Abstract
Green stem disorder (GSD) of soybean is characterized by delayed leaf and stem maturation despite normal pod maturation. Previous studies have suggested that GSD occurrence is promoted by a high source–sink ratio, which is produced by thinning or shade removal at the R5 growth stage (the beginning of seed filling). Here the effects of different times and durations of shade removal after the R5 stage on GSD severity were analyzed. First, shade removal for more than 28 days after R5 increased GSD severity by more than 0.4 point in GSD score. Thinning treatment at R5 increased specific leaf weight by 23%, suppressed stem dry weight reduction, and upregulated 19 genes including those encoding vegetative storage proteins at R5 + 28d, indicating excess source ability relative to sink size. On the contrary, shade removal for 14 days after R5 decreased GSD severity by 0.5 point in GSD score. In this treatment, seed size was smaller, while seed number was significantly larger than control, suggesting that shortage of source ability relative to sink size. These results implied that soybean plants regulate GSD occurrences either positively or negatively according to a source-sink ratio during the R5 to R5 + 28d growth stages.
Similar content being viewed by others
Introduction
Green stem disorder (GSD) of soybean (Glycine max (L.) Merr.) is a disorder wherein stems and leaves remain green and retain moisture, regardless of mature pods and seeds, at the time of harvesting1,2 (medium and sever GSD symptoms were shown in Fig. 1b,c, respectively). GSD is a serious problem for farmers because it negatively affects the harvesting efficiency and seed appearance during combine harvesting of soybean3,4,5. It is important to identify the factors that determine GSD occurrence in soybean3.
Photographs of the experimental plots in the soybean fields. Soybean cultivation of the shade treatments in Experiments 1 and 2 (a–d) and the thinning treatments in Experiment 3 (e–h). (a) Photograph on October 3, 2018. (b, c, d) Photographs on the days when the first plant reached the R8 growth stage. (b) The full shading (Control), average GSD score of 3.9 on October 26, 2018. (c) The shade removal from R5 to R8 (Treatment 3), average GSD score of 4.4 on October 29, 2018. (d) The no shade (Treatment 6), average GSD score of 2.2 on October 25, 2018. (e, g) The dense treatment plot. (f, h) The thinning treatment plot. (e, f) R5 growth stage on September 6, 2018. (g, h) R8 growth stage on November 1, 2018. Photographs were taken by R. Yamazaki.
Previous studies have shown that removing pods (depodding) at the pod setting and filling stages promotes GSD occurrence6,7,8,9,10,11,12. Depodding resulted in an upregulated gene expression and accumulation of vegetative storage proteins (VSPs) in the stem and leaves13,14,15. Furthermore, other biotic or abiotic factors that possibly promote GSD, such as high soil moisture content and diseases during the reproductive period16,17 and pest attack18, result in sink limitation at the pod setting and filling stages. According to these studies, a high source–sink ratio induced by sink limitation could result in GSD occurrence8,13,14. The same logic was applied in the GSD occurrence promoted by source surplus without sink limitation. Islam et al.19 reported that high-N treatments suppressed leaf senescence from the stages R6 (the beginning of full seed stage) to R7 (the beginning of pod maturity) in soybean, resulting in GSD. The number of seeds and pods per plant, representing sink size, was not affected by the N treatments, but seed size was increased. These results are consistent with the hypothesis that the high ratio of source affects GSD.
Furthermore, our previous studies reported that increasing light availability by thinning (after dense cultivation) or shade removal (after side-shading cultivation) at the R5 stage (the beginning of seed filling) increased GSD occurrence in soybean compared to the control plants, which were grown at a dense plant population or were exposed to shading treatments20,21. The plants in the thinning treatment at the R5 stage showed more severe GSD symptoms than those in the thinning treatment at the R1 stage (the beginning of flowering)20,21. The sink size (seed and pod number) was not significantly affected by thinning or shade removal at the R5 stage, while the sink size significantly increased after these treatments at the R1 stage20, suggesting that the R5 stage is critical for determining the source–sink ratio and then GSD.
The period after the R5 stage includes developmentally important events, such as the determination of sink size (seed and pod number)22,23, decrease in photosynthetic activity24, and the redistribution of assimilates from the stem and leaves to seeds25,26. Thus, it is necessary to narrow down the critical or effective growth period after the R5 stage for GSD occurrence under improved light conditions. The objective of this study was to identify the important time and duration of improved light conditions for increased GSD severity and to reveal the effects of light availability on growth parameters and gene expression. First, we employed a side-shade equipment that reversibly altered the assimilation ability of the source by attaching or detaching a shade-sheet to identify the critical growth period for GSD occurrence. Second, plant growth traits and transcriptomes of the stems at the critical growth stage were analyzed under the field conditions, wherein the plant growth habits were different from those in a growth chamber27.
Results
Effects of shading during the whole growth period on GSD severity and plant growth
Growth traits (seed weight, seed number, stem and pod dry weights) in the no shade showed significantly higher scores (Table 1) than the other five treatments because of different light condition before the R5 growth stage. Since the objective of this study was to see the effects of light improvement after the R5 stage, comparison between the control and treatment 6 was shown just for reference.
Shading in entire growth periods (full shade) significantly increased GSD severity, with higher GSD score and number of leaves per node at the R8 stage (full pod maturation), and higher percentage of GSD ratio (= 5), and lower percentage of GSD ratio (≤ 3), compared to the treatment 6 (no shade) (Table 1). As an indicator of source-sink ratio, the sum of seed weight, stem and pod dry weights (source ability) per seed number (sink size) was calculated (Table 1). There was no significant difference between the control and treatment 6. There was also no significant difference in seed size (100 seeds weight) or timing of the R8 growth stage (Supplementary Table S1 online) between the two treatments.
Effects of the time and duration of shade removal on GSD severity
The treatment 1 (shade removal from R5 to R5 + 14 d) showed significantly lower GSD score, fewer number of leaves per node at the R8 stage, and significantly higher GSD ratio (≤ 3) (47.2%) than the control (full shade) (Table 2), suggesting that shade removal from R5 to R5 + 14 d suppressed GSD severity.
The treatments 2 (shade removal from R5 to R5 + 28 d) and 3 (shade removal from R5 onwards) showed significantly higher GSD score and significantly higher number of leaves per node at the R8 stage than the control (Table 2). The GSD ratio (= 5) was significantly higher in the treatments 2 (33.3%) and 3 (50.8%) than the control (Table 2). However, the GSD severity parameters in the treatments 4 (shade removal from R5 + 28 d onwards) and 5 (shade removal from R5 + 42 d onwards) were not significantly different from the control (Table 2). These results indicated that shade removal for 28 d at and after the R5 stage increased the severity of GSD symptoms, but short-term shade removal treatment, such as 14 d at and after the R5 stage, decreased GSD severity. In contrast, shade removal at and after R5 + 28 d had no effect on GSD severity.
In the timing of R8 growth stage, there were no significant differences between control and each treatment by Tukey’s test, although there were significant differences in treatment and interaction by ANOVA (Supplementary Table S2 online). Thus, the effect of each partial shade treatment on the timing of R8 was not considered in this study.
Effects of the time and duration of shade removal on growth parameters
Seed number was significantly increased in the shading treatments 1, 2, and 3 compared to the control, treatments 4, and 5 (Table 3). There were no significant differences in the seed number between the treatments 1, 2, and 3, indicating that shade removal from R5 to R5 + 14 d increased seed number, while shade removal after R5 + 14 d had no further effect. Moreover, seed weight significantly increased in the treatments 2 and 3 (Table 3). However, there was no significant difference in the seed weight between the treatments 2 and 3. The treatment 1 showed no significant difference in seed weight, causing a significant decrease in seed size (Table 3). Therefore, seed weight and number were differentially regulated by shade removal during 28 d at and after the R5 stage, while shade removal after R5 + 28 d had no effect on the two growth parameters.
In contrast, stem dry weight was significantly higher in the treatment 3 compared to the control, treatments 1, and 2 (Table 3). Additionally, the stem dry weight of the treatment 2 was significantly higher than the control, suggesting that stem dry weight increased with the duration of shade removal at and after the R5 stage. The treatments 4 and 5 showed no significant differences in stem dry weight.
Effects of thinning at the R5 growth stage on plant growth
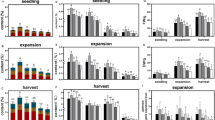
The thinning treatment at the R5 stage increased GSD severity in the two-year experiments (Table 4, Fig. 1h), which agrees with the results of previous studies20,21. After thinning treatment, leaf number decreased from R5 to R8 (Fig. 2a), indicating that leaf abscission progressed after the R5 stage. However, at R5 + 28 d, the number of leaves in the thinning treatment was significantly higher than the dense treatment (Fig. 2a), indicating that the progression of leaf abscission was suppressed by thinning.
Effects of thinning at the R5 growth stage on growth characteristics. The scale of x-axis indicates days after the R5 stage. (a) leaf number, (b) leaf dry weight, (c) specific leaf weight, and (d) stem dry weight. Dense indicates that the plant population was maintained at 22.2 plants m−2 throughout the cultivation period. Thinning indicates that the plant population was changed from 22.2 plants m−2 to 5.56 plants m−2 at the R5 growth stage. The values are the average of two-year experiments. Error bars represent standard error. ** indicates statistically significant difference between dense- and thinning-treatment plots (t-test, p < 0.01). Statistical analyses were performed using the statistical software BellCurve for Excel version 3.21.
Although leaf number decreased in both the treatments, the leaf dry weight per plant did not decrease from R5 to R5 + 28 d in the thinning treatment (Fig. 2b) because the leaf dry weight per leaf area (specific leaf weight) increased after thinning, while it remained unaltered in the dense treatment (Fig. 2c). Moreover, the stem dry weight decreased from R5 to R8 in the dense treatment, whereas the decrease in the stem dry weight at R5 + 28 d and R8 was significantly suppressed by thinning (Fig. 2d).
Effects of thinning at the R5 growth stage on transcriptome
A total of 54,878 transcriptionally active regions were identified in the stem based on the annotated sequences in the soybean genome. After filtering, the significantly upregulated or downregulated genes were identified in plants exposed to thinning using 211 and 528 probes for the R5 + 14 d and R5 + 28 d datasets, respectively (Fig. 3). The filtered probes were further screened to obtain 126 and 132 probes for the R5 + 14 d and R5 + 28 d datasets, respectively, because the genes with a low expression level scattered quite differently between the dense and thinning treatments (Fig. 3). The analyses of the low expression genes will be reported elsewhere.
Scatter plots of all the differentially expressed genes (DEGs) in the stem identified by RNA-seq exposed to thinning and dense treatments. Vertical and horizontal axes represent mean expression level in plants exposed to the thinning and the dense treatment, respectively. One plot indicates one gene. (a, b) Scatter plot of all expressed genes at R5 + 14 d (a) and R5 + 28 d (b). Warm and cold colors in the plots indicate high and low overlapping levels, respectively. (c, d) Scatter plots of all DEGs at R5 + 14 d (c) and R5 + 28 d (d). Colored points indicate DEGs. Red colored points above the horizontal (y = − 2) and vertical lines (x = − 2) indicate the genes filtered using intensity difference test (p < 0.05) and screened using a logarithmic value of > − 2.0. Figures were produced with the software SeqMonk v1.38.2 and Java (1.8.0_151).
GO enrichment analysis showed that thinning-induced differentially expressed genes (DEGs) were mainly associated with photosynthesis (44.8% in the R5 + 14 d and 56.1% in the R5 + 28 d datasets) (Table 5). The second group of genes induced by thinning were involved in carbohydrate metabolism (24.1% in the R5 + 14 d and 9.8% in the R5 + 28 d datasets). However, the GO enrichment analysis of approximately half of the transcriptionally active genes (26,836 genes) with a logarithmic value above -2.0 revealed that the photosynthesis-related genes were not the most overrepresented genes (7.2% in the R5 + 14 d and 6.9% in the R5 + 28 d datasets). This indicated that the thinning treatment markedly altered the expression of photosynthesis-related genes in the stem.
The filtered and screened DEGs were then compared between the R5 + 14 d and R5 + 28 d datasets and a set of common 19 upregulated and 8 downregulated genes were identified in both the datasets (Table 6). Of the 18 upregulated genes, eight genes were related to photosynthesis, including those encoding the 22 kDa-protein of photosystem II (PsbS), chlorophyll a/b-binding protein (CP24), chloroplast pigment-binding protein (CP26), and early light-induced proteins (ELIPs). The remaining four upregulated genes encode VSPs, such as 28 and 31 kDa stem glycoproteins (VspA and VspB) and lipoxygenase. VSPs and lipoxygenase were also upregulated by depodding and accumulated in paravenial mesophyll cells as a temporary storage for N28.
A NAC transcription factor (Glyma.16G043200) gene was downregulated in the R5 + 14 d and R5 + 28 d datasets (Table 6). Amino acid homology analysis using BLASTP revealed that Glyma.16G043200 was a homolog of the NAC domain-containing protein 47 (ANAC047) of Arabidopsis, which belongs to the stress-responsive NAC-B (SNAC-B) subfamily29. ANAC047 is a senescence-associated gene that promotes senescence and programmed cell death in leaves30. The genes encoding endo-1,4-β-mannanase and xyloglucan endotransglucosylase/hydrolase were also downregulated under thinning treatment (Table 6), which are senescence-associated enzymes in leaf abscission zones and degrade cell wall31.
Discussion
The methodologies to identify the critical growth period under improved light conditions
Turner et al.32 reported that depodding resulted in gene expression patterns derived from both sink-limited effects and stress responses to injuries. Meanwhile, in the present study, thinning treatment probably caused a response to high light stress, irrespective of the increased assimilation ability of the source. Transcriptome analysis revealed that photosynthesis-related genes were significantly altered by the thinning treatment (Table 5); eight out of the 19 upregulated genes were related to photosynthesis, such as those encoding PsbS, CP24, CP26, and ELIPs (Table 6). These proteins absorb excess light energy to prevent photo-oxidative stress caused by reactive oxygen species generated under high light intensities33,34,35. Dihydroflavonol reductase (Table 6), i.e., an anthocyanin synthase, also prevents oxidative stress36, suggesting that high light intensity-responsible genes were induced by the thinning treatment.
In contrast, shading treatment throughout the cultivation period (full shade) increased GSD severity as well (Table 1). This phenomenon was also observed in the previous study20 although shading strength was different from one another. Shading treatment from sowing to R8 significantly reduced the sink capacity and the assimilation ability of the source by approximately 75% (Table 1). There was no significant difference between the control and treatment 6 in the indicator of source-sink ratio (above ground dry matter/seed number, Table 1). It suggested that GSD promoted by continuous shading had different mechanisms which could not be explained by source-sink ratio, as suggested in the previous study20.
An intermediate GSD score of full shade enabled to analyze whether the light availability treatment in experiment 2 promotes or suppresses GSD occurrence. Thus, these characteristics coupled with the reversibility of the shading treatment helped in the identification of the critical period for GSD occurrence between 14 and 28 d after the R5 stage.
Evidence of high source–sink ratio and delayed senescence at the critical period
Depodding results in a rapid increase in dry weights of leaf13 and stem9,16, suggesting an alteration in the leaf function from an assimilation organ to a storage organ for excess assimilates. In experiment 3, suppressed leaf abscission, increased leaf specific weight, and reduced loss of stem dry weight (redistribution of assimilates from the stem to seeds) from R5 to R5 + 28 d in the thinning treatment indicated that improved light conditions from R5 to R5 + 28 d resulted in excess assimilation products relative to the sink demand (Fig. 2).
Brown and Hudson27 attempted to distinguish the effect of sink limitation from that of injury stress responses by analyzing the transcriptomes of depodded and male-sterile soybean plants. They thought that the common upregulated and downregulated genes in both types of plants were related to the effects of sink-limitation. The results were that lipoxygenase genes were highly expressed in both types of sink-limited plants. Lipoxygenases have distinct roles in stress responses37 and as storage proteins28. In the present study, four out of the 19 genes upregulated in the stem upon thinning encoded VspA (Glyma.07G014500), VspB (Glyma.08G200100) and two lipoxygenases (Glyma.15G026400 and Glyma.15G026500). These results are consistent with those of Brown and Hudson27.
The gene encoding NAC transcription factor (Glyma.16G043200), which was significantly decreased upon thinning in the present study (Table 6), was also suppressed in the leaves of both depodded and male-sterile soybean plants27. In soybean, NAC transcription factors GmNAC81 and GmNAC30 promote leaf senescence38. As D gene in sorghum induces programmed cell death and determines stem water content39, Glyma.16G043200 may also promote senescence and programmed cell death in soybean stem and may be related to GSD occurrence.
GSD severity was affected by source-sink ratio determined by the time and duration of light improvement
Based on the comparison about the effects of the time and duration of shade removal after the R5 stage on sink size, source ability, and GSD severity, it was indicated that the modulation of the source–sink ratio was associated with GSD severity, which corresponds to the source-sink balance hypothesis. In this context, we proposed a model on the relative potential of source and sink (Fig. 4), which was constructed using the seed number (sink potential) and the seed weight, dry weights of stem and pod (source potential) at the R8 stage in experiments 1 and 2 (Tables 1, 3). The model indicates how the sink and source potentials are determined and how GSD is affected by those potentials. In this model, we employed two assumptions. One is that relative potentials of sink and source were constant during shading. The other one is that the slope (increasing rate) of relative sink and source potentials was identical between treatments during no shade only until a specific grain filling stage, after that the slope becomes zero. The specific stages are around R5 + 14 d and R5 + 28 d for sink and source potential, respectively. For example, the relative potentials of sink and source at R5 were determined by those at R8 in the control and common between the control and treatments 1–5 because the growth before R5 was same between those treatments. Sink size, represented by the seed number, increased in the treatments 1, 2, and 3 compared to the control, while the seed number had no significant difference between the treatments 1, 2, and 3 (Table 3), suggesting that shade removal from R5 to R5 + 14 d determined sink size, while shade removal after R5 + 14 d had almost no further effect on sink size (Fig. 4b,c,d). Similar findings have been reported, in which soybean plants had the ability to adjust sink size in response to light conditions through the abortion of flowers, pods, and seeds until approximately 10–12 or 14–21 d after the R5 stage22,23. In contrast, the assimilation ability of the source, represented by the sum of seed weight, dry weights of stem and pod (Table 3), was significantly increased between the treatments 1 and 2 (Fig. 4b,c). Although the treatment 3 showed significantly increased stem dry weight than the treatment 2 (Table 3), there were no significant differences in the seed weight between the treatments 2 and 3. This indicated that an increased supply of assimilates resulting from shade removal from R5 to R5 + 28 d was sufficient to fulfill sink demands, but the sink size did not increase after R5 + 14 d (Fig. 4c,d). Consequently, the balance between the relative potentials of sink and source at R8 determines GSD.
Schematic diagram of relative sink and source potentials to no shade treatment after the R5 growth stage. Control (a), treatment 1 (b), treatment 2 (c), treatment 3 (d), treatment 4 (e), and treatment 5 (f) of Experiment 2. Relative sink potential to no shade was estimated by seed number per plant relative to that of the no shade treatment (treatment 6) at the R8 growth stage (Tables 1, 3). Relative source potential to no shade was estimated by the seed weight, dry weights of pod and stem per plant relative to that of the no shade treatment at the R8 growth stage (Tables 1, 3). In this model, the relative potentials are supposed to be unchanged under shading. The sink potential is increased by shade removal until around R5 + 14d, while the source potential is increased by shade removal until around R5 + 28 d. Shade removal after R5 + 28 d has no or negative effect. The increasing rate of relative potentials under shade removal is supposed to be same between treatments. Gray area indicates the period covered with a shade sheet.
The treatment 1 had significantly smaller seeds and relatively low GSD scores than those of the control treatment (Tables 2, 3). Previous studies reported that source restriction by defoliation or shading reduced seed size40,41. Therefore, the reduction in seed size in the treatment 1 suggested shortage of assimilates to fulfill sink demands, which in turn, indicated that shade removal for 14 d after the R5 stage increased sink size without fulfilling sink demands (Fig. 4b).
The photosynthetic ability of soybean decreases with age throughout the pod filling stage24. The results of the experiment 3 (Fig. 2) showed that leaf abscission proceeded after the R5 stage and was promoted by mutual shading under a dense plant population, suggesting that the shading treatment in the experiment 2 also promoted the decrease in the photosynthetic activity of plants. Therefore, in the shade removal after R5 + 28 d, plants lost the ability to increase photosynthetic activity in response to improved light conditions (Fig. 4e,f).
It is notable that the increasing rate was higher in the relative potential of sink than that of source under the shade removal until R5 + 14 d (Fig. 4). This model would be further tested to elucidate the molecular mechanism underlying GSD. In the present study, increased GSD severity in soybean upon exposure to improved light conditions at and after the R5 stage was attributed to a high source–sink ratio owing to increased photosynthesis relative to sink size, which was based on the source–sink balance hypothesis.
Conclusions
Green stem disorder (GSD) of soybean was promoted by a high source–sink ratio, that was increased by either sink limitation or source ability enhancement. Source ability was upregulated by light availability improvement such as thinning and shade removal. The increase in sink and source was, however, possible only by around R5 + 14 d and R5 + 28 d growth stages, respectively. Source and sink abilities could be represented by the sum of seed weight, dry weights of stem and pod and by seed number at maturity, respectively.
Materials and methods
Plant material and experimental site
Experiments 1, 2 and 3 were conducted using the soybean cultivar ‘Sachiyutaka’ (Maturity group 6)42 in the experimental fields of National Agriculture and Food Research Organization (NARO), Western Region Agricultural Research Center, Hiroshima, Japan (34°30′N, 133°23′E; Elevation: 2 m asl; Soil: Typic fluvaquent). The groundwater level in all the experimental fields was maintained at 30 cm below the surface using the farm-oriented enhancing aquatic system FOEAS43, and 3 g m−2 N, 10 g m−2 P2O5, and 10 g m−2 K2O were supplemented to the soil one day before sowing. To check biotic stress, insecticides and fungicides were regularly sprayed in the fields.
Experiment 1: Shading for the whole growth duration
Seeds were sown on June 28, 2017, and June 29, 2018. The shade equipment in each plot surrounded seven plants in a single row (plant spacing: 0.3 m), without any shade-sheet on the upper part (width: 0.1 m) of the equipment, which mimicked a canopy where some of the uppermost leaves are not shaded by the neighboring plants under field conditions (Fig. 1a)20. The shade-sheets used in the equipment were black-colored polypropylene sheets, with a shade strength of 82% (eliminating an average of 82% photosynthetically active radiation in sunlight)20 and were raised when one of the uppermost leaves in the plots grew 3 cm taller than the uppermost side of the shade equipment. Thus, the height of the equipment paralleled plant heights throughout the experiment. The shading treatment was applied from sowing to R8 (full shade, control) or never applied (no shade, treatment 6). There were two replicates in 2017 and four replicates in 2018. The main stem of each plant was supported using a gardening pole to prevent lodging (Fig. 1b,c,d).
Experiment 2: Time and duration of shade removal at and after the R5 growth stage
Five treatments (treatments 1–5) as well as the control treatment were conducted with shading from sowing. The control treatment was common with Experiment 1. In the treatments 1, 2, and 3, shade-sheets were removed at the R5 stage. Then, shading treatment was resumed 14 d and 28 d after the initiation of the R5 stage in the treatments 1 and 2, respectively. The shade-sheets were not resumed after R5 in the treatment 3. In the treatments 4 and 5, shade sheets were removed after 28 d of the R5 stage (R5 + 28 d) and 42 d of the R5 stage (R5 + 42 d), respectively.
Experiment 3: Thinning at the R5 growth stage
Seeds were sown in 3.0 m × 1.2 m plots on June 27, 2016 (two replicates) and 3.0 m × 2.1 m plots on June 25, 2018 (four replicates). There were two types of plots with different plant population size: dense (Fig. 1e,g) and thinning (Fig. 1f,h) plots. In the dense plots, plant populations were maintained at 22.2 plants m−2, with a plant spacing of 0.15 m in 0.3 m rows, throughout the cultivation period. In contrast, the plant populations in the thinning plots were changed from dense to sparse (5.56 plants m−2 in 0.6 m rows and 0.3 m plant spacing) at the R5 stage. In the thinning plots, the aboveground parts of the plants in every alternate row and those of every alternate plant in the remaining rows were excised. To prevent lodging, plants were supported by poles standing on both ends of the rows and greenhouse polyethylene bands, which were stretched out between the poles at 40 cm above the ground (Fig. 1g,h).
Measurements of growth parameters
Seven plants from each plot were sampled at the R8 stage for experiments 1 and 2 during both years. For experiment 3, six (2016) or nine (2018) plants from each dense and thinning treatment plot, except the plants at the plot borders, were randomly selected after the R8 and 28 d of the R5 stage (R5 + 28 d) for the analysis of growth parameters. Moreover, six (2016) or nine (2018) thinned plants, except the plants at the plot borders, were randomly sampled at the R5 stage to analyze the growth parameters of the plants at the start of the thinning treatment.
Growth parameters, including leaf area, leaf number, dry weight of leaflets, and stem dry weight, were measured for each sampled plant of experiment 3. The leaf area was measured using an area meter LI-3100C (LI-COR, Lincoln, NA, USA), and the dry weights of each plant part were measured after drying them at 80 °C for 3 d before measurements.
Development and GSD score
The dates of the R5 and R8 stages for each plant were determined using the method of Fehr and Caviness44. The severity of GSD was determined using the three indexes reported in the previous study, namely GSD score, leaf number per node at the R8 stage, and GSD ratio20. GSD score was calculated using a method modified from that of Furuya and Umezaki45, and each plant was assigned a GSD score (1–5) based on the number of remaining leaves per node and the color of the stem at the R8 stage, where a high GSD score represented more severe GSD symptoms. In addition, the ratio of the number of plants with no leaves (GSD score: < 3) and the number of plants with one-third of the leaves remaining at the R8 stage (GSD score: 5) to the number of sampled plants were calculated as GSD ratio (≤ 3) and GSD ratio (= 5), respectively for each plot. After the R8 stage, seed weight of 100 seeds and seed number were measured for each sampled plant. Dry weights of the stems and pods were measured after drying them at 80 °C for 3 d.
Transcriptome analysis
For transcriptome analysis as part of experiment 3, two replicates with three main plant stems, each from dense and thinning treatments after 14 and 28 d of treatment (R5 + 14 d and R5 + 28 d), were collected and freeze-stored in liquid nitrogen at − 80 °C in 2016. Total RNA was extracted using phenol/SDS and treated with DNase I, followed by column purification. The quality and quantity of RNA were ensured using Nanodrop and Agilent 2100 Bioanalyzer, respectively, and RNA-Seq libraries were constructed using Illumina’s Truseq Stranded mRNA LT Sample Prep Kit (as per the manufacturer's instructions) and sequenced using Illumina HiSeq 4000 paired-end sequencing technology, with a read length of 100 and sample size of 7.46 giga base. The sequences were then aligned with the soybean reference genome (Gmax_275_v2.0) using TopHat2 2.0.946.
For the data analysis, filtering and analysis were performed using the software SeqMonk v1.38.2 (http://www.bioinformatics.bbsrc.ac.uk/projects/seand) and Java (1.8.0_151) (https://www.oracle.com/java/). Gene expressions were quantified using the RNA-Seq pipeline quantitation based on merged transcripts, counting reads over exons and correcting for feature length, using log-transformed data. A total of 54,878 transcriptionally active regions (probes) were generated for analysis. However, for the R5 + 14 d dataset, only the first field replicate was used for analysis because of the low RNA quality of the second replicate, whereas both the field replicates of the R5 + 28 d dataset were grouped for analysis. The probes were filtered using the intensity difference test (p < 0.05) and then further screened for a logarithmic value (to the base 10) above -2.0. The filtered and screened probes were then analyzed using gene ontology (GO) enrichment analysis of the web service of SoyBase (https://www.soybase.org/) with annotations from the Wm82.a2. v1 (Glyma 2.0).
Statistical analyses
A completely randomized design was used for each experiment. Statistical significance of differences (p < 0.05 or p < 0.01) was tested using the analysis of variance (ANOVA) or t-test. Dunnett and Tukey’s tests were used (p < 0.05 or p < 0.01) to compare the mean values of the control and other treatments and the mean values of plant growth parameters between the treatments, respectively. GSD scores and GSD ratio were analyzed after the Box-Cox and angular transformations, respectively, to meet the assumption that the data be approximately normally distributed. The analyses were performed using the statistical software BellCurve for Excel version 3.21 (Social Survey Research Information Co. Ltd., https://bellcurve.jp/ex/).
Ethical approval
The soybean cultivar ‘Sachiyutaka’ was provided by National Agriculture and Food Research Organization (NARO). The cultivar, which was developed by NARO and registered as the registration number of 11,367, was used in accordance with the plant variety protection and seed act of Japan. The plant resource can be accessed via NARO Genebank project (https://www.gene.affrc.go.jp/index_en.php). This research was approved by the Western Region Agricultural Research Center, NARO as the research number of 1,040,101, 2016–2018. All the experiments carried out on plants in this study were in compliance with relevant institutional, national, and international guidelines and legislation.
Data availability
All data supporting the findings of this study are available within the paper. The transcriptome reads are available in the DDBJ database as accession # DRA008086 under BioProject # PRJDB7775.
References
Harbach, C. J. et al. Delayed senescence in soybean: Terminology, research update, and survey results from growers. Plant Health Progress 17, 76–83 (2016).
Hobbs, H. A. et al. Green stem disorder of soybean. Plant Dis. 90, 513–518 (2006).
Hill, C. B., Hartman, G. L., Esgar, R. & Hobbs, H. A. Field evaluation of green stem disorder in soybean cultivars. Crop Sci. 46, 879–885 (2006).
Morita, K. et al. (2006) Effect of green stem on soiled bean index at harvest of soybean by combine harvester. Hokuriku Crop Sci. 41, 107–109 (2006) (in Japanese).
Ogiwara, H. Delayed leaf senescence. In: Agriculture, Forestry and Fisheries Research Council of Japan, ed. Soybean-technical development for improving national food self-sufficiency ratio. Annotated bibliography of Agriculture, Forestry, and Fisheries Research, vol. 27, 291–294 (2002). (in Japanese).
Crafts-Brandner, S. J. & Egli, D. B. Sink removal and leaf senescence in soybean. Plant Physiol. 85, 662–666 (1987).
Crafts-Brandner, S. J., Below, F. E., Harper, J. E. & Hageman, R. H. Effects of pod removal on metabolism and senescence of nodulating and nonnodulating soybean isolines. Plant Physiol. 75, 311–317 (1984).
Egli, D. B. & Bruening, W. P. Depodding causes green-stem syndrome in soybean. Crop Manag. 5(1), 1–9. https://doi.org/10.1094/CM-2006-0104-01-RS (2006).
Htwe, N. M. P. S. et al. Leaf senescence of soybean at reproductive stage is associated with induction of autophagy-related genes, GmATG8c, GmATG8i and GmATG4. Plant Prod. Sci. 14, 141–147 (2011).
Leopold, A. C., Niedergang-Kamien, E. & Janick, J. Experimental modification of plant senescence. Plant Physiol. 34, 570–573 (1959).
Mondal, M. H., Brun, W. A. & Brenner, M. L. Effects of sink removal on photosynthesis and senescence in leaves of soybean (Glycine max L.) plants. Plant Physiol. 61, 394–397 (1978).
Wittenbach, V. A. Effect of pod removal on leaf senescence in soybean. Plant Physiol. 70, 1544–1548 (1982).
Wittenbach, V. A. Effect of pod removal on leaf photosynthesis and soluble protein composition of field-grown soybeans. Plant Physiol. 73, 121–124 (1983).
Wittenbach, V. A. Purification and characterization of a soybean leaf storage glycoprotein. Plant Physiol. 73, 125–129 (1983).
Staswick, P. E. Developmental regulation and the influence of plant sinks on vegetative storage protein gene expression in soybean leaves. Plant Physiol. 89, 309–315 (1989).
Sato, J., Shiraiwa, T., Sakashita, M., Tsujimoto, Y. & Yoshida, R. The occurrence of delayed stem senescence in relation to trans-zeatin riboside level in the xylem exudate in soybeans grown under excess-wet and drought soil conditions. Plant Prod. Sci. 10, 460–467 (2007).
Takehara, T. et al. Occurrence of delayed leaf senescence of soybean caused by Rhizoctonia aerial blight in Japan. Jpn. Agric. Res. Q. 50, 201–208 (2016).
Boethel, D. J. et al. Delayed maturity associated with southern green stink bug (Heteroptera: Pentatomidae) injury at various soybean phenological stages. J. Econ. Entomol. 93, 707–712 (2000).
Islam, M. M. et al. Nitrogen manipulation affects leaf senescence during late seed filling in soybean. Acta Physiol. Plant. 39, 42 (2017).
Yamazaki, R., Katsube-Tanaka, T. & Shiraiwa, T. Effect of thinning and shade removal on green stem disorder in soybean. Plant Prod. Sci. 21, 83–92 (2018).
Yamazaki, R., Katsube-Tanaka, T., Kawasaki, Y., Katayama, K. & Shiraiwa, T. Effect of thinning on cultivar differences of green stem disorder in soybean. Plant Prod. Sci. 22, 311–318 (2019).
Board, J. E. & Tan, Q. Assimilatory capacity effects on soybean yield components and pod number. Crop Sci. 35, 846–851 (1995).
Egli, D. B. Soybean reproductive sink size and short-term reductions in photosynthesis during flowering and pod set. Crop Sci. 50, 1971–1977 (2010).
Wells, R., Schulze, L. L., Ashley, D. A., Boerma, H. R. & Brown, R. H. Cultivar differences in canopy apparent photosynthesis and their relationship to seed yield in soybean. Crop Sci. 22, 886–890 (1982).
Islam, M. M. et al. Nitrogen redistribution and its relationship with the expression of GmATG8c during seed filling in soybean. J. Plant Physiol. 192, 71–74 (2016).
Zhao, X., Zheng, S. H. & Arima, S. Influence of nitrogen enrichment during reproductive growth stage on leaf nitrogen accumulation and seed yield in soybean. Plant Prod. Sci. 17, 209–217 (2014).
Brown, A. W. & Hudson, K. A. Transcriptional profiling of mechanically and genetically sink-limited soybeans. Plant Cell Environ. 40, 2307–2318 (2017).
Tranbarger, T. J., Franceschi, V. R., Hildebrand, D. F. & Grimes, H. D. The soybean 94-kilodalton vegetative storage protein is a lipoxygenase that is localized in paraveinal mesophyll cell vacuoles. Plant Cell 3, 973–987 (1991).
Melo, B. P. et al. Revisiting the soybean GmNAC superfamily. Front. Plant Sci. 9, 1864 (2018).
Kim, H. J. et al. Gene regulatory cascade of senescence-associated NAC transcription factors activated by ETHYLENE-INSENSITIVE2-mediated leaf senescence signaling in Arabidopsis. J. Exp. Bot. 65, 4023–4036 (2014).
Tucker, M. L., Burke, A., Murphy, C. A., Thai, V. K. & Ehrenfried, M. L. Gene expression profiles for cell wall-modifying proteins associated with soybean cyst nematode infection, petiole abscission, root tips, flowers, apical buds, and leaves. J. Exp. Bot. 58, 3395–3406 (2007).
Turner, G. W. et al. Experimental sink removal induces stress responses, including shifts in amino acid and phenylpropanoid metabolism, in soybean leaves. Planta 235, 939–954 (2012).
Roach, T. & Krieger-Liszkay, A. The role of the PsbS protein in the protection of photosystems I and II against high light in Arabidopsis thaliana. Biochim. Biophys. Acta Bioenerg. 1817, 2158–2165 (2012).
Horton, P., Ruban, A. V. & Walters, R. G. Regulation of light harvesting in green plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 47, 655–684 (1996).
Hutin, C. et al. Early light-induced proteins protect Arabidopsis from photooxidative stress. Proc. Natl. Acad. Sci. U.S.A. 100, 4921–4926 (2003).
Wang, H. et al. Functional characterization of dihydroflavonol-4-reductase in anthocyanin biosynthesis of purple sweet potato underlies the direct evidence of anthocyanins function against abiotic stresses. PLoS ONE 8, e78484 (2013).
Saravitz, D. M. & Siedow, J. N. The differential expression of wound-inducible lipoxygenase genes in soybean leaves. Plant Physiol. 110, 287–299 (1996).
Pimenta, M. R. et al. The stress-induced soybean NAC transcription factor GmNAC81 plays a positive role in developmentally programmed leaf senescence. Plant Cell Physiol. 57, 1098–1114 (2016).
Fujimoto, M. et al. Transcriptional switch for programmed cell death in pith parenchyma of sorghum stems. Proc. Natl. Acad. Sci. U.S.A. 115, 8783–8792 (2018).
Egli, D. B. Variation in leaf starch and sink limitations during seed filling in soybean. Crop Sci. 39, 1361–1368 (1999).
Board, J. E. & Harville, B. G. Late-planted soybean yield response to reproductive source/sink stress. Crop Sci. 38, 763–771 (1998).
Fatichin, Zheng, S. H., Narasaki, K. & Arima, S. Genotypic adaptation of soybean to late sowing in southwestern Japan. Plant Prod. Sci. 16, 123–130 (2013).
Wakasugi, K. & Fujimori, S. Subsurface Water Level Control System “FOEAS” that promotes the full use of paddy fields. J. Jpn. Soc. Irrig. Drain. Rural Eng. 77, 705–708 (2009) (in Japanese).
Fehr, W. R. & Caviness, C. E. Stages of soybean development. Spec. Rep. 80. Iowa Agric. Home Econ. Exp. Stn. Iowa State Univ., Ames. (1977).
Furuya, T. & Umezaki, T. Simplified distinction method of degree of delayed stem maturation of soybean plants. Jpn. J. Crop Sci. 62, 126–127 (1993) (in Japanese with English abstract).
Kim, D. et al. TopHat2: Accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 14, R36 (2013).
Acknowledgements
We gratefully acknowledge Dr. Katsuyuki Katayama, Dr. Hideo Hamaguchi, Dr. Satoko Yasumoto, Dr. Gen Ishioka, Dr. Takayuki Mitsunaga, Dr. Yu Tanaka, the members of the Technical Support Center Operations Unit-1, and the staff of the Western Region Agricultural Research Center, National Agriculture and Food Research Organization (NARO) for their technical assistance and useful comments on this study. This study was supported by JSPS KAKENHI Grant No. 21H02330 to T.S.
Author information
Authors and Affiliations
Contributions
R.Y., T.K., Y.K., and T.S. conceptualized and designed experiments. R.Y. performed field experiments, sampling, and took a photograph. E.O. performed RNA-seq experiments. R.Y., T.K., Y.K., and T.S. analyzed data of field experiments. T.K. and E.O. analyzed RNA-seq experiments. R.Y., T.K., and T.S. produced figures and wrote the manuscript. All the authors have read and edited the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yamazaki, R., Katsube-Tanaka, T., Ogiso-Tanaka, E. et al. High source–sink ratio at and after sink capacity formation promotes green stem disorder in soybean. Sci Rep 12, 10440 (2022). https://doi.org/10.1038/s41598-022-14298-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-14298-4
This article is cited by
-
Leafhopper transmits soybean stay-green associated virus to leguminous plants
Phytopathology Research (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.