmann, D. K. N., LaPoint, S., Wikelski, M. & Hertel, M. Profound reversible seasonal changes of individual skull size in a mammal. Curr. Biol. 27, R1106–R1107 (2017).
Lázaro, J. & Dechmann, D. K. Dehnel’s phenomenon. Ecol. Evol. 31, R463–R465 (2021).
Bronstein, J. L. The evolution of facilitation and mutualism. J. Ecol. 97, 1160–1170 (2009).
Leigh, J. The evolution of mutualism. J. Environ. Biol. 23, 2507–2528 (2010).
Liu, C., Yang, D. R. & Peng, Y. Q. Body size in a pollinating fig wasp and implications for stability in a fig-pollinator mutualism. Entomol. Exper. Appl. 138, 249–255 (2011).
Pires, M. M., Guimarães, P. R., Galetti, M. & Jordano, P. Pleistocene megafaunal extinctions and the functional loss of long-distance seed-dispersal services. Ecography 41, 153–163 (2018).
Boucher, D., James, S. & Keeler, K. The ecology of mutualism. Annu. Rev. Ecol. Syst. 13, 315–347 (1982).
Irwin, R. E. & Brody, A. K. Nectar robbing in Ipomopsis aggregata: effects on pollinator behavior and plant fitness. Oecologia 116, 519–527 (1998).
Allen, G. The Anemonefishes: their classification and biology (T.F.H. Publications, 1972).
Fautin, D.G. & Allen, G.R. Field guide to anemonefishes and their host sea anemones. (Western Australian Museum, Perth, 1992).
Ollerton, J., McCollin, D., Fautin, D. G. & Allen, G. R. Finding NEMO: nestedness engendered by mutualistic organization in anemonefish and their hosts. Proc. R. Soc. B Biol. Sci. 274, 591–598 (2006).
Fricke, H. & Fricke, S. Monogamy and sex change by aggressive dominance in coral reef fish. Nature 266, 830–832 (1977).
Buston, P. M. Size and growth modification in clownfish. Nature 424, 145–146 (2003).
Mariscal, R. N. The nature of the symbiosis between Indo-Pacific anemone fishes and sea anemones. Mar. Biol. 6, 58–65 (1970).
Elliott, J. K., Elliott, J. M. & Mariscal, R. N. Host selection, location, and association behaviors of anemonefishes in field settlement experiments. Mar. Biol. 122, 377–389 (1995).
Verde, A. E., Cleveland, A. & Lee, R. W. Nutritional exchange in a tropical tripartite symbiosis II: direct evidence for the transfer of nutrients from host anemone and zooxanthellae to anemonefish. Mar. Biol. 162, 2409–2429 (2015).
Cleveland, A., Verde, E. A. & Lee, R. W. Nutritional exchange in a tropical tripartite symbiosis: direct evidence for the transfer of nutrients from anemonefish to host anemone and zooxanthellae. Mar. Biol. 158, 589–602 (2011).
Sale, P. F. Effect of cover on agonistic behavior of a reef fish: a possible spacing mechanism. Ecology 53, 753–758 (1972).
Fricke, H. W. & Holzberg, S. Social units and hermaphroditism in a pomacentrid fish. Naturwissenschaften 61, 367–368 (1974).
Fricke, H. W. Control of different mating systems in a coral reef fish by one environmental factor. Anim. Behav. 28, 561–569 (1980).
Mitchell, J. S. & Dill, L. M. Why is group size correlated with the size of the host sea anemone in the false clown anemonefish?. Canad. J. Zool. 83, 372–376 (2005).
Chausson, J., Srinivasan, M. & Jones, G. P. Host anemone size as a determinant of social group size and structure in the orange clownfish (Amphiprion percula). PeerJ 6, e5841 (2018).
Reed, C., Branconi, R., Majoris, J., Johnson, C. & Buston, P. Competitive growth in a social fish. Biol. Lett. 15, 20180737 (2019).
Buston, P. M. Mortality is associated with social rank in the clown anemonefish (Amphiprion percula). Mar. Biol. 143, 811–815 (2003).
Branconi, R. et al. Ecological and social constraints combine to promote evolution of non-breeding strategies in clownfish. Comm. Biol. 3, 1–7 (2020).
Schmiege, P. F., D’Aloia, C. C. & Buston, P. M. Anemonefish personalities influence the strength of mutualistic interactions with host sea anemones. Mar. Biol. 164, 24 (2017).
Barbasch, T. A. & Buston, P. M. Plasticity and personality of parental care in the clown anemonefish. Anim. Behav. 136, 65–73 (2018).
Abramoff, M. D., Magalhaes, P. J. & Ram, S. J. Image PROcessing with ImageJ. Biophoto. Int. 11, 36–42 (2004).
R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/ (2020).
Goodrich, B., Gabry, J., Ali I. & Brilleman, S. Rstanarm: Bayesian applied regression modeling via Stan. R package version 2.21.1 https://mc-stan.org/rstanarm (2020).
Weatherley, A. H. Approaches to understanding fish growth. Trans. Am. Fish. Soc. 119, 662–672 (1990).
Gabry, J. Shinystan: interactive visual and numerical diagnostics and posterior analysis for Bayesian models. R package version 2.5.0. https://CRAN.R-project.org/package=shinystan (2018).
Gelman, A., Goodrich, B., Gabry, J. & Vehtari, A. R-squared for Bayesian regression models. Am. Stat. 3, 307–309 (2018).
Lüdecke, D., Ben-Shachar, M. S., Patil, I., Waggoner, P. & Makowski, D. Performance: an R package for assessment, comparison and testing of statistical models. J. Open Sour. Softw. 6, 60 (2021).
Gabry, J., Simpson, D., Vehtari, A., Betancourt, M. & Gelman, A. Visualization in Bayesian workflow. J. R. Stat. Soc. Ser. A Stat. Soc. 182, 389–402 (2019).
Gabry, J. & Mahr, T. Bayesplot: plotting for bayesian models. R package version 1.8.0. https://mc-stan.org/bayesplot/ (2021).
Elliott, J. K. & Mariscal, R. N. Coexistence of nine anemonefish species: differential host and habitat utilization, size and recruitment. Mar. Biol. 138, 23–36 (2001).
Buston, P. M. Forcible eviction and prevention of recruitment in the clown anemonefish. Behav. Ecol. 14, 576–582 (2003).
Fautin, D. G. & Allen, G. R. Anemone fishes and their host sea anemones: a guide for aquarists and divers. Sea Challengers (1997).
Beldade, R., Blandin, A., O’Donnell, R. & Mills, S. C. Cascading effects of thermally-induced anemone bleaching on associated anemonefish hormonal stress response and reproduction. Nat. Commun. 8, 1–9 (2017).
Cortese, D. et al. Physiological and behavioural effects of anemone bleaching on symbiont anemonefish in the wild. Funct. Ecol. 35, 663–674 (2021).
Scherbatskoy, E. C. et al. Characterization of a novel picornavirus isolated from moribund aquacultured clownfish. J. Gen. Virol. 101, 735–745 (2020).
Saenz-Agudelo, P., Jones, G. P., Thorrold, S. R. & Planes, S. Mothers matter: contribution to local replenishment is linked to female size, mate replacement and fecundity in a fish metapopulation. Mar. Biol. 162, 3–14 (2014).
Barbasch, T. A. et al. Substantial plasticity of reproduction and parental care in response to local resource availability in a wild clownfish population. Oikos 129, 1844–1855 (2020).
Sebens, K. P. The ecology of indeterminate growth in animals. A. Rev. Ecol. Syst. 18, 371–407 (1987).
Buston, P. M. & García, M. B. An extraordinary life span estimate for the clown anemonefish Amphiprion percula. J. Fish Biol. 70, 1710–1719 (2007).
Chamberlain, S. A., Kilpatrick, J. R. & Holland, J. N. Do extrafloral nectar resources, species abundances, and body sizes contribute to the structure of ant–plant mutualistic networks?. Oecologia 164, 741–750 (2010).
Marting, P. R., Kallman, N. M., Wcislo, W. T. & Pratt, S. C. Ant-plant sociometry in the Azteca-Cecropia mutualism. Sci. Rep. 8, 1–15 (2018).
Fordyce, J. A. The evolutionary consequences of ecological interactions mediated through phenotypic plasticity. J. Exp. Biol. 209, 2377–2383 (2006).
West-Eberhard, M. J. Developmental plasticity and evolution (Oxford University Press, 2003).
West-Eberhard, M. J. Phenotypic accommodation: adaptive innovation due to developmental plasticity. J. Exp. Zool. B Mol. Develop. Evol. 304, 610–618 (2005).
Moczek, A. P. et al. The role of developmental plasticity in evolutionary innovation. Proc. R. Soc. B Biol. Sci. 278, 2705–2713 (2011).
Acknowledgements
We would like to thank Robin Francis, E Schlatter, Kurt Castro and Leah Desrochers for assistance with larval rearing and feeding. Thanks to Nelson Sikatua for field assistance, the Tamare-Kilu communities for granting access to their reefs and Mahonia Na Dari Research and Conservation Centre and Walindi Resort for supporting field work in Kimbe Bay, Papua New Guinea. This project received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement No. 841263. ET was supported by Boston University’s Undergraduate Research Opportunities Program and a Lara Vincent Student Research Award.
Author information
Authors and Affiliations
Contributions
T.R., A.K.B., E.T. and P.M.B. conceived of the research. T.R. and T.A.B. conducted field work. T.R., A.K.B., E.T., I.T. and B.D. conducted the experiments. T.R. performed data analysis. All authors contributed to the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rueger, T., Bhardwaj, A.K., Turner, E. et al. Vertebrate growth plasticity in response to variation in a mutualistic interaction. Sci Rep 12, 11238 (2022). https://doi.org/10.1038/s41598-022-14662-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-14662-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.