Abstract
Expansion of oxygen minimum zones in the world's oceans is likely to enhance the production of anaerobic metabolites by marine microorganisms. Here we show that toluene is present throughout the year in shelf waters of the upwelling ecosystem off Concepción (36° S), Chile, and it is a product of microbial anaerobic metabolism. The intra-annual variability in toluene concentrations is consistent with seasonal variability in the strengths of suboxic equatorial and oxygenated subantarctic water masses. Laboratory incubations of oxygen minimum zone water showed microbial production of toluene in the absence of O2. Toluene concentrations were elevated (up to 96 nM) in deeper O2-depleted waters and followed a seasonal pattern in oceanographic conditions. There is evidence to hypothesize that microbial production of toluene could be a homeostatic biochemical mechanism to thrive in the more acidic oxygen minimum zone waters. On the other hand, evidence indicates that microbial anaerobic degradation of toluene may be a source of NO2− by partial denitrification, as shown for aquifer sediments. Since toluene production was not detected in incubations under aerobic conditions, we hypothesize that oxygen minimum zone waters export toluene to surrounding oxygenated waters. Expansion of hypoxia in the ocean will certainly enhance the production and export of anaerobic metabolites by marine microorganisms.
Similar content being viewed by others
Introduction
Across the world’s oceans, anaerobic metabolism presently occurs in marine sediments, anoxic basins, and areas known as oxygen minimum zones (OMZ). These OMZ regions are open ocean waters1, occupying approximately 1% of the global ocean volume, but with a disproportionate influence on biogeochemical cycles through the removal of nitrogen and greenhouse gases from the ocean to the atmosphere2. Documented expansion of OMZ regions across the world's oceans3 will expand anaerobic microbial metabolism in the water column and the associated production of reduced metabolites4,5,6,7,8 readily available for further microbial degradation.
Toluene is an aromatic hydrocarbon that occurs as a minor component of gasoline and is also produced by bacteria9,10 through anaerobic decarboxylation of phenylacetate derived from the catabolism of the amino acid phenylalanine10,11. Biological production of toluene has been reported in anaerobic bacterial isolates12, anaerobic sewage sludge13,14, anoxic freshwater sediments15,16, and the anoxic hypolimnion of stratified lakes17. Marine phytoplankton is also a potential source of toluene, as shown in monocultures of coccolithophores and diatoms under oxidative stress. They could be a potential source of benzenoids to the atmosphere18.
In oceanic OMZ waters, biogenic production of toluene is promoted both by low-O2 conditions and by the high availability of proteinaceous organic matter (phenylalanine). As a permanent feature of the modern ocean, these OMZ waters are maintained by elevated fluxes of plankton-derived material from the photic zone, mainly in the form of protein carbon that in turn promotes oxygen depletion19. The biological production of toluene from phenylalanine has been shown to involve transamination, decarboxylation, and oxidation to form phenylacetate10, which is the substrate for a glycyl radical decarboxylase enzyme that catalyzes phenylacetate decarboxylation to toluene9,10,20, a process that is irreversibly inactivated in the presence of O210,19,21. Genomic evidence indicates that glycyl radical enzymes are widespread amongst facultative and obligate anaerobic microorganisms9,22,23,24,25.
Toluene is produced in bulk for industrial applications and consumer products and is found in concentrations of 2–4 mg/L in sewage sludge13,23. Toluene is a molecule of interest as a health hazard to humans24, and can account for a significant fraction of volatiles in the environment (compounds of high vapor pressure and low water solubility, with boiling points ranging between 69 and 317 °C)25. Toluene has been quantified in Narraganset Bay waters on the east coast of the US (0.2–60 nM)26, in the vicinity of a production platform in the Northwest Gulf of Mexico (21 nM)27, and Kuwait Bay (0.4–37 nM)28. These concentrations of toluene were attributed to the result of human activity and are higher than those reported in surface waters of the Caribbean Sea (0.5–4 nM)29, Resurrection Bay in the Gulf of Alaska (1–2 nM)30, Croatian coastal marine waters (2–9 nM)31, surface coastal waters of the Gulf of Mexico (0.05–4 nM)32, and the Chemotaxis Dock in Woods Hole (0.1 nM)29,33.
In the present study, we examined the microbial production of toluene in the water column of the upwelling ecosystem off Concepción in central Chile, both through laboratory incubations of OMZ waters and through monthly field oceanographic campaigns over an annual cycle. We hypothesized that toluene is a secondary metabolic product of microbial degradation of proteinaceous derived precursors under anaerobic conditions in the water column of the OMZ.
Results
Experimental microbial synthesis of toluene and decay of phenylalanine
Toluene was detected both in anoxic incubations with phenylalanine and in the field by GC–MS, as evidenced by m/z fragments 65 ([M − H+]–C2H2), the base peak tropylium cation 91 [M − H+] and the molecular cation 92 [M+] (Fig. 2A,D). Likewise, deuterated toluene (C6D5–CH3, toluene-d5) was detected in anoxic incubation bottles amended with deuterated phenylalanine. Mass spectra showed the diagnostics m/z fragments 68 ([M − H+]–C2D2), tropylium cation 96 [M − H+], and molecular ion 97 [M+] (Fig. 2A,E). In the 5-day anoxic incubations with phenylalanine (Fig. 3), toluene net production peaked on the third day, reaching 131 ± 3 µM toluene and 102 ± 2 µM toluene-d5 under treatment conditions for denitrification (Fig. 3A); 26 ± 1 µM toluene and 35 ± 8 µM toluene-d5 under conditions for sulfate reduction (Fig. 3B); and 82 ± 7 µM toluene and 94 ± 9 µM toluene-d5 in the absence of added external electron acceptors (Fig. 3C). Toluene and toluene-d5 were below the limit of detection and quantification in oxic incubations (Fig. 3D) and in control incubations (Supplementary Fig. 1A). Net maximum production of toluene and toluene-d5 in the presence of SO42− was ca. 3 times lower than in incubations with NO3− and incubations without electron acceptors (Wilcoxon test p < 0.05).
Most (95%) phenylalanine and phenyl-d5-alanine initially present decayed within 5 days of incubation under all conditions (Fig. 3), without significant differences within and between experimental treatments (Wilcoxon test, p > 0.05). A significantly higher decay of phenylalanine and phenyl-d5-alanine was observed under oxic incubations (100% decay in 3 days) compared with ca. 16% remaining after 3 days under anoxic incubations (Wilcoxon test p < 0.05, Fig. 3). Decay of phenylalanine and phenyl-d5-alanine was not observed in abiotic controls nor in blank incubations (Supplementary Fig. 1B).
Experimental microbial decay of toluene
In the presence of either NO3− or SO42−, about 80% of the deuterated and non-deuterated toluene produced by day three had disappeared within 1 day (Fig. 3A,B), whereas no decay was detected in the absence of electron acceptors, where toluene and toluene-d5 remained at ca. 80 µM until the end of the experiment (Fig. 3C, Wilcoxon < 0.05). Evidence of sulfate reduction through production of HS− during the incubation with SO42− is provided by the mass spectrum (Supplementary Fig. 2).
Year-round and depth variability of toluene in the water column
The water column of the study area (Station 18, 90 m depth, Fig. 1) exhibits a distinct annual cycle where suboxic conditions (lower than 22 µM O2) below 40 m depth are observed for most of the year. However, during austral winter, O2 concentrations increase throughout the water column (Fig. 4A), as a result of the intrusion of the well-oxygenated and less saline ESPTW (Fig. 4B) on the continental shelf off Concepción. During austral summer, salinity increases due to evaporation and entrainment from below, producing summer ESPTW (Fig. 4B). This pattern is consistent with previous investigations on the dynamics of the OMZ off Concepción8,34,35,36,37,38,39.
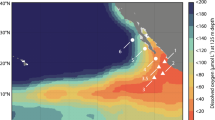
Geographical location of the study site at Station 18, off Concepción in central Chile. Color scale represents the average chlorophyll-a concentration observed between November 2009 and January 2011. The black solid line shows the 200 m isobath. The map was generated using Ocean Data View40).
Toluene concentrations varied between 5 and 96 nM, being lower throughout the whole water column during austral autumn–winter (< 20 nM), but much higher in deeper waters (> 40 m) during austral spring–summer between November 2009, January–March and September–December 2010, and January 2011 (Fig. 5A), consistent with a stratified distribution linked to water mass dynamics (Fig. 5B). Thus, lower concentrations of toluene tend to be associated more with ESPTW, whereas higher concentrations are observed in ESSW (Fig. 5B).
Discussion
Our incubation experiments clearly demonstrated that the microbial assemblage from OMZ waters synthesizes toluene from phenylalanine as an organic substrate under anaerobic conditions (Figs. 2, 3). This corroborates previous observations in bacterial cultures of Clostridium12 and Tolumonas16, in anoxic lake waters41 and sediments15, during anaerobic sludge digestion13,14, and phytoplankton cultures18. Furthermore, the biosynthesis of toluene-d5 from the deuterated aromatic ring of phenyl-d5-alanine shows that phenylalanine was indeed the precursor of toluene (Fig. 2A,D,E). The production of labeled toluene from the aromatic ring of the deuterated phenylalanine suggests a glycyl radical toluene synthase attacks on phenylalanine or phenylacetate methylene β-carbon42. Glycyl radical enzymes are proteins that belong to the enzymes of the Fe–S cluster, one of the oldest proteins of the planet43,44. The gene encoding for Fe–S proteins assembly was recently identified in the metagenome of Marinimicrobia bacteria (MAG-SAR406), a pervasive OMZ bacterium, detected in the Oxygen Minimum Zone of the Tropical Pacific Ocean45,46,47, with the capability for hydrogen and carbon monoxide oxidation, arsenic metabolism, cellulose degradation, and partial denitrification. Being a permanent feature of the modern ocean, we hypothesize that these radical enzymes are widely distributed in the microbial assemblage inhabiting the OMZ. Additionally, bacteria from the Acidobacteria phylum have been detected in OMZ's waters of northern Chile48, and the toluene-producing enzyme phenylacetate decarboxylase (PhdB) and its cognate, a radical S-adenosylmethionine activating enzyme (PhdA), and the genes encoding for the enzyme were identified from the metaproteome and metagenome of an Acidobacteria isolated from an anaerobic sewage sludge9. Acidobacteria were found exclusively in OMZ waters compared to surface and deeper water masses in northern Chile and are therefore a potential producer of toluene; however, their abundance is lower than other pervasive bacterial groups present in these waters48.
(A) Overlaid gas chromatograms showing retention times of toluene and toluene-d5 in incubations anoxic-1 (green line), anoxic-2 (red line), and anoxic-3 (blue line). (B and C) Overlaid chromatograms from control incubations of synthetic seawater (black line) and treatments without inocula (green, red and blue lines, as in caption A) amended with phenylalanine (B) and phenyl-d5-alanine (C). (D) Mass spectrum of toluene (retention time 11.9 min) detected in both environmental samples and in anoxic incubations with phenylalanine as substrate. (E) Mass spectrum of deuterated toluene (retention time 10.2 min) produced in anoxic incubations with l-phenyl-d5-alanine.
Reactions catalyzed by phenylacetate decarboxylases are endothermic; therefore, microbial production of toluene has been associated with gaining selective advantages other than energy conservation9. Toluene-producing bacteria of the OMZ could produce toluene as a mechanism of negative allelopathy against nanoheterotrophs predators abundant in OMZ waters49,50.
Toluene could also be synthesized for intracellular pH homeostasis regulation since phenylacetate decarboxylation removes protons from the cytoplasm, thus promoting cellular alkalinization9. This mechanism could represent a selective advantage for toluene-synthesizing OMZ microorganisms to thrive in the moderately acidic conditions of OMZ waters (pH 7.8) due to the intrusion of the more acidic ESSW51,52,53, and the co-occurrence of metabolic protongenic reactions during glucose, acetate, and Stickland fermentation8. In addition, depletion of protons in the cytoplasm allows the development of proton-motive force that will generate metabolic energy for toluene-producers microbes54.
Although the experiment with deuterated phenylalanine unequivocally confirmed phenylalanine as a precursor for toluene biosynthesis, an expected 1:1 stoichiometric production of toluene from phenylalanine10 was not demonstrated. The observed toluene production accounted for only 5–21% of phenylalanine decay under all anoxic treatments (Fig. 3A–C). Phenylalanine can undergo oxidation to CO255, thus partially explaining the relatively low toluene yield. Anaerobic catabolism of phenylalanine without production of toluene has been shown to produce phenylacetate, phenylpropionate, and phenylacetate in fermenting bacteria Clostridium, and producing phenylacetate in denitrifier Thauera aromatic11,56,57. Thus, decay of phenylalanine in our incubations could result from the activity of a toluene-producing glycyl radical enzyme and from anaerobic catabolism mediated by benzoyl-coenzyme A11,56,57,58, with phenylacetate as the intermediate substrate in both pathways. Another factor that could influence the 1:1 stoichiometry in our incubations is the concurrent microbial oxidation of toluene as it is being produced, as already shown for other environments59,60,61,62,63,64,65,66. In consequence, observed changes in toluene concentrations during incubations will correspond to net production.
Toluene microbial decay by anaerobic oxidation coupled to denitrification and partial denitrification are the most thermodynamically favorable reactions (ΔG > 2500 kJ mol−1) compared with toluene oxidation with NO2− and SO42− and fermentation (Table 1). Metagenome-assembled genomes from the eastern tropical Pacific OMZ contain genes involved in partial denitrification67, supporting the feasibility of this metabolism in OMZ waters.
In anaerobic toluene-degrading communities in aquifer sediments, variability in NO2− and NO3− concentrations determines the fate of reduction of NO3− (NO2− or N2) by modulating changes in the microbial community of denitrifiers that totally or partially denitrifies67. In our study site, the distribution and availability of NO2− showed a clear vertical and temporal pattern with the highest concentrations during austral spring and summer and restricted to the deepest layers, that resembles the vertical distribution of toluene concentrations throughout a year cycle (Supplementary Fig. 3A,C), both significantly correlated (Supplementary Table 1), suggesting the occurrence of partial denitrification. On the contrary, NO3− concentration decreases in the deepest layer during austral summer (Supplementary Fig. 3B), and the year-round pattern resembles the upwelling and productivity cycles in the study area, as shown by negative correlations (Supplementary Table 1) with temperature (an indicator of upwelling) and fluorescence (an indicator of phytoplankton productivity). Taken together, it suggests that partial denitrification during degradation of toluene could be occurring in these waters, apparently associated with the year cycle in nutrient availability, thus providing NO2− to anammox bacteria in these waters68, unveiling a novel and hitherto unknown connection of C and N cycling in OMZ waters.
The anoxic incubations also revealed that ~ 80% of produced toluene disappeared within 1 day in the presence of NO3− or SO42−, whereas in the absence of these electron acceptors, toluene remained at ~ 80 ± 4 µM on day 3 and decayed slightly to less than 75 ± 6 µM by day 5 (Fig. 3C). Thermodynamic considerations support this since fermentation of toluene is endergonic under both experimental and field conditions (Table 1) due to the production of H2 during its disproportionation that drives ΔG to be > 0. However, partial pressure of H2 can be reduced by an electron-acceptor microorganism in a microbial syntrophic consortium, thus rendering an overall exothermic fermentation reaction, as shown in laboratory co-cultures66. Moreover, in a syntrophic coculture, Meckenstock et al.69 also detected that syntrophic degradation is 2–3 times slower than toluene oxidation by sulfate. Our relatively short experimental incubations preclude conclusions regarding whether toluene would remain at 80 ± 4 μM (Fig. 3C) or would slowly decay in the absence of external electron acceptors, given sufficient microbial consortium growth.
Our study site (Station 18) is located 18 nautical miles from the coast of Concepción, a major city of Chile, and therefore input from the urban, maritime, port and industrial activities is conceivable since toluene is universally used in industry (principally as a solvent and gasoline additive) and in domestic products70. However, depth profiles of toluene in the water column (Fig. 5A and Supplementary Fig. 4) indicate natural biogeochemical processes. In austral spring, summer, and autumn, increased concentrations with depth are consistent with a deeper source for toluene in O2-depleted waters (Figs. 4A and 5A). Despite our evidence for potential toluene production in the water column, diffusion from sediments is an additional source that cannot be ruled out, as shown in other coastal anoxic sediments70,71.
(A) Dissolved oxygen time-series data from November 2009 throughout January 2011. The white line represents the 10 µM O2 isoline. Black dots represent sampling points. (B) Potential temperature (ϴ)/salinity diagram from depth profiles taken at Station 18, off Concepción. Data points are color-coded (side color bar) for oxygen concentrations. Rectangles indicate temperature and salinity ranges for Equatorial Subsurface Water (ESSW) and Eastern South Pacific Transition Water (ESPTW) in austral winter and summer. Grey dotted lines represent isopycnals as sigma-t (σ0).
(A) Toluene time-series data from November 2009 to January 2011. White lines represent the 10 and 75 µM toluene isolines. Black dots represent sampling points. (B) Potential temperature (ϴ)/salinity diagram from depth profiles taken at Station 18, off Concepción. Data points are color-coded (side color bar) for toluene concentrations. Rectangles indicate temperature and salinity ranges for Equatorial Subsurface Water (ESSW) and Eastern South Pacific Transition Water (ESPTW) in austral winter and summer. Grey dotted lines represent isopycnals as sigma-t (σ0).
Circulation of water masses forced by atmospheric conditions represents a fundamental control of the annual cycle at the study site. Oceanographic Station 18 (Fig. 1) is at the southernmost limit of the extensive OMZ of the Humboldt Current System72. It is subject to alternate intrusions of oxygenated subantarctic waters during austral autumn–winter and of anoxic and hypoxic waters associated with Equatorial Subsurface Waters (ESSW)37. Concentrations of toluene exceeding 50 nM occurred principally below the 10 µM O2 isoline (Fig. 4A), conditions favorable for the activity of the glycyl radical decarboxylase enzyme9,10, otherwise irreversibly inactivated by O210,19,21.
The vertical distribution of toluene in the water column appears to be inversely related to dissolved oxygen at the study site (Figs. 4A and 5A), with a significant negative correlation between the two (Spearman correlation, ρ = − 0.6, p < 0.05, Supplementary Table 1). Moreover, toluene concentrations correlate negatively with temperature and positively with salinity (Spearman correlation, p < 0.05, Supplementary Table 1), indicative of the annual cycle of upwelling of ESSW and intrusions of ESPTW (Fig. 5B), as previously observed8,37. Significant associations of toluene concentrations with NO2−, PO43−, and volatile fatty acids (VFA; Spearman correlations; p < 0.05; Supplementary Table 1) were also observed. These are considered to be molecular indicators of fermentation8 and denitrification6,73 and provide the chemical environment for toluene production in the water column.
Biosynthesis of toluene from deuterated and non-deuterated phenylalanine was not detected in oxic incubations (Fig. 3D), as might be expected10. This corroborates our observations that the lowest concentrations of toluene occurred in well-oxygenated and low salinity subantarctic waters that form ESPTW in winter and summer (Fig. 5B). Because subantarctic waters are well oxygenated, we would anticipate that no production of toluene occurs in these waters, and we suggest that the relatively small inventory of toluene in ESPTW results from mixing with OMZ waters (Fig. 5). We cannot rule out that OMZ’s pervasive bacterial groups SAR202 and SAR406 may degrade aromatics and high molecular weight recalcitrant dissolved organic matter47,74 using monooxygenase enzymes when oxygenated waters intrude the continental shelf. Therefore, the lower concentrations of toluene observed in the most oxygenated waters of the study site (e.g., ESPTW), could be the net result of input from OMZ waters and microbial aerobic degradation as O2 availability increases during mixing (Fig. 4A,B).
The average depth-integrated toluene inventory of 3 mmol toluene m−2 (Supplementary Fig. 3) represents an estimated reservoir of about 2 million tons of toluene for the whole OMZ off Chile of ca. 10 million km272, is about 50% higher than the inventory of CH4 and near 20 times lower than that of VFA (Supplementary Table 2), putative representative molecules of anaerobic metabolism. The estimated inventory of toluene is a significant quantity compared with the annual toluene production of the European Union of 1–10 million tons71.
Conclusions
In laboratory incubations of OMZ waters off central Chile, microbial assemblages anaerobically biosynthesize toluene from phenylalanine, a finding consistent with the year-round pattern of toluene concentrations in the water column in the upwelling ecosystem of the Humboldt Current System. A fraction of this toluene is exported to surrounding oxygenated waters, namely the subantarctic Eastern South Pacific Transition Water (ESPTW).
Microbial production of toluene increases cellular pH, perhaps counteracting the external acidic media of the OMZ (pH 7.8). The field and laboratory data analysis suggest that, as shown for toluene-degrading communities in aquifer sediments, the availability of NO2− to oxidize toluene could determine the fate of the reduction of NO3− (NO2− or N2).
Our estimate of the potential reservoir of toluene in the OMZ off Chile is in the order of 2 million tons, within the range of CH4 and VFA inventories, putative representative molecules of anaerobic metabolism, as well as in the range of annual industrial production of toluene for the European Union. Warming and eutrophication of coastal waters are likely to further promote hypoxic conditions, enhance anaerobic metabolism, and potentially reveal reservoirs of various organics produced by the OMZ microbiome.
Methods
Sampling
Water samples
Water samples were collected onboard the research vessel Kay-Kay II (Universidad de Concepción) quasi-monthly between November 2009 and January 2011 (13 sampling dates). Seawater was collected at depths of 0, 10, 30, 65, 70, and 80-m using a rosette system equipped with 10-L Niskin bottles. Subsamples of 50 mL volume were transferred onboard to pre-combusted (450 °C, 4 h) gas tight borosilicate Wheaton bottles (darkened with aluminum foil) under a N2 atmosphere—using glove bags (Aldrich AtmosBag) to avoid O2 contamination—then immediately poisoned with HgCl2 (0.001%) to arrest microbial activity. Bottles were crimp-sealed with ultra-pure bromobutyl stoppers (Wheaton) and stored in the dark at 4 °C until further analysis of toluene in the laboratory (within 12 h). Data for temperature, salinity, O2, NO3−, NO2−, PO43−, NH4+ and fluorescence were provided by the COPAS Center Time Series Oceanographic Station 18 (FONDAP COPAS) and the program Microbial Initiative in Low Oxygen off Concepción and Oregon (Moore Foundation http://mi_loco.coas.oregonstate.edu). Volatile fatty acid (VFA) data were taken from Srain et al.8.
Inocula and incubation media
Inocula of seawater for laboratory experiments were obtained on January 18, 2011, from 65 m depth (10.3 °C, 34.6 PSU, 4.0 µM O2) and transferred to 10 mL serum vacuum-tubes BD. Tubes were maintained at 10 °C in darkness until arrival at the laboratory. Inocula and reagents were manipulated in a laminar flow hood LABCONCO Class II Type IIA under a N2-saturated atmosphere (glove bags Aldrich Atmosbag) achieved by bubbling with N2 (99.9% purity). O2-sensitive methylene blue (Resazurin 0.0001%, Wolfe75; McDonald et al.76) was added to anoxic incubation vessels to detect traces of O2 contamination (> 0.7 µM). Artificial seawater for anoxic incubations was prepared according to Lovley77.
Experimental setup
Experimental incubations were conducted in duplicate by transferring 30 mL of artificial seawater into darkened pre-combusted (450 °C, 4 h) borosilicate glass bottles (60 mL, Wheaton), under a N2 saturated atmosphere. l-Phenylalanine in excess (500 µM, CAS 63–91-2) and d-glucose (10 µM, CAS 50-99-7), were added as carbon and nitrogen sources, and inoculated with 10% v/v of seawater collected from the OMZ. Bottles were sealed with ultra-pure bromobutyl stoppers, keeping a headspace volume of 20 mL for sampling of volatile compounds. Incubation of bottles was carried out at 10 °C in darkness, with constant orbital agitation (180 rpm). Incubations were conducted for 5 days with the headspace sampled (500 µL with a Hamilton gas-tight syringe 1750) every ca. 24 h.
Experimental treatments were (in parenthesis the initial final concentration added).
-
(1)
Anoxic-1 incubation under denitrifying conditions: Phenylalanine (500 µM), glucose (10 µM) and NO3− (800 µM). This same concentration of NO3− was subsequently added daily under a N2-saturated atmosphere using a microliter syringe Hamilton (500 µL) previously flushed with N2.
-
(2)
Anoxic-2 incubation under sulfate reduction conditions: Phenylalanine (500 µM), glucose (800 µM) and SO42− (450 µM), amended with 5% v/v of reducing solution (Na2O2S × 5H2O and cysteine) to promote sulfate reduction. This same concentration of SO42− was subsequently added daily under N2-saturated atmosphere using a microliter syringe Hamilton (500 µL) previously flushed with N2.
-
(3)
Anoxic-3 incubation under fermentative conditions: Phenylalanine (500 µM) and glucose (800 µM) without external electron acceptors.
-
(4)
Oxic incubation: Phenylalanine (500 µM), glucose (800 µM) and O2 (370 µM). Incubation flasks were oxygenated to saturation (8.9 mL L−1 at 10 °C and 0.21 atm, Henry’s Law) with synthetic air (ca. 20% O2, 80% N2, 99.99% purity). Five mL aliquots of pure synthetic air were added daily to the incubation flasks using a 1000 µL gas-tight syringe Hamilton.
-
(5)
Experiments Anoxic-1, Anoxic-2, Anoxic-3, and Oxic were also repeated using deuterated phenylalanine (500 µM l-phenyl-d5-alanine, CAS 284664-89-7), instead of phenylalanine, to confirm that toluene synthesis came from phenylalanine.
Anoxic conditions were achieved by gently bubbling N2 into incubation bottles for 15 min to displace traces of O2. Abiotic controls with substrates were incubated without OMZ inocula for each treatment.
Analysis of toluene in ambient and experimental samples
Analysis of toluene was carried out through Headspace Solid Phase Micro–Extraction coupled to Gas Chromatography with MSD detection (HS–SPME–GCMS). Twenty-five mL of water was removed from each sample (n = 3), under a N2 saturated atmosphere, to generate a headspace. Environmental and experimental samples were placed on a thermostatic hotplate at 20 °C, with constant stirring (using an acid-cleaned and sterilized stirring bar) for 5 min to reach partition equilibrium between aqueous and gas phases. Polar and non-polar volatile organic compounds were adsorbed using a fiber of 85 μm Carboxen/PDMS Stable Flex (SUPELCO). The fiber was inserted into the insertion septum of the gas bottle and exposed to the gas phase (headspace). After adsorption of analytes, the fiber was placed in the injection port of the gas chromatograph and exposed for 5 min at 250 °C for desorption of the collected gaseous analytes. GC–MS analyses were conducted using a gas chromatograph Agilent 6890 N series coupled to a mass spectrometer Agilent 5973 Network. The mass spectrometer was operated in electron impact mode (70 eV) and spectra of toluene standards and incubation samples were acquired in full scan mode (m/z 40–600, 2.6 s−1), with spectra for environmental samples acquired using selective ion monitoring (SIM): toluene (tropylium ion m/z 91). Chromatographic separation was achieved using a HP–Plot/Q column 30 m (0.32 mm diameter, 0.20 μm film thickness), using He as carrier gas. The oven temperature program started at 100 °C (5 min), ramped to 230 °C at 50 °C min−1 (held 2 min), to 250 °C at 50 °C min−1 (held 7 min).
Quantification of toluene and deuterated toluene (toluene-d5) in both scan and selective ion monitoring (SIM) modes was determined using calibration curves (R2 > 0.994) for which toluene standards (HPLC grade, ≥ 98.8% purity, Fisher) were added to artificial seawater in the range 1 nmol L–1 –1 mmol L−1. Concentrations of toluene in the gas phase were calculated as CHS = Cap/(K + (VHS/VS)) with the partition coefficient K = CAP/CHS. CHS is the concentration in the headspace, CAP is the concentration in the aqueous phase, and VHS and VS are headspace and sample volumes78, resulting in a partition coefficient of 0.9. Detection limits were calculated from the slopes and residual standard deviations derived from linear regressions of calibration curves (3 times residual error times slope)79. Limit of detection for toluene was 1 nmol L−1, whilst limit of quantification was 5 nmol L−1. Synthetic seawater was analyzed for toluene.
Analyses of dissolved free amino acids (DFAA)
Concentrations of DFAA from incubations, and from Station 18, were quantified as OPA-derivatized adducts80 with a Shimadzu LC-10AT HPLC coupled to a Shimadzu RF-10Axl fluorescence detector (set at excitation/emission of 340/450 nm), column oven CTO 10As and autosampler (Shimadzu SIL 10 ADvp). Aliquots of 0.6 mL, mixed with 0.4 mL methanol, were derivatized in the autosampler with 60 μL ortho-phthalaldehyde/2-mercaptoethanol reagent and 100 μL sodium acetate buffer 0.1 N, pH 5, and then injected (50 μL) into the HPLC. Fifteen amino acids (asp, glu, ser, his, gly, thr, arg, ala, tyr, val, met, phe, ile, leu, lys) were separated using an Alltima C18 (5 mm, 250 × 4.6 mm) column maintained at 40 °C, with a mobile phase of 5% tetrahydrofuran in 25 mM sodium acetate and methanol, and at a flow rate of 1 mL min−1. A gradient of 25–30% methanol in 35 min, 30–50% in 7 min, 50–60% in 18 min, 60–100% in 12 min was used. Initial conditions were restored within 7 min, and the column equilibrated for 10 min between injections. Amino acids were identified and quantified by comparison with chromatograms of a standard amino acid mix (Pierce 20088) run under the same conditions every 10 injections. The coefficient of variation for quantification of duplicate samples was 9.4%.
Statistical analysis
Since homogeneity of variances (Levene test) and normality of variables (Shapiro–Wilk test) were not fulfilled, we tested for significant differences between environmental data using the non-parametric Kruskal–Wallis ANOVA test. Statistical differences between experimental treatments were determined by using the Wilcoxon matched pairs test. Correlations were examined using Spearman R coefficients.
Thermodynamic calculations
Calculated Gibbs Energy values (ΔG) for proposed oxidation reactions of toluene in the suboxic water column were calculated using activities of substrates and products, temperature and pH of the water depth of inoculum collection in austral spring or from literature. The following data and sources were used: temperature (10.2 °C), NO2− (5 µM) and NO3− (25 µM) from the COPAS Center Oceanographic Time Series database (W. Schneider, curator), pH (7.8, this study), toluene (0.09 µM, this study), SO42− (28 mM)81, HCO3− (2 mM)81, N2 (389 µM)82, HS− (1 µM)83,84. Activities of N2, NO2−, NO3−, SO42− and HS− in experimental incubations were determined stoichiometrically from the chemical equations of the proposed reactions. Thermodynamic calculations were carried out using THERMODYN software85.
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information files.
References
Levin, L. A. et al. Effects of natural and human-induced hypoxia on coastal benthos. Biogeosciences. https://doi.org/10.5194/bg-6-2063-2009 (2009).
Ulloa, O. & Pantoja, S. The oxygen minimum zone of the eastern South Pacific. Deep. Res. Top. Part II Stud. Oceanogr. https://doi.org/10.1016/j.dsr2.2008.12.004 (2009).
Stramma, L., Johnson, G. C., Sprintall, J. & Mohrholz, V. Expanding oxygen-minimum zones in the tropical oceans. Science https://doi.org/10.1126/science.1153847 (2008).
Levipan, H. A., Quiñones, R. A., Johansson, H. E. & Urrutia, H. Methylotrophic methanogens in the water column of an upwelling zone with a strong oxygen gradient off Central Chile. Microbes Environ. https://doi.org/10.1264/jsme2.22.268 (2007).
Gutiérrez, M. H., Pantoja, S., Tejos, E. & Quiñones, R. A. The role of fungi in processing marine organic matter in the upwelling ecosystem off Chile. Mar. Biol. https://doi.org/10.1007/s00227-010-1552-z (2011).
Galán, A., Thamdrup, B., Saldías, G. S. & Farías, L. Vertical segregation among pathways mediating nitrogen loss (N2 and N2O production) across the oxygen gradient in a coastal upwelling ecosystem. Biogeosciences. https://doi.org/10.5194/bg-14-4795-2017 (2017).
Plominsky, A. M. et al. Metabolic potential and in situ transcriptomic profiles of previously uncharacterized key microbial groups involved in coupled carbon, nitrogen and sulfur cycling in anoxic marine zones. Environ. Microbiol. https://doi.org/10.1111/1462-2920.14109 (2018).
Srain, B. M. et al. Fermentation and anaerobic oxidation of organic carbon in the oxygen minimum zone of the upwelling ecosystem off concepción Central Chile. Front. Mar. Sci. https://doi.org/10.3389/fmars.2020.00533 (2020).
Beller, H. R. et al. Discovery of enzymes for toluene synthesis from anoxic microbial communities. Nat. Chem. Biol. https://doi.org/10.1038/s41589-018-0017-4 (2018).
Zargar, K. et al. In vitro characterization of phenylacetate decarboxylase, a novel enzyme catalyzing toluene biosynthesis in an anaerobic microbial community. Sci. Rep. https://doi.org/10.1038/srep31362 (2016).
Carmona, M. et al. Anaerobic catabolism of aromatic compounds: A genetic and genomic view. Microbiol. Mol. Biol. Rev. https://doi.org/10.1128/mmbr.00021-08 (2009).
Pons, J. L., Rimbault, A., Darbord, J. C. & Leluan, G. Biosynthèse de toluène chez Clostridium aerofoetidum souche WS. Ann. l’Institut Pasteur Microbiol. https://doi.org/10.1016/S0769-2609(84)80029-0 (1984).
Mrowiec, B., Suschka, J. & Keener, T. C. Formation and biodegradation of toluene in the anaerobic sludge digestion process. Water Environ. Res. https://doi.org/10.2175/106143005x41852 (2005).
Marczak, M., Wolska, L. & Namiesnik, J. Determination of toluene formed during fermentation of sewage sludge. Int. J. Environ. Stud. https://doi.org/10.1080/00207230600662310 (2006).
Jüttner, F. Formation of toluene by microorganisms from anoxic freshwater sediments. Fresenius. J. Anal. Chem. https://doi.org/10.1007/BF00321745 (1991).
Fischer-Romero, C., Tindall, B. J. & Jüttner, F. Tolumonas auensis gen. nov., sp. nov., a toluene-producing bacterium from anoxic sediments of a freshwater lake. Int. J. Syst. Bacteriol. https://doi.org/10.1099/00207713-46-1-183 (1996).
Jüttner, F. & Henatsch, J. J. Anoxic hypolimnion is a significant source of biogenic toluene. Nature https://doi.org/10.1038/323797a0 (1986).
Middelburg, J. J. & Levin, L. A. Coastal hypoxia and sediment biogeochemistry. Biogeosciences. https://doi.org/10.5194/bg-6-1273-2009 (2009).
Heider, J., Spormann, A. M., Beller, H. R. & Widdel, F. Anaerobic bacterial metabolism of hydrocarbons. FEMS Microbiol. Rev. https://doi.org/10.1016/S0168-6445(98)00025-4 (1998).
Rodrigues, A. V., Tantillo, D. J., Mukhopadhyay, A., Keasling, J. D. & Beller, H. R. Insight into the mechanism of phenylacetate decarboxylase (PhdB), a toluene-producing glycyl radical enzyme. ChemBioChem https://doi.org/10.1002/cbic.201900560 (2020).
Selmer, T., Pierik, A. J. & Heider, J. New glycyl radical enzymes catalysing key metabolic steps in anaerobic bacteria. Biol. Chem. https://doi.org/10.1515/BC.2005.114 (2005).
Sawers, G. Biochemistry, physiology and molecular biology of glycyl radical enzymes. FEMS Microbiol. Rev. https://doi.org/10.1016/S0168-6445(98)00020-5 (1998).
Klenk, H. P. et al. The complete genome sequence of the hyperthermophilic, sulphate-reducing archaeon Archaeoglobus fulgidus. Nature https://doi.org/10.1038/37052 (1997).
Smith, D. R. et al. Complete genome sequence of Methanobacterium thermoautotrophicum ΔH: Functional analysis and comparative genomics. J. Bacteriol. https://doi.org/10.1128/jb.179.22.7135-7155.1997 (1997).
Lee, C., Wakeham, S. & Arnosti, C. Particulate organic matter in the sea: The composition conundrum. Ambio https://doi.org/10.1579/0044-7447-33.8.565 (2004).
Wakeham, S. G., Goodwin, J. T. & Davis, A. C. Distributions and fate of volatile organic compounds in Narragansett Bay, Rhode Island. Can. J. Fish. Aquat. Sci. https://doi.org/10.1139/f83-336 (1983).
Wiesenburg, D. A., Bodennec, G. & Brooks, J. M. Volatile liquid hydrocarbons around a production platform in the Northwest Gulf of Mexico. Bull. Environ. Contam. Toxicol. https://doi.org/10.1007/BF01611003 (1981).
Saeed, T., Khordagui, H. & Al-Bloushi, A. Distribution of volatile liquid hydrocarbons in the vicinity of power/desalination plants in Kuwait. Water Sci. Technol. https://doi.org/10.1016/S0273-1223(99)00588-0 (1999).
Sauer, T. C. Volatile liquid hydrocarbons in waters of the Gulf of Mexico and Caribbean Sea. Limnol. Oceanogr. https://doi.org/10.4319/lo.1980.25.2.0338 (1980).
Button, D. K., Robertson, B. R., McIntosh, D. & Juttner, F. Interactions between marine bacteria and dissolved-phase and beached hydrocarbons after the Exxon Valdez oil spill. Appl. Environ. Microbiol. https://doi.org/10.1128/aem.58.1.243-251.1992 (1992).
Karačonji, B., Skender, L. & Karačić, V. Benzene, toluene, ethylbenzene, and isomeric xylenes in various water samples in Croatia. Bull. Environ. Contam. Toxicol. https://doi.org/10.1007/s00128-006-0943-9 (2006).
Sauer, T. C., Sackett, W. M. & Jeffrey, L. M. Volatile liquid hydrocarbons in the surface coastal waters of the Gulf of Mexico. Mar. Chem. https://doi.org/10.1016/0304-4203(78)90039-7 (1978).
Gschwend, P. M., Zafiriou, O. C., Mantoura, R. F. C., Schwarzenbach, R. P. & Gagosian, R. B. Volatile organic compounds at a coastal site. 1. Seasonal variations. Environ. Sci. Technol. https://doi.org/10.1021/es00095a010 (1982).
Schneider, W., Fuenzalida, R., Rodríguez-Rubio, E., Garcés-Vargas, J. & Bravo, L. Characteristics and formation of Eastern South Pacific Intermediate Water. Geophys. Res. Lett. https://doi.org/10.1029/2003GL017086 (2003).
Schneider, W., Donoso, D., Garcés-Vargas, J. & Escribano, R. Water-column cooling and sea surface salinity increase in the upwelling region off central-south Chile driven by a poleward displacement of the South Pacific High. Prog. Oceanogr. https://doi.org/10.1016/j.pocean.2016.11.004 (2017).
Escribano, R. & Schneider, W. The structure and functioning of the coastal upwelling system off central/southern Chile. Prog. Oceanogr. https://doi.org/10.1016/j.pocean.2007.08.020 (2007).
Sobarzo, M., Bravo, L., Donoso, D., Garcés-Vargas, J. & Schneider, W. Coastal upwelling and seasonal cycles that influence the water column over the continental shelf off central Chile. Prog. Oceanogr. https://doi.org/10.1016/j.pocean.2007.08.022 (2007).
Letelier, J., Pizarro, O. & Nuñez, S. Seasonal variability of coastal upwelling and the upwelling front off central Chile. J. Geophys. Res. Ocean. https://doi.org/10.1029/2008JC005171 (2009).
Escribano, R. & Morales, C. E. Spatial and temporal scales of variability in the coastal upwelling and coastal transition zones off central-southern Chile (35–40° S). Prog. Oceanogr. https://doi.org/10.1016/j.pocean.2011.07.019 (2012).
Schlitzer, R. Ocean Data View Version 4.3.6. (2010) Bremerhaven: Alfred Wegener Institute. http://odv.awi.de/
Niggemann, J., Ferdelman, T. G., Lomstein, B. A., Kallmeyer, J. & Schubert, C. J. How depositional conditions control input, composition, and degradation of organic matter in sediments from the Chilean coastal upwelling region. Geochim. Cosmochim. Acta. https://doi.org/10.1016/j.gca.2006.12.012 (2007).
Blattner, F. R. et al. The complete genome sequence of Escherichia coli K-12. Science https://doi.org/10.1126/science.277.5331.1453 (1997).
Weiss, M. C. et al. The physiology and habitat of the last universal common ancestor. Nat. Microbiol. https://doi.org/10.1038/nmicrobiol.2016.116 (2016).
Imlay, J. A. Iron-sulphur clusters and the problem with oxygen. Mol. Microbiol. https://doi.org/10.1111/j.1365-2958.2006.05028.x (2006).
Beman, J. M. et al. Biogeochemistry and hydrography shape microbial community assembly and activity in the eastern tropical North Pacific Ocean oxygen minimum zone. Environ. Microbiol. https://doi.org/10.1111/1462-2920.15215 (2021).
Pajares, S., Varona-Cordero, F. & Hernández-Becerril, D. U. Spatial distribution patterns of bacterioplankton in the oxygen minimum zone of the Tropical Mexican Pacific. Microb. Ecol. https://doi.org/10.1007/s00248-020-01508-7 (2020).
Bertagnolli, A. D., Padilla, C. C., Glass, J. B., Thamdrup, B. & Stewart, F. J. Metabolic potential and in situ activity of marine Marinimicrobia bacteria in an anoxic water column. Environ. Microbiol. https://doi.org/10.1111/1462-2920.13879 (2017).
Ganesh, S., Parris, D. J., Delong, E. F. & Stewart, F. J. Metagenomic analysis of size-fractionated picoplankton in a marine oxygen minimum zone. ISME J. https://doi.org/10.1038/ismej.2013.144 (2014).
Böttjer, D. & Morales, C. E. Nanoplanktonic assemblages in the upwelling area off Concepción (∼ 36°S), central Chile: Abundance, biomass, and grazing potential during the annual cycle. Prog. Oceanogr. https://doi.org/10.1016/j.pocean.2007.08.024 (2007).
Cuevas, L. A. & Morales, C. E. Nanoheterotroph grazing on bacteria and cyanobacteria in oxic and suboxic waters in coastal upwelling areas off northern Chile. J. Plankton Res. https://doi.org/10.1093/plankt/fbi124 (2006).
Vargas, C. A. et al. Influences of riverine and upwelling waters on the coastal carbonate system off Central Chile and their ocean acidification implications. J. Geophys. Res. Biogeosci. https://doi.org/10.1002/2015JG003213 (2016).
Feely, R. A., Sabine, C. L., Hernandez-Ayon, J. M., Ianson, D. & Hales, B. Evidence for upwelling of corrosive ‘acidified’ water onto the continental shelf. Science (80-). https://doi.org/10.1126/science.1155676 (2008).
Torres, R. et al. Air-sea CO2 fluxes along the coast of Chile: From CO2 outgassing in central northern upwelling waters to CO2 uptake in southern Patagonian fjords. J. Geophys. Res. Ocean. https://doi.org/10.1029/2010JC006344 (2011).
Michels, P. A. M., Michels, J. P. J., Boonstra, J. & Konings, W. N. Generation of an electrochemical proton gradient in bacteria by the excretion of metabolic end products. FEMS Microbiol. Lett. https://doi.org/10.1111/j.1574-6968.1979.tb03339.x (1979).
Mohamed, M. E. S., Seyfried, B., Tschech, A. & Fuchs, G. Anaerobic oxidation of phenylacetate and 4-hydroxyphenylacetate to benzoyl-coenzyme A and CO2 in denitrifying Pseudomonas sp.—Evidence for an α-oxidation mechanism. Arch. Microbiol. https://doi.org/10.1007/BF00249036 (1993).
Barker, H. A. Amino acid degradation by anaerobic bacteria. Annu. Rev. Biochem. https://doi.org/10.1146/annurev.bi.50.070181.000323 (1981).
Schneider, S., Mohamed, M. E. S. & Fuchs, G. Anaerobic metabolism of l-phenylalanine via benzoyl-CoA in the denitrifying bacterium Thauera aromatica. Arch. Microbiol. https://doi.org/10.1007/s002030050504 (1997).
Debnar-Daumler, C., Seubert, A., Schmitt, G. & Heider, J. Simultaneous involvement of a tungsten-containing aldehyde: Ferredoxin oxidoreductase and a phenylacetaldehyde dehydrogenase in anaerobic phenylalanine metabolism. J. Bacteriol. https://doi.org/10.1128/JB.00980-13 (2014).
Wakeham, S. G., Canuel, E. A. & Doering, P. H. Geochemistry of volatile organic compounds in seawater: Mesocosm experiments with 14C-model compounds. Geochim. Cosmochim. Acta. https://doi.org/10.1016/0016-7037(86)90399-6 (1986).
Lovley, D. R. et al. Oxidation of aromatic contaminants coupled to microbial iron reduction. Nature https://doi.org/10.1038/339297a0 (1989).
Dolfing, J., Zeyer, J., Binder-Eicher, P. & Schwarzenbach, R. P. Isolation and characterization of a bacterium that mineralizes toluene in the absence of molecular oxygen. Arch. Microbiol. https://doi.org/10.1007/BF00276528 (1990).
Evans, P. J., Mang, D. T., Kim, K. S. & Young, L. Y. Anaerobic degradation of toluene by a denitrifying bacterium. Appl. Environ. Microbiol. https://doi.org/10.1128/aem.57.4.1139-1145.1991 (1991).
Rabus, R., Nordhaus, R., Ludwig, W. & Widdel, F. Complete oxidation of toluene under strictly anoxic conditions by a new sulfate-reducing bacterium. Appl. Environ. Microbiol. https://doi.org/10.1128/aem.59.5.1444-1451.1993 (1993).
Beller, H. R., Spormann, A. M., Sharma, P. K., Cole, J. R. & Reinhard, M. Isolation and characterization of a novel toluene-degrading, sulfate-reducing bacterium. Appl. Environ. Microbiol. https://doi.org/10.1128/aem.62.4.1188-1196.1996 (1996).
Harms, G., Rabus, R. & Widdel, F. Anaerobic oxidation of the aromatic plant hydrocarbon p-cymene by newly isolated denitrifying bacteria. Arch. Microbiol. https://doi.org/10.1007/s002030050784 (1999).
Meckenstock, R. U., Krieger, R., Ensign, S., Kroneck, P. M. H. & Schink, B. Acetylene hydratase of Pelobacter acetylenicus: Molecular and spectroscopic properties of the tungsten iron-sulfur enzyme. Eur. J. Biochem. https://doi.org/10.1046/j.1432-1327.1999.00600.x (1999).
Zhu, B., Friedrich, S., Wang, Z., Táncsics, A. & Lueders, T. Availability of nitrite and nitrate as electron acceptors modulates anaerobic toluene-degrading communities in aquifer sediments. Front. Microbiol. https://doi.org/10.3389/fmicb.2020.01867 (2020).
Sun, X. & Ward, B. B. Novel metagenome-assembled genomes involved in the nitrogen cycle from a Pacific oxygen minimum zone. ISME Commun. https://doi.org/10.1038/s43705-021-00030-2 (2021).
Meckenstock, R. U. Fermentative toluene degradation in anaerobic defined syntrophic cocultures. FEMS Microbiol. Lett. https://doi.org/10.1016/S0378-1097(99)00290-6 (1999).
Bravo-Linares, C. M. & Mudge, S. M. Analysis of volatile organic compounds (VOCs) in sediments using in situ SPME sampling. J. Environ. Monit. https://doi.org/10.1039/b617215f (2007).
Guigue, C. et al. Remobilization of polycyclic aromatic hydrocarbons and organic matter in seawater during sediment resuspension experiments from a polluted coastal environment: Insights from Toulon Bay (France). Environ. Pollut. https://doi.org/10.1016/j.envpol.2017.06.090 (2017).
Fuenzalida, R., Schneider, W., Garcés-Vargas, J., Bravo, L. & Lange, C. Vertical and horizontal extension of the oxygen minimum zone in the eastern South Pacific Ocean. Deep. Res. Part II Top. Stud. Oceanogr. https://doi.org/10.1016/j.dsr2.2008.11.001 (2009).
Galán, A., Fauńdez, J., Thamdrup, B., Santibáñez, J. F. & Farías, L. Temporal dynamics of nitrogen loss in the coastal upwelling ecosystem off Central Chile: Evidence of autotrophic denitrification through sulfide oxidation. Limnol. Oceanogr. https://doi.org/10.4319/lo.2014.59.6.1865 (2014).
Landry, Z., Swa, B. K., Herndl, G. J., Stepanauskas, R. & Giovannoni, S. J. SAR202 genomes from the dark ocean predict pathways for the oxidation of recalcitrant dissolved organic matter. MBio https://doi.org/10.1128/mBio.00413-17 (2017).
Wolfe, R. S. Techniques for cultivating methanogens. Methods Enzymol. https://doi.org/10.1016/B978-0-12-385112-3.00001-9 (2011).
McDonald, J. E., Rooks, D. J. & McCarthy, A. J. Methods for the isolation of cellulose-degrading microorganisms. Methods Enzymol. https://doi.org/10.1016/B978-0-12-415931-0.00019-7 (2012).
Lovley, D. Dissimilatory Fe(III)- and Mn(IV)-reducing prokaryotes. Prokaryotes Prokaryotic Physiol. Biochem. https://doi.org/10.1007/978-3-642-30141-4_69 (2013).
Slack, G. C., Snow, N. H. & Kou, D. Extraction of volatile organic compounds from solids and liquids. Sample Preparation Tech. Analyt. Chem. https://doi.org/10.1002/0471457817.ch4 (2003).
Shrivastava, A. & Gupta, V. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chronicles Young Sci. https://doi.org/10.4103/2229-5186.79345 (2011).
Lindroth, P. & Mopper, K. High performance liquid chromatographic determination of subpicomole amounts of amino acids by precolumn fluorescence derivatization with o-phthaldialdehyde. Anal. Chem. https://doi.org/10.1021/ac50047a019 (1979).
Pilson, M. E. Q. An introduction to the chemistry of the sea. Introduction Chem. Sea. https://doi.org/10.1017/cbo9781139047203 (2012).
Craig, H., Weiss, R. F. & Clarke, W. B. Dissolved gases in the equatorial and South Pacific Ocean. J. Geophys. Res. https://doi.org/10.1029/jz072i024p06165 (1967).
Lavik, G. et al. Detoxification of sulphidic African shelf waters by blooming chemolithotrophs. Nature https://doi.org/10.1038/nature07588 (2009).
Schunck, H. et al. Giant Hydrogen sulfide plume in the oxygen minimum zone off peru supports chemolithoautotrophy. PLoS ONE https://doi.org/10.1371/journal.pone.0068661 (2013).
Damgaard, L. R. & Hanselmann, K. Thermodyn©—A spread sheet for the calculation of free reaction energies under actual conditions. Based on K.W. Hanselmann. Microbial energetics applied to waste repositories. Experientia 47, 645–687. https://doi.org/10.1007/BF01958816 (1991).
Acknowledgements
This research was funded by the Center for Oceanographic Research in the eastern South Pacific (COPAS FONDAP 15010007), COPAS Sur-Austral (AFB170006 and ACE210004), COPAS Coastal (FB210021), the Gordon and Betty Moore Foundation (Oregon-Concepción, Grant 1661) and FONDECYT 1200252. We acknowledge the support provided by the COPAS Oceanographic Time Series St. 18 off Concepción. We are grateful to the crew of the L/C Kay-Kay II for help during sampling, the personnel of the Marine Organic Geochemistry Laboratory at University of Concepción, and Dr. Giovanni Testa for providing Figure 1.
Author information
Authors and Affiliations
Contributions
B.M.S., designed experiments and fieldwork, conducted thermodynamic, statistical and chemical analyses and laboratory incubations, and drafted and edited the manuscript. S.P.G., co-designed experiments and fieldwork, supervised analyses, and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Srain, B.M., Pantoja-Gutiérrez, S. Microbial production of toluene in oxygen minimum zone waters in the Humboldt Current System off Chile. Sci Rep 12, 10669 (2022). https://doi.org/10.1038/s41598-022-14103-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-14103-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.