Abstract
There is conflicting evidence on the role of lipid biomarkers in breast cancer (BC), and no study to our knowledge has examined this association among African women. We estimated odds ratios (ORs) and 95% confidence intervals (95% CI) for the association of lipid biomarkers—total cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), and triglycerides—with odds of BC overall and by subtype (Luminal A, Luminal B, HER2-enriched and triple-negative or TNBC) for 296 newly diagnosed BC cases and 116 healthy controls in Nigeria. Each unit standard deviation (SD) increase in triglycerides was associated with 39% increased odds of BC in fully adjusted models (aOR: 1.39; 95% CI: 1.03, 1.86). Among post-menopausal women, higher total cholesterol (aOR: 1.65; 95% CI: 1.06, 2.57), LDL cholesterol (aOR: 1.59; 95% CI: 1.04, 2.41), and triglycerides (aOR: 1.91; 95% CI: 1.21, 3.01) were associated with increased odds of BC. Additionally, each unit SD increase in LDL was associated with 64% increased odds of Luminal B BC (aOR 1.64; 95% CI: 1.06, 2.55). Clinically low HDL was associated with 2.7 times increased odds of TNBC (aOR 2.67; 95% CI: 1.10, 6.49). Among post-menopausal women, higher LDL cholesterol and triglycerides were significantly associated with increased odds of Luminal B BC and HER2 BC, respectively. In conclusion, low HDL and high LDL are associated with increased odds of TN and Luminal B BC, respectively, among African women. Future prospective studies can definitively characterize this association and inform clinical approaches targeting HDL as a BC prevention strategy.
Similar content being viewed by others
Introduction
Breast cancer (BC) in Nigeria, like in other West African countries and among Black patients in the United States (US), is characterized by disproportionately high rates of the triple-negative (TN) molecular subtype1,2,3. TNBCs are aggressive cancers, described by estrogen (ER), progesterone (PR), and human epidermal growth factor receptor 2 (HER2) negativity and associated with poor clinical outcomes4,5. Africa suffers from the highest age-standardized BC mortality rate globally6, and the past few decades have observed increasing BC incidence on the African continent7. An understanding of the risk factors contributing to the higher prevalence of TNBCs among women of African descent is crucial to the development of preventive interventions that may reduce the BC burden within this population. In addition to increasing BC incidence, the African continent has also experienced significantly increasing rates of obesity, diabetes, and dyslipidemia (abnormally elevated blood cholesterol or lipid levels), so called “diseases of affluence” due to globalization and the epidemiologic transition8,9. Prior studies have documented a positive association between measures of excess adiposity and BC incidence10,11, but none to our knowledge has examined specific biomarkers associated with dyslipidemia with BC risk by molecular subtype on the African continent.
Prior studies in the US, Europe and parts of Asia evaluating the relationship between serum lipids and risk of BC have been inconclusive, and several review papers have summarized published results on this topic. A recent systematic review of prospective studies reported an inverse association between biomarkers of total cholesterol and high-density lipoprotein (HDL) cholesterol and risk of breast cancer, but no significant associations with low-density lipoprotein (LDL) cholesterol12. This study noted significant heterogeneity among included studies for total cholesterol based on geographical location. The inverse association for HDL cholesterol was replicated in a separate systematic review which also reported a positive association for LDL cholesterol13. A third meta-analysis found that higher triglyceride levels, but not total cholesterol, HDL cholesterol or LDL cholesterol levels was inversely associated with BC risk14. It is worth noting that the majority of studies on this topic have been conducted among White populations in the United States and Europe. Studies among African American populations are limited and conflicting. While one study among African Americans in the United States found a statistically significant reduction in BC risk with high levels of total cholesterol and a significant increase in risk associated with low HDL cholesterol15; another study reported no significant association with total cholesterol16. Research on this topic deserves further study to more clearly elucidate the association between lipid biomarkers and BC risk. To our knowledge, studies on this topic have not been conducted in Nigeria or West Africa.
Importantly, few epidemiological studies have examined the association between lipids and BC molecular subtype. One study in Korea noted that low HDL cholesterol and high levels of triglycerides were associated with an increased risk of developing hormone receptor negative tumors17. Another study in Spain found that the risk of postmenopausal Luminal A BC significantly increased with higher circulating levels of triglycerides18. However, no study to our knowledge has examined this association among African women or in African American women, despite the higher risk of TNBC in these populations. To our knowledge, ours is the first study to evaluate the association between total cholesterol, LDL cholesterol, HDL cholesterol, and triglycerides with BC molecular subtypes among Nigerian women. Blood lipids are easily measurable markers that are routinely assessed in clinical practice. Thus, further insight on this relationship by molecular subtype may enable the development of preventative strategies that are well-suited to the Nigerian and African context.
Methods
Study design
The Mechanisms for Established and Novel Risk Factors for Breast Cancer in Women of Nigerian Descent (MEND) study has been previously described in detail19. Briefly, MEND enrolled newly diagnosed BC patients from four hospitals in southwestern Nigeria. At each hospital site, a trained nurse explained the study requirements to suspected BC patients during their clinical visits. Interested participants were evaluated for eligibility. Reasons for exclusion included an inability to communicate in English to complete the required baseline survey, prior diagnosis and/or treatment for cancer, and other medical conditions that may have interfered with participation in the study. All study participants gave written and verbal informed consent, and then completed a questionnaire that covered information on sociodemographic characteristics, reproductive history, and past personal and family history of cancer. Anthropomorphic measurements were taken, and blood samples and tumor biopsy samples were collected. All samples were obtained at the time of biopsy prior to receipt of any surgery, chemotherapy, or radiation treatment. After collection and processing, tissue and blood samples were stored in −80 °C freezers until shipment to the United States for assays and further analysis. For their participation in this study, participants received an N500 telephone recharge card (valued at US $1.50) in addition to the supplies necessary for their biopsy. Healthy controls were selected from a cohort of 4,000 healthy, community-based women recruited as part of the Human Heredity and Health (H3) Africa Chronic Kidney Disease (CKD) Case–Control study20. The CKD study recruited from Nigeria and Ghana between 2015 and 2017, overlapping with case recruitment. Controls were recruited from churches, communities, and business offices. The present analysis was restricted to controls recruited from Nigeria due to significant country-level differences in cholesterol. Recruitment of Nigerian controls occurred in the South-Western region of the country, overlapping with the case recruitment region. Extensive socio-demographic, clinical, family history and behavioral risk factor data was collected, and blood samples were collected and processed at clinical labs following a standardized protocol. Serum samples for cases and controls were assayed for lipid biomarkers at the Duke Molecular Pathology Institute at the same time, and the laboratory technician was blinded to case status. These procedures were approved by the Institutional Review Boards at Duke University and the participating hospitals. Among MEND cases, there were only 15 refusals and 1 withdrawal, and similarly low rates were observed among controls.
Breast cancer cases and subtyping
BC diagnosis was ascertained either through pathology reports of clinical biopsy samples evaluated by a trained pathologist from the diagnosing hospital in Nigeria, or from biopsy samples that were shipped to the US for review by a trained US pathologist. If either indicated a cancer diagnosis, the sample was considered a confirmed case. Confirmed samples underwent immunohistochemistry in Nigeria as part of regular standard of care procedures, or at the Duke University BioRepository and Precision Pathology Center. Due to infrastructural limitations, it was not possible to complete immunohistochemistry within routine clinical care locally in Nigeria for all patients. If results from both sources were available, US typing was used as it constituted most of the available immunohistochemistry information on cases. Estrogen receptor (ER) and progesterone receptor (PR) status was scored using the Allred method21,22. The intensity of staining was categorized as 0 (none), 1 (mild), 2 (moderate), or 3 (strong), and the proportion of nuclear positivity was scored into 0 (0%), 1 (< 1%), 2 (1–10%), 3 (11–33%), 4 (33–66%) or 5 (67–100%). The numbers from these two scores were summed to positive (3–8) or negative (0–2). HER2 status was categorized as negative (scores = 0–1) or positive (score = 3) based on immunohistochemistry membrane staining; intense membrane staining of 30% of tumor cells constituted a positive result23. There were no equivocal (score = 2) results in our sample. Based on these categorizations, cancer subtype was determined: Luminal A (ER+ and/or PR+ /HER2-), Luminal B (ER+ and/or PR+ /HER2 +), TN (ER-/PR-/HER2-), or HER2 (ER-/PR-/HER2+). In all, there were 124 cases with available data on ER/PR/HER2 status for classification into a molecular subtype. There was no systematic selection of cases for subtyping. Cases with available molecular subtypes were similar to all cases by demographic, clinical, and reproductive characteristics.
Measures
Measurements of total cholesterol, HDL, LDL, and triglycerides for cases and controls were performed using a Beckman DxC 600 clinical analyzer with assays that utilized standard reagents also from Beckman (Brea, CA). There was no systematic selection of participants for lipid measurements. Cases with available lipid results were similar to all cases by demographic, clinical, and reproductive characteristics. Following the joint harmonized criteria for metabolic syndrome and guidelines set by the National Cholesterol Education Program, high total cholesterol was defined as >200 mg/dL24; low HDL was defined as <50 mg/dL25; high LDL was defined as >100 mg/dL24; and high triglycerides was defined as >150 mg/dL25. In addition, lipid measures were specified as standard deviation (SD) change by subtracting the sample mean from individual measurements and dividing by the sample standard deviation. Other covariates included in analysis were staff assessed height, weight, blood pressure; participants self-reported reproductive and clinical history, including age at menarche, number of pregnancies, number of births, and menopausal status. Participants who self-reported a history of cancer and those missing this information were excluded from the present analysis, in addition to participants who were missing information on their menopausal status. Missing values for variables with <10% missing for both cases and controls were replaced with the median (for continuous variables) or modal (for categorical variables) value of their respective group. For variables with more than >10% missing, missing values were not imputed (age at menarche).
Analytical approach
The sample was characterized via descriptive statistics, and results were reported as frequencies and proportions for categorical variables and medians (first quartile, third quartile) for continuous variables. Differences in associations by case/control status were tested using chi-square (χ2) tests or Fisher exact tests for categorical variables and Kruskal–Wallis nonparametric tests for continuous variables. We estimated the association between each lipid biomarker (total cholesterol, HDL, LDL, and triglycerides) and odds of BC using logistic regression models. Each measure was analyzed separately in the following three models: unadjusted, adjusted for age only, and adjusted for age, body mass index (BMI), age at menarche, number of pregnancies, number of births, hypertension at enrollment, and menopausal status. In a final model, we mutually adjusted for all lipid measures in addition to all previous covariates. Selection of covariates was based on a priori knowledge regarding the relationships between these factors and exposure and outcome. In each model, we specified each lipid biomarker as a categorical variable (high vs. low for total cholesterol, LDL, and triglycerides; and low vs. high for HDL), and also evaluated continuous measures of total cholesterol, HDL, LDL, and triglycerides based on one-unit SD increase (for total cholesterol, LDL, and triglycerides) or decrease (for HDL). We stratified our analysis of the continuous lipid profile measures by menopausal status. We further analyzed the subset of cases with cancer subtyping data available via multinomial logistic regression models. Control status was specified as the outcome reference group, and the fully adjusted model was repeated here to predict the odds of having Luminal A, Luminal B, TN, HER2 cancer subtypes. To address the issue of multiple comparisons, we applied the Bonferroni correction, and set significance at α = 0.0125 (0.05/4) for these associations. SAS v9.4 (SAS Institute, Cary, NC) was used for all analyses and significance was broadly set at α = 0.05.
Ethical approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the Institutional Review Board of Duke University (Pro00102004). This article does not contain any studies with animals performed by any of the authors. Informed consent was obtained from all individual participants included in the study.
Results
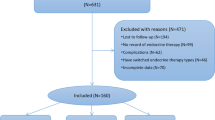
The present analysis includes 296 BC cases and 116 healthy controls (Fig. 1). Cases were slightly older than controls—the median age at diagnosis for cases was 48.5 years, and the median age at enrollment for controls was 46 years (Table 1). Cases and controls were similar in terms of reproductive characteristics: number of pregnancies (5 vs. 5), number of births (4 vs. 4), and menopausal status (pre/peri-menopausal 48% vs. 49%). However, cases were more likely than controls to have high diastolic blood pressure (79.7 vs. 75.0), while controls were more likely to have higher BMI (25.4 vs. 26.5). Across total cholesterol quartiles (Table 2), those in the highest cholesterol group were older (p = 0.0003), and more likely to be a higher weight (p = 0.0593), have a higher blood pressure (systolic: p = 0.0067; diastolic: p = 0.0202) and be post-menopausal (p = 0.0003). A higher proportion of controls relative to cases were within the lowest total cholesterol quartiles among participants who were 60 years or older (Fig. 2).
In fully adjusted multivariable logistic regression models (Table 3), one-unit SD increase in triglycerides was associated with 39% increased odds of BC overall (aOR: 1.39; 95% CI: 1.03, 1.86). Each SD increase in triglycerides remained significantly associated with odds of BC (aOR 1.47; 95% CI 1.06, 2.03) in the mutually adjusted model including all four lipid profile measures. Among post-menopausal women, one-unit SD increases in total cholesterol (aOR: 1.65; 95% CI: 1.06, 2.57), LDL cholesterol (aOR: 1.59; 95% CI: 1.04, 2.41), and triglycerides (aOR: 1.91; 95% CI: 1.21, 3.01) were associated with increased odds of BC in fully adjusted models. No significant associations were observed among pre/peri-menopausal women.
In multinomial logistic regression models predicting the odds of each molecular subtype relative to controls (Table 4), clinically low HDL was associated with 2.7 times the odds of TNBC (aOR: 2.67; 95% CI: 1.10, 6.49). Additionally, each unit SD increase in LDL was associated with 64% increased odds of Luminal B BC (aOR: 1.64; 95% CI: 1.06, 2.55). These associations were both significant at α = 0.05 without accounting for multiple comparisons; however, they were not significant following the Bonferroni correction (α = 0.0125). Among post-menopausal women, one-unit SD increases in LDL cholesterol and triglycerides were significantly associated with increased odds of Luminal B BC (aOR: 3.52; 95% CI: 1.48, 8.35) and HER2 BC (aOR: 4.15; 95% CI: 1.71, 10.05), after accounting for multiple comparisons. No significant associations were observed among pre/peri-menopausal women in the subtype analysis.
Discussion
For the first time, we describe the results of a case-control analysis of lipid biomarkers (total cholesterol, HDL cholesterol, LDL cholesterol, and triglycerides) and odds of BC and molecular subtypes among African women. Among cases and controls, those who were older, had high BMI and high blood pressure at enrollment were more likely to have high cholesterol. Higher triglycerides were associated with increased odds of BC in fully adjusted models. Among post-menopausal women, higher total cholesterol, LDL cholesterol, and triglycerides were all associated with increased odds of BC. In the analysis of molecular subtypes, low HDL and high LDL were associated with increased odds of the TNBC and Luminal B subtypes, respectively. Among post-menopausal women, higher LDL and triglycerides were significantly associated with increased odds of the Luminal B and HER2 subtypes, respectively.
Several past studies among populations from the US, Europe, and Asia have evaluated the association between lipid biomarkers and BC risk, however results have been inconsistent. For total cholesterol, one study in Korea noted a positive association with BC risk26, but others in the US and Europe, like ours, have found no association16,27, and one study additionally observed an inverse association28. In the context of LDL, a case-control study among African American women in the US found a 59% reduction in risk among those who had clinically high levels of LDL cholesterol15. Other studies in the US, Asia, and Europe, like ours, have also found no association14,29, although one Mendelian randomization study among those of European descent documented a positive association30. We did not observe a significant association between HDL cholesterol and odds of BC. One study in Europe found an inverse association between HDL cholesterol and BC risk28, while a Mendelian randomization analysis in Europe found that an increase in genetically-predicted HDL was associated with increased BC risk31. However, others in the US and Europe have failed to find an association with HDL29,32. Regarding triglycerides, one study using the Swedish AMORIS database noted a weak protective association with risk of BC33, while others still have reported no association28,34. On the contrary, two small case–control studies in India and the US, like ours, found a positive association between triglycerides and BC35,36. Ultimately, there is inconclusive evidence regarding the role of lipid biomarkers in BC risk, suggesting that additional studies on this topic are still warranted, and importantly, studies from diverse populations will be needed to determine if region-specific associations may explain the disparate findings.
Our analysis of the association between lipid measures and BC subtypes revealed that low HDL cholesterol level is associated with increased odds of TNBC, and that higher LDL is associated with increased odds of Luminal B BC. We also found that higher LDL is associated with increased odds of Luminal B BC and that higher triglycerides is associated with increased odds of HER2 BC among post-menopausal women, but not among pre/peri-menopausal women. Our results are inconsistent with findings from a study from Korea reporting that low HDL cholesterol and high triglycerides were associated with an increased risk of developing hormone receptor negative tumors among premenopausal women17. Consistent with our results, a study among patients from the US found that dyslipidemia, investigated as part of metabolic syndrome, was associated with TNBC, and specifically, low HDL was associated with TNBC37. Given that epidemiologic studies evaluating the association of lipid biomarkers and BC subtypes are very limited, our findings provide important initial evidence upon which future studies can expand.
The biological mechanisms underlying the association between lipids and BC remain unclear and is an active area of research. Studies have suggested that elevated serum cholesterol levels may advance tumor progression38, and a recent review of laboratory studies suggests that cholesterol is capable of regulating proliferation, migration, and signaling pathways in BC39. Research on mechanisms underlying risk by molecular BC subtype is limited, however, as suggested by Llanos et al.15, it is possible that HDL influences overall BC risk by moderating biologically active estradiol40, a risk factor for BC among postmenopausal women41. Low HDL cholesterol may reflect an unfavorable hormonal profile, and the conversion of androgens to estrogens within adipose tissues may represent a causal mechanism for the inverse association between HDL and BC risk40. Fernandez and Murillo demonstrated that HDL is inversely correlated with waist circumference and higher BMI42, providing support for the mediating role of adiposity. In a previous study of the same population, we found that higher BMI was associated with reduced odds of breast cancer43. It is also possible that HDL plays a key role in reverse cholesterol transport that may contribute to the blocking of tumor progression and ultimately BC incidence. Although reverse cholesterol transport may be its primary role, HDL has also been shown to possess antimicrobial, antioxidant, antiglycation, anti-inflammatory, antiatherogenic, and immunosuppressive properties44,45,46. The numerous functions of HDL provide a plethora of opportunities for novel research, but also make pinpointing the exact mechanism by which it may confer protection against BC difficult. Some of the conflicting results in the epidemiology of HDL and BC risk may be explained, in part, by the observation that the environment in which HDL exists in the body may influence its effect on BC cells. Pan and colleagues used in vivo and in vitro models of BC and observed that oxidized HDL and HDL derived from diabetic patients were associated with the promotion of metastasis and invasion to surrounding tissues47,48,49. Future studies on the role of cholesterol oxidation products and signaling pathways may shed additional insights into these mechanisms50,51. Still, these explanations are not specific to TNBC, and further studies are needed to fully characterize these mechanisms by BC subtype.
Understanding the mechanism by which HDL has shown an inverse association with TNBC is further complicated by challenges related to sample size as TNBC typically accounts for an estimated 15–20% of all BCs. Further, although an estimated 80% of TNBC are classified as the basal BC intrinsic subtype52, new research suggests that TNBC may actually be quite heterogenous with respect to cellular and molecular features53. African American women tend to demonstrate patterns of TNBC occurrence that map more closely with women from western and sub-Saharan Africa than they do with women from east Africa, implicating a role of genetic factors54,55. That clinically low HDL was associated with TNBC provides a possibility of a therapeutic target for the BC subtype that is the most aggressive, has a poor prognosis, and by definition, cannot be targeted with pharmaceutical therapy designed for ER + cancers. Still as we point out, the mechanisms underlying the inverse association between HDL and TNBC risk require vigorous investigations, perhaps pooled analyses across existing studies may provide additional insight.
There are several strengths and limitations of this study that may impact the interpretation of these results. Many covariates were self-reported by participants, potentially introducing recall bias into our analysis. However, our main exposures of interest, namely total cholesterol, HDL, LDL, and triglycerides were assayed for cases and controls at the same time following the same standard assay protocol, thus minimizing batch effects. Moreover, due to the case-control study design, we are unable to rule out the possibility of reverse causality. It is possible that lipid levels may be influenced by the presence of BC, producing the observed association. Furthermore, due to lack of available data, we were unable to incorporate the use of drug treatment for dyslipidemia and hypertension in our analysis; it is possible that treatment for these conditions may influence results. We were also unable to adjust our models for waist circumference, as this variable was not available for controls. Although we adjusted for BMI, we acknowledge that there may be residual confounding by adiposity. In future studies, we additionally hope to evaluate potential confounding by socioeconomic status. Strengths of our study include the use of histologically confirmed cancer cases and pathologically verified molecular subtypes assessed by a single pathologist, the availability of data on critical reproductive history and clinical characteristics for covariate adjustment, and the unique study population of Nigerian women—adding to the diversity of study populations for this topic. Although our sample size is modest compared with other large cohorts, we emphasize that our study is the first to characterize the association between lipid profile measures and BC risk in Nigeria, and one of very few studies worldwide to evaluate the association between lipid profile measures and BC risk by subtype. We lay important groundwork for future large prospective studies among African women.
Conclusions
We evaluated the association between lipid profile biomarkers and odds of BC for the first time among Nigerian women, a population that is disproportionately affected by aggressive TNBC. Past research on this topic is highly conflicting, and few studies worldwide have evaluated associations by specific BC molecular subtypes. We report a positive association between triglycerides and odds of BC, and between low HDL with TNBC, and high LDL with Luminal B BC. Lipids are easily measured in clinical settings, making this an attractive target for cancer prevention strategies that may reduce the risk of BC among Nigerian women.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- 95% CI:
-
95% Confidence interval
- aOR:
-
Adjusted odds ratio
- BMI:
-
Body mass index
- BC:
-
Breast cancer
- CKD:
-
Chronic kidney disease
- ER:
-
Estrogen receptor
- H3A:
-
Human Heredity and Health Africa
- HDL:
-
High-density lipoprotein cholesterol
- HER2:
-
Human epidermal growth factor-2
- IHC:
-
Immunohistochemistry
- LDL:
-
Low-density lipoprotein cholesterol
- MEND:
-
Mechanisms for Established and Novel Risk Factors for Breast Cancer in Women of Nigerian Descent
- OR:
-
Odds ratio
- PR:
-
Progesterone receptor (PR)
- SD:
-
Standard deviation
- TME:
-
Tumor microenvironment
- TN:
-
Triple-negative
- US:
-
United States
References
Dietze, E. C., Sistrunk, C., Miranda-Carboni, G., O’Regan, R. & Seewaldt, V. L. Triple-negative breast cancer in African-American women: Disparities versus biology. Nat. Rev. Cancer 15(4), 248–254 (2015).
Newman, L. A. et al. Hereditary susceptibility for triple negative breast cancer associated with western sub-saharan african ancestry: Results from an international surgical breast cancer collaborative. Ann. Surg. 270(3), 484–492 (2019).
Nwagu, G. C., Bhattarai, S., Swahn, M., Ahmed, S. & Aneja, R. Prevalence and mortality of triple-negative breast cancer in West Africa: Biologic and sociocultural factors. JCO Glob. Oncol. 7, 1129–1140 (2021).
Wright, N., Rida, P., Rakha, E., Agboola, A. & Aneja, R. Panoptic overview of triple-negative breast cancer in Nigeria: current challenges and promising global initiatives. J. Glob. Oncol. 4, 1–20 (2018).
Kumar, P. & Aggarwal, R. An overview of triple-negative breast cancer. Arch. Gynecol. Obstet. 293(2), 247–269 (2016).
Azubuike, S. O., Muirhead, C., Hayes, L. & McNally, R. Rising global burden of breast cancer: the case of sub-Saharan Africa (with emphasis on Nigeria) and implications for regional development: a review. World J. Surg. Oncol. 16(1), 63 (2018).
Adeloye, D. et al. Estimating the incidence of breast cancer in Africa: A systematic review and meta-analysis. J. Glob. Health 8(1), 010419 (2018).
McKeown, R. E. The epidemiologic transition: Changing patterns of mortality and population dynamics. Am. J. Lifestyle Med. 3(1 Suppl), 19s–26s (2009).
Fitzmaurice, C. et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: A systematic analysis for the global burden of disease study. JAMA Oncol. 3(4), 524–548 (2017).
Okobia, M. N. et al. Anthropometry and breast cancer risk in Nigerian women. Breast J. 12(5), 462–466 (2006).
Ogundiran, T. O. et al. Body fat distribution and breast cancer risk: findings from the Nigerian breast cancer study. Cancer Causes & Control: CCC. 23(4), 565–574 (2012).
Touvier, M. et al. Cholesterol and breast cancer risk: A systematic review and meta-analysis of prospective studies. Br. J. Nutr. 114(3), 347–357 (2015).
Cedó, L., Reddy, S. T., Mato, E., Blanco-Vaca, F. & Escolà-Gil, J. C. HDL and LDL: Potential new players in breast cancer development. J. Clin. Med. 8(6), 853 (2019).
Ni, H., Liu, H. & Gao, R. Serum lipids and breast cancer risk: A meta-analysis of prospective cohort studies. PLoS ONE 10(11), e0142669 (2015).
Llanos, A. A. et al. Cholesterol, lipoproteins, and breast cancer risk in African American women. Ethn. Dis. 22(3), 281–287 (2012).
Bosco, J. L., Palmer, J. R., Boggs, D. A., Hatch, E. E. & Rosenberg, L. Cardiometabolic factors and breast cancer risk in U.S. black women. Breast Cancer Res. Treatment 134(3), 1247–1256 (2012).
Kim, Y. et al. Serum high-density lipoprotein cholesterol and breast cancer risk by menopausal status, body mass index, and hormonal receptor in Korea. Cancer Epidemiol., Biomark. & Prevent.: A Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prevent. Oncol. 18(2), 508–515 (2009).
Gago-Dominguez, M., Calaza, M., Muñoz-Garzon, V., Martinez, M. E., Castelao, J.E. Abstract 2269: Circulating lipids and breast cancer subtypes in a Spanish population. 2017;77(13 Supplement):2269.
Akinyemiju, T. et al. Collaborative molecular epidemiology study of metabolic dysregulation, DNA methylation, and breast cancer risk among Nigerian women: MEND study objectives and design. J. Glob. Oncol. 5, 1–9 (2019).
Osafo, C. et al. Human heredity and health (H3) in Africa kidney disease research network: A focus on methods in Sub-Saharan Africa. Clin. J. Am. Soc. Nephrol.: CJASN. 10(12), 2279–2287 (2015).
Mukherjee, T. Interpretation of ER and Her2neu hormonal receptor in breast cancer. Med. J. Armed Forces India. 72(1), 99 (2016).
Hammond, M. E. et al. American society of clinical oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer (unabridged version). Arch. Pathol. Lab. Med. 134(7), e48-72 (2010).
Wolff, A. C. et al. American society of clinical oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch. Pathol. Lab. Med. 131(1), 18–43 (2007).
Third Report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation. 106(25), 3143–421 (2002).
Alberti, K. G. et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 120(16), 1640–1645 (2009).
Kitahara, C. M. et al. Total cholesterol and cancer risk in a large prospective study in Korea. J. Clin. Oncol.: Off. J. Am. Soc. Clin. Oncol. 29(12), 1592–1598 (2011).
Martin, L. J. et al. Serum lipids, lipoproteins, and risk of breast cancer: A nested case-control study using multiple time points. J. Natl. Cancer Inst. 107(5), djv032 (2015).
His, M. et al. Prospective associations between serum biomarkers of lipid metabolism and overall, breast and prostate cancer risk. Eur. J. Epidemiol. 29(2), 119–132 (2014).
Chandler, P. D. et al. Lipid biomarkers and long-term risk of cancer in the Women’s Health Study. Am. J. Clin. Nutr. 103(6), 1397–1407 (2016).
Nowak, C. & Ärnlöv, J. A Mendelian randomization study of the effects of blood lipids on breast cancer risk. Nat. Commun. 9(1), 3957 (2018).
Beeghly-Fadiel, A. et al. A Mendelian randomization analysis of circulating lipid traits and breast cancer risk. Int. J. Epidemiol. 49(4), 1117–1131 (2020).
His, M. et al. Associations between serum lipids and breast cancer incidence and survival in the E3N prospective cohort study. Cancer Causes & Control : CCC. 28(1), 77–88 (2017).
Melvin, J. C. et al. Lipid profiles and risk of breast and ovarian cancer in the Swedish AMORIS study. Cancer Epidemiol., Biomark. & Prevent. : A Publ. Am. Assoc. Cancer Res., Cospons. Am. Soc. Prevent. Oncol. 21(8), 1381–1384 (2012).
Inoue, M. et al. Impact of metabolic factors on subsequent cancer risk: Results from a large-scale population-based cohort study in Japan. Eur. J. Cancer Prevent.: The Off. J. Eur. Cancer Prevent. Organ. (ECP). 18(3), 240–247 (2009).
Ray, G. & Husain, S. A. Role of lipids, lipoproteins and vitamins in women with breast cancer. Clin. Biochem. 34(1), 71–76 (2001).
Agurs-Collins, T., Kim, K. S., Dunston, G. M. & Adams-Campbell, L. L. Plasma lipid alterations in African-American women with breast cancer. J. Cancer Res. Clin. Oncol. 124(3–4), 186–190 (1998).
Maiti, B., Kundranda, M. N., Spiro, T. P. & Daw, H. A. The association of metabolic syndrome with triple-negative breast cancer. Breast Cancer Res. Treat. 121(2), 479–483 (2010).
Llaverias, G. et al. Role of cholesterol in the development and progression of breast cancer. Am. J. Pathol. 178(1), 402–412 (2011).
Danilo, C. & Frank, P. G. Cholesterol and breast cancer development. Curr. Opin. Pharmacol. 12(6), 677–682 (2012).
Furberg, A. S. et al. Metabolic and hormonal profiles: HDL cholesterol as a plausible biomarker of breast cancer risk. The Norwegian EBBA Study. Cancer Epidemiol. Biomark. Prevent. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prevent. Oncol. 14(1), 33–40 (2005).
Hankinson, S. E. Endogenous hormones and risk of breast cancer in postmenopausal women. Breast Dis. 24, 3–15 (2005).
Fernandez, M. L. & Murillo, A. G. Postmenopausal women have higher HDL and decreased incidence of low HDL than premenopausal women with metabolic syndrome. Healthcare (Basel, Switzerland). 4(1), 20 (2016).
Akinyemiju, T. et al. Association of body composition with odds of breast cancer by molecular subtype: Analysis of the Mechanisms for Established and Novel Risk Factors for Breast Cancer in Nigerian Women (MEND) study. BMC Cancer 21(1), 1051 (2021).
von Eckardstein, A., Hersberger, M. & Rohrer, L. Current understanding of the metabolism and biological actions of HDL. Curr. Opin. Clin. Nutr. Metab. Care 8(2), 147–152 (2005).
Soran, H., Hama, S., Yadav, R. & Durrington, P. N. HDL functionality. Curr. Opin. Lipidol. 23(4), 353–366 (2012).
Yvan-Charvet, L., Wang, N. & Tall, A. R. Role of HDL, ABCA1, and ABCG1 transporters in cholesterol efflux and immune responses. Arterioscler. Thromb. Vasc. Biol. 30(2), 139–143 (2010).
Pan, B. et al. HDL of patients with type 2 diabetes mellitus elevates the capability of promoting breast cancer metastasis. Clin. Cancer Res.: An Off. J. Am. Assoc. Cancer Res. 18(5), 1246–1256 (2012).
Pan, B. et al. Hypochlorite-induced oxidative stress elevates the capability of HDL in promoting breast cancer metastasis. J. Transl. Med. 10, 65 (2012).
Pan, B. et al. High-density lipoprotein of patients with type 2 diabetes mellitus elevates the capability of promoting migration and invasion of breast cancer cells. Int. J. Cancer 131(1), 70–82 (2012).
Nazih, H., Bard, J. M. Cholesterol, oxysterols and LXRs in breast cancer pathophysiology. Int. J. Mol. Sci. 21(4), 1356 (2020).
Kloudova, A., Guengerich, F. P. & Soucek, P. The role of oxysterols in human cancer. Trends Endocrinol. Metab. 28(7), 485–496 (2017).
Newman, L. A. & Kaljee, L. M. Health disparities and triple-negative breast cancer in African American Women: A review. JAMA Surg. 152(5), 485–493 (2017).
Garrido-Castro, A. C., Lin, N. U. & Polyak, K. Insights into molecular classifications of triple-negative breast cancer: Improving patient selection for treatment. Cancer Discov. 9(2), 176–198 (2019).
Jiagge, E. et al. Comparative analysis of breast cancer phenotypes in African American, White American, and West versus East African patients: Correlation between African ancestry and triple-negative breast cancer. Ann. Surg. Oncol. 23(12), 3843–3849 (2016).
Newman, L. A. Disparities in breast cancer and african ancestry: A global perspective. Breast J. 21(2), 133–139 (2015).
Acknowledgements
We acknowledge the role of the H3Africa Consortium in making this research possible though the sharing of data. The National Institutes of Health (USA) and Wellcome Trust (UK) have provided the core funding for the H3Africa Consortium. We thank the many MEND investigators who contributed substantially to the inception and design of the study, and the patients and families who participated in the MEND study for their vital contribution in advancing the science of cancer in Nigeria and globally. We acknowledge the important contribution of the MEND research nurses: Cordelia Ibeneme, Peju Olabanji, Rebecca Israel, Esther Akinwale, Deborah Awodeyi, and Shukurat Oduola.
Funding
This research was specifically funded by National Institutes of Health, National Cancer Institute, Fogarty International Center (K01TW010271, T.A.), and National Institutes of Health (NIH 1P30DK124723-01). The views expressed in this paper do not represent the views of the National Institutes of Health, H3Africa Consortium or their funders.
Author information
Authors and Affiliations
Consortia
Contributions
Conceptualization, T.A.; methodology, T.A.; formal analysis, A.G., T.O., T.A.; resources, T.A.; writing—original draft preparation, A.G.; writing—review and editing, A.G., V.S., A.D. T.O., K.D.J., O.S., A.D., A.H., O.A., G.O., A.A., O.A., T.O., O.O., O.A., A.A., O.A., A.O., A.A., T.O.T., D.A., M.J.M, C.B.N., T.A.; funding acquisition, C.B.N., T.A. All authors have read and agreed to the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gupta, A., Saraiya, V., Deveaux, A. et al. Association of lipid profile biomarkers with breast cancer by molecular subtype: analysis of the MEND study. Sci Rep 12, 10631 (2022). https://doi.org/10.1038/s41598-022-13740-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-13740-x
This article is cited by
-
Coenzyme Q10 in the eye isomerizes by sunlight irradiation
Scientific Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.