Abstract
Past studies indicate that men are more likely to smoke and be at higher risk of smoking-related conditions than women. Our research aimed, through meta-analysis, to assess the association between smoking and fracture risk in men. The following databases were searched, including MEDLINE, EMBASE, Scopus, PsycINFO, ISI Web of Science, Google Scholar, WorldCat, and Open Grey, for identifying related studies. A random-effects model was used to pool the confounder-adjusted relative risk (R.R.). Frequentist and Bayesian hierarchical random-effects models were used for the analysis. The heterogeneity and publication bias were evaluated in this study. Twenty-seven studies met the inclusion criteria. Overall, smoking is associated with a significantly increased risk of fracture in both the frequentist approach (R.R., 1.37; 95% confidence interval: 1.22, 1.53) and the Bayesian approach (R.R., 1.36; 95% credible interval: 1.22, 1.54). Significant heterogeneity was observed in the meta-analysis (Higgin's I2 = 83%) and Cochran's Q statistic (p < 0.01). A significant association was also observed in multiple pre-specified sensitivity and subgroup analyses. Similar results were observed in the group containing a large sample size (≥ 10,000 participants), and the group has a small sample size (< 10,000 participants); the pooled R.R was 1.23 (95% confidence interval, 1.07–1.41) and 1.56 (95% confidence interval, 1.37–1.78), respectively. With the Bayesian method, the effect size was 1.23 (95% credible interval, 1.05, 1.45) for the large sample size group and 1.57 (95% credible interval, 1.35, 1.82) for the small sample size group. Smoking is associated with a significant increase in fracture risk for men. Thus, smoking cessation would also greatly reduce fracture risk in all smokers, particularly in men.
Similar content being viewed by others
Introduction
Osteoporotic fractures are a major cause of morbidity and disability in older people, often leading to their premature death1. As the global population ages, osteoporotic fractures are expected to increase significantly in the coming decades2. Worldwide, the number of people aged 50 years or older and who were at high risk of osteoporotic fracture was around 158 million in 2010, and that number is expected to double by 20403. In the United States, data from 2013–2014 indicated that around 8.3% of adults should have received osteoporosis treatment because they were at a 20% or greater 10-year risk of major osteoporotic fractures3. From 2006 to 2025, annual osteoporotic fracture events and costs for affected populations in the United States are projected to grow by more than 48%2. Thus, osteoporotic fracture prevention is essential for both high-risk individuals and for society in general.
Smoking is the single most preventable cause of disease, disability, and death in the United States4. Recent data suggested that there are still approximately 34.2 million adult smokers in this country5. Specifically, men are more likely to smoke than women in the US since 16.7% of adult males and 13.6% of adult females smoke cigarettes6. In addition, smoking accounted for an estimated 3.1 million years of potential life lost for male smokers and 2.0 million years for female smokers during 2000–20047, suggesting that men are at higher risk of smoking-related conditions than women. Prior studies found that smoking was associated with a significantly increased risk of fractures8,9. Smoking increases the risk of spine and hip fracture to 32% and 40% in men, respectively10. The one-year mortality rates were as high as 20.6%11 and 37.1%12 among male smokers with spine and hip fractures. Hence, a reliable estimate of the association between smoking and fractures in men is crucial, which might help to improve their recognition of the dangers of smoking.
Although a prior meta-analysis was conducted and found a significant association between smoking and hip fractures in men13, that study was completed five years ago. As a result, several large-scale eligible cohort studies published in recent years were not included14,15,16,17,18,19. As well, the prior meta-analysis only focused on hip fracture13, while more current related research has well documented that smoking harms overall bone physiology, thus leading to increased fractures in many other skeletal regions20. Therefore, a comprehensive and updated meta-analysis about the association between smoking and fractures in men is needed. Therefore, our current meta-analysis aimed to include both frequentist and Bayesian approaches in order to quantify all eligible cohort studies that assessed the association between smoking and fractures in men. With the Bayesian method, we can estimate the probabilities that smoking increases fracture risk by more than 0%, 10%, and 20%, which the frequentist approach is unable to provide. Therefore, using both classical and advanced methodology in our meta-analysis allows us to gather more comprehensive and accurate information about the effects of smoking on fractures among men.
Methods
This meta-analysis was conducted in accordance with the Meta-Analysis of Observational Studies in Epidemiology (MOOSE) guidelines21, with reference to Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA)22. The study objectives, primary outcomes, literature search strategy, inclusion and exclusion criteria, study selection methods, data extraction, and data synthesis were all defined in advance in the meta-analysis research protocol (in Supplementary). We also pre-specified the sensitivity and subgroup analyses that we planned to conduct this meta-analysis in the protocol.
Data sources and searches
Two investigators (Y.X. and Y.B.) conducted a comprehensive literature search. Electronic searches were conducted on MEDLINE, using the following terms: men, fractures, osteoporosis, smoking, cigarette, and tobacco, with no restrictions on language, year of publication, or publication status. Using the same strategy, we also conducted literature searches of EMBASE, PsycINFO, SCOPUS, and ISI Web of Science. The above search terms were adapted for other database searches, according to the syntax of each specific database. The last literature search was conducted on April 28, 2021. We also searched Google Scholar, WorldCat Dissertations, and Open Grey. Experienced librarians were consulted to ensure the comprehensiveness of the literature search. Two investigators (M.W. and Y.B.) independently examined reference lists from the original studies and related meta-analyses and reviews.
Study selection
The following criteria were used to screen relevant references: (1) prospective or retrospective cohort studies designs; (2) reported smoking status (never, ever, or current smoker); (3) had risk estimates for any fracture or provided sufficient information to estimate fracture risk; and (4) reported results for men. At the initial selection stage, the two investigators independently screened each article's title and abstract retrieved from the electronic search. Only those citations that both reviewers deemed irrelevant were excluded. References with a disagreement between the two reviewers were included for a further full review. In the second phase of the study selection, each reference's full content obtained during the screening stage was reviewed and assessed by the investigators independently. For duplicate publications from the same study cohort, we included in our meta-analysis the study with the largest sample size or effect size adjusted for the largest number of confounders. Disagreements or uncertainties were discussed and resolved through adjudication from a third investigator (Y.X.) when needed. We only included studies that reported relative risk (R.R.) or hazard ratio (H.R.) of fracture associated with smoking or those with the necessary data for calculating R.R. in the current meta-analysis. The agreement between investigators was evaluated using the κ statistic, a robust statistic for inter-rater reliability testing.
Study appraisal
The methodologic quality of each included study was scored independently by the two researchers (M.W. and Y.B.), using the Newcastle–Ottawa Scale23. No major disagreements or discrepancies arose between the two investigators; minor differences were resolved by rechecking the original reports and by discussion. As recommended by the MOOSE study group21, the quality scores were not used as weights in the meta-analysis. However, quality scores were used in the subgroup analysis (score > 7 versus ≤ 7).
Data abstraction
The two reviewers (M.W. and Y.B.) performed data extraction independently. Before the study, a standard data abstraction form was developed. The following information was recorded: titles, authors, types of publication (journal article, abstract, or unpublished data), characteristics of study (year of publication, country of origin, inclusion and exclusion criteria, number of participants, number of cases, and duration of follow-up), characteristics of participants (age and race, if applicable), assessment of exposure (smoking), method of ascertainment of outcomes, outcomes (fractures, along with the corresponding regions), and risk estimates (adjusted R.R. and H.R., corresponding 95% confidence intervals, adjustment of confounders, and stratification abstraction). When multiple estimates were presented in the original studies, the estimates with most confounders were adjusted, and the estimate of current smokers was chosen for overall pooled analysis when applicable. Corresponding estimates from the subgroup analyses in the original studies were abstracted when appropriate. One study24 did not report adequate data to compute the effect size. We attempted to contact the corresponding author for additional information but were unsuccessful.
Statistical analysis
The summary measures used in this meta-analysis were confounder-adjusted R.R. or H.R. for fractures. For studies that reported the estimates by subgroups only, the overall effect size was estimated by a meta-analysis of the reported subgroup's estimates. Before we pooled the data, R.R. or H.R. was transformed into their natural logarithms in order to stabilize the variance and normalize the distribution. We derived H.R. or R.R. natural logarithm variance from the corresponding 95% CIs provided in the original reports. Both frequentist and Bayesian hierarchical random-effects models were utilized for the synthesis analysis. In the frequentist meta-analysis, the DerSimonian-Laird method25 was used to calculate the pooled R.R. and variance. In the Bayesian meta-analysis, Gaussian distribution with an unknown effect size (θi) and known within-study variance \({\delta }_{i}^{2}\) was assumed for each log R.R. (denoted as φi). The set of θi across the original studies was also assumed to follow a Gaussian distribution, with an unknown mean (μ) and across-study variance (τ2), where μ was the estimate of the overall log R.R. and τ2 was a measure of the between-study variation. The prior distribution of τ2 was assumed to follow an improper uniform distribution, and the prior distribution for τ2 was assumed to be non-informative. The probabilities that current smoking use increases fracture risk by more than 0%, 10%, or 20% were estimated and reported. Heterogeneity was assessed with Cochran's Q statistic and Higgins's index26,27. Univariate and multiple meta-regression analysis was performed to explore heterogeneity. Baujat plot was used to identify studies that had high heterogeneity28, and the effect size was estimated after removing outlying/influential studies.
Several pre-specified sensitivity analyses were conducted to assess the robustness of our estimates. The effects of current smoking on fracture risk were calculated with different inclusion criteria, including reporting R.R./H.R., using medical records/hospital dataset, using hip fracture as the outcome, using clinical vertebral fracture as the outcome, and studies focusing on people older than 60 years old. Subgroup meta-analysis stratified by characteristics identified study location, length of follow-up, sample size, year of publication, and quality score. We also conducted a cumulative meta-analysis by performing sequential random-effects pooling, beginning with the earliest qualified report. Each subsequent meta-analysis summarized all eligible reports from the preceding years. To demonstrate the effect of adding reports on the pooled effect size, we presented results chronologically in a forest plot.
A funnel plot created by plotting R.R.s against their standard errors was utilized to examine the potential for publication bias. We also used the Egger test to examine the significance of publication bias. Furthermore, the trim-and-fill method was employed to estimate and adjust unpublished studies' potential effects on the estimated effect size. We used R statistical software (Version 4.0, Core Team, Vienna, Austria) for the data analysis. A p-value of 0.05 or less was considered statistically significant.
Results
Literature search
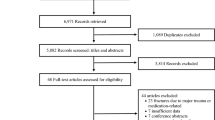
The study flow diagram is illustrated in Fig. 1. After removing duplicate references from different databases, we found a total of 6945 potential references. After investigators Y.B. and M.W. screened titles and abstracts of all these references, 58 full-text research articles were retrieved and assessed for eligibility. The agreement between the two investigators was modest at this initial screening stage (κ = 0.75). After reviewing all full-text articles, twenty-eight studies with fracture data met the inclusion criteria. However, two study reports from the same study team used the same data source but focused on different outcomes29,30. Therefore, we combined the two studies as one, and twenty-seven studies were included in the current meta-analysis. Three original studies31,32,33 by Drs. Nguyen et al.31, Felsenberg et al.33, and De Laet et al.32, included in a meta-analysis conducted by Dr. Kanis et al.34, also met our inclusion criteria. However, the three studies were updated by Drs. Nguyen et al.35, Roy et al.36, and van der Klift et al.37 with larger study samples, respectively. Thus we included the three corresponding updated studies35,36,37 in the meta-analysis. The agreement between the two investigators was good at this second stage (κ = 0.83). All included studies were published in English.
Study characteristics
The characteristics of the 27 included studies are summarized in Table 18,14,15,16,17,18,19,29,30,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53. For these included studies, the median follow-up time was 9 years, with a range of 2.7–30 years, and the median sample size was 6582, with a range from 258 to 1,174,232. Except for two studies17,52, the other twenty-five studies reported using R.R./H.R. However, one of the 25 studies reported H.R. using current smokers rather than non-smokers as the reference16. Therefore, we used non-smokers as the reference group and calculated the R.R. based on the provided data for the three mentioned studies16,17,52. The potential confounding effects of age were adjusted in 21 studies, and these studies also reported effect size with multiple risk factors adjusted8,14,19,29,30,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,53. Twenty-one studies were conducted outside of North and South America8,14,15,16,18,19,29,30,35,36,37,38,40,41,42,44,45,46,48,49,51,52. Thirteen studies only focused on the association between current smoking and hip fractures8,14,19,29,30,38,39,40,41,45,47,49,52,53. The quality score of the 27 included studies ranged from 4 to 9.
Meta-analysis
Figure 2 shows the pooled R.R. and corresponding 95% confidence interval (CI) based on the frequentist method and the pooled R.R. with corresponding 95% credible interval (CrI) based on Bayesian approaches. Compared to non-smokers, smokers (including former and current smokers) had an overall R.R. of 1.37 (95% CI, 1.22–1.53) in the frequentist approach and 1.36 (95% CrI, 1.22–1.54) in the Bayesian method. The results from the Bayesian hierarchical random-effects model suggest that the probabilities that smoking increased fracture risk by more than 0%, 10%, and 20% were 99%, 99%, and 98%, respectively. Significant heterogeneity was observed among the twenty-seven studies in this meta-analysis, as the Cochran Q statistic was significant (p < 0.01), and the Higgins I2 index was 83%. The pooled estimates varied slightly but remained significant when they included studies with different eligibility criteria for the analysis (Table 2). Similar effect size was observed among studies that reported R.R./H.R., and the estimate was 1.38 (95% CI, 1.27–1.50). The effect size slightly increased when studies used hip fracture (R.R., 1.46; 95% CI, 1.24–1.72) or vertebral fracture (R.R., 1.48; 95% CI, 1.28–1.72) as the outcome. When we restricted the analysis to the eight studies that included only participants over 60 years of age, the overall R.R. increased to 1.48 (95%CI, 1.27–1.72). The cumulative meta-analysis further demonstrated an association between current smoking and fracture risk (see Fig. 3). By sequentially accumulating studies according to their publication year, the pooled estimates fluctuated during 1993–2009, and the estimate remained stable afterward.
Subgroup analysis
Table 3 summarizes the effects of smoking on fracture risk in the subgroup analyses. The fracture risk was slightly higher among studies conducted in North/South America (R.R., 1.54; 95% CI, 1.29–1.84) than studies completed in Europe and other regions. We also found studies with follow-ups of more than five years had a lower R.R. (1.31; 95% CI, 1.15–1.50) than studies with less than five follow-up years (R.R., 1.49; 95% CI, 1.33–1.67). Notably, the group with a large sample size (≥ 10,000 participants) had a significantly lower R.R. (1.23; 95% CI, 1.07–1.41) compared to the group with a small sample size (< 10,000 participants) (R.R., 1.56; 95% CI, 1.37–1.78); the between-group difference was significant (p = 0.01). Moreover, studies published after 2010 had a similar R.R. (1.39; 95% CI, 1.25–1.54) to those published before or in 2010 (1.37; 95% CI, 1.08–1.75). Except for the sample size group, no significant between-group difference was observed in the subgroup analyses. The estimated effect sizes in the subgroup with the Bayesian method are shown in Supplementary Table 1, all of which were quite similar to the results from the frequentist, which indicated that heterogeneity remained high in most subgroup analyses. In the univariate meta-regression, the results of R2 indicate that two variables, location and reported R.R./H.R., could explain the, 31.34% and 58.61% of the heterogeneity, respectively. There was no multicollinearity between the two mentioned variables, and the heterogeneity in this study was further assessed with multiple meta-regression. After adjusting the two variables and their interaction, the significant heterogeneity among included studies was still observed (I2, 58.7%; p-value = 0.0006). The Baujat plot was then used to detect outlier/influential studies, and two16,41 were identified (Supplementary Fig. 1). After excluding the two studies, the pooled effect size for the remaining 25 studies was 1.33 (95%CI, 1.24–1.42) in the frequentist approach, and the I2 was 23.4%, while the p-value for the heterogeneity test was 0.14 (Supplementary Fig. 2).
Publication bias
Publication bias was examined by plotting the log R.R.s between smokers and non-users against their standard errors for each study (Fig. 4). Visual inspection of the funnel plot indicated that publication bias might be present. The Egger test (p = 0.0024) also indicated significant publication bias in our current meta-analysis. Hence, we employed the trim-and-fill correction to adjust for the publication bias. However, the overall effect size remained significant after the correction (R.R., 1.20; 95% CI, 1.06–1.35).
Discussion
This meta-analysis summarizes 27 cohort studies to assess the association between smoking and fracture risk among men. Both the frequentist method (R.R., 1.37; 95% CI, 1.22, 1.53) and the Bayesian method (R.R., 1.36; 95% CrI, 1.22, 1.54) showed a significant association between smoking and increased fracture risk. The association between smoking and fracture risk was consistent in all sensitivity analyses with different inclusion criteria, various subgroup analyses, and analysis after excluding two outlier/influential studies, which suggests consistency and robustness of findings in this meta-analysis. Furthermore, although the cumulative meta-analysis showed that the pooled estimate fluctuated during 1993–2009, the study also showed a consistent and significant association between smoking and an increased risk of fractures since 2010. This finding suggests that the addition of future studies would have a limited impact on the overall estimate. Additionally, the 95% CIs were increasingly narrower when studies were organized chronologically, which further demonstrates the robustness of our results.
Our meta-analysis results are consistent with the previous meta-analysis conducted by Dr. Wu et al., that smoking increases the risk of hip fracture in men13. However, the previous meta-analysis was published five years ago and thus was unable to integrate findings from recently published, more extensive studies14,15,16,17,18,19,54,55,56,57. Moreover, the previous meta-analysis only assessed smoking and hip fracture association. Therefore, the association between smoking and other, more specific fracture outcomes remains unknown. However, we updated the meta-analysis by including the most recent qualified study reports in the present study. We also quantified the association between smoking and overall fracture, along with vertebral fractures. In addition, we included two crucial updated study reports in the present meta-analysis, both with large sample sizes. Dr. Forsen and colleagues published an eligible study in 199458, which was included in the previous meta-analysis. The same research group published another updated report with the same data in 1998; the corresponding updates were included in the present meta-analysis. We also replaced the original study by Paganini-Hill et al.59 in the previous meta-analysis, with an updated report by White et al.43, in the present meta-analysis. Although both study reports used the same data source, the newer version was more comprehensive. It assessed the association between smoking and hip, wrist, and spine fracture, while the older one only focused on hip fracture, so the current study included the updated findings. Compared to the previous meta-analysis, we employed both the frequentist and Bayesian approaches to evaluate the association between smoking and fracture risk. The results from the two methods were consistent in our study. In addition, the Bayesian meta-analysis provided the probabilities that smoking increases fracture risk by 10% and 20%; such results help male smokers to recognize that smoking is linked to elevated fracture risk. Our findings are also consistent with a prior meta-analysis by Dr. Kanis and his colleagues34 that current male smokers had a significantly higher fracture risk than non-current smokers. Compared to Dr. Kanis and colleagues' meta-analysis, our meta-analysis not only included all additional recent eligible studies but also replaced three original studies by Drs. Nguyen et al.31, Felsenberg et al.33, and De Laet et al.32 in Dr. Kanis's meta-analysis with the corresponding updated studies by Drs. Nguyen et al.35, Roy et al.36, and van der Klift et al.37, respectively. The three updated studies had a larger sample size, which might contribute to a more precise estimate because the variance and standard error decrease as the sample size increaseds60. Thus, our meta-analysis is likely to yield a more accurate estimate of the effect size.
The underlying mechanism of how smoking influences fracture risk is not fully understood. One potential reason could be the decreased bone mineral density (BMD) caused by smoking61. Low BMD is the primary cause of osteoporotic fracture risk and is a measure widely used in clinical practice to identify patients at an increased risk of fracture56. The biological plausibility of BMD loss due to smoking can be linked to the effects of nicotine and cadmium in cigarette smoke on bone cells61. In addition, smoking is associated with decreased vitamin D levels. People with low vitamin D are more likely to have low BMD and are at a higher risk of suffering a fracture62. On the other hand, smoking is also associated with reducing calcium absorption54, also leading to increased fracture risk. Another potential reason is that smoking has been considered a risk factor for injury57, which is linked to fractures. A study in elderly persons found a 28% increase in smokers' accidental injury over non-smokers, and smoking and nicotine are inhibitory factors in wound and fracture healing55. Smoking also interferes with tissue repair processes, leaving tissue more susceptible to injury and fracture55.
Our study has several limitations. First, two studies with self-reported data and one without specification about the outcome measures were included. The data from the three studies might be less reliable compared to other data derived directly from medical records. After removing the three studies, the effect size of smoking on fracture risk decreased slightly. Second, due to the different questionnaire designs from the included studies, we could not examine the dose–response relationship between smoking and the risk of fractures. Third, publication bias is suspected in the current meta-analysis, as indicated by the funnel plot and Egger test. However, the pooled estimate remained significant after we adjusted for publication bias by using the trim-and-fill method. Finally, the adjustment for confounders in all the included articles varies, which may exaggerate or underestimate the findings. Nevertheless, this limitation unlikely altered our meta-analyses conclusion; the consistent findings from sensitivity and subgroup analyses suggested that our current study findings are reliable and robust.
Conclusion
In summary, our comprehensive meta-analysis found a significant association between smoking and increased risk of fractures. Our findings were consistent in both frequentist and Bayesian approaches, as well as all subgroup analyses, sensitivity analysis, and the analysis with publication bias correction. More importantly, our results have crucial implications in public health, with the most apparent being that quitting smoking can reduce an individual's risk of bone fracture, both now and later in life.
References
Colón-Emeric, C. S. & Saag, K. G. Osteoporotic fractures in older adults. Best Pract. Res. Clin. Rheumatol. 20, 695–706. https://doi.org/10.1016/j.berh.2006.04.004 (2006).
Burge, R. et al. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J. Bone Miner. Res. 22, 465–475. https://doi.org/10.1359/jbmr.061113 (2007).
Odén, A., McCloskey, E. V., Kanis, J. A., Harvey, N. C. & Johansson, H. Burden of high fracture probability worldwide: secular increases 2010–2040. Osteoporos. Int. J. Establ. Result Coop. Between Eur. Found. Osteoporos. Natl. Osteoporos. Found. USA 26, 2243–2248. https://doi.org/10.1007/s00198-015-3154-6 (2015).
Centers for Disease C et al. How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General (Centers for Disease Control and Prevention, US, 2010).
Creamer, M. R. et al. Tobacco product use and cessation indicators among adults — United States, 2018. MMWR Morb. Mortal. Wkly. Rep. 2019 68, 1013–1019 (2019).
Jamal, A. et al. Current cigarette smoking among adults – United States, 2005–2015. MMWR Morb. Mortal. Wkly Rep. 65, 1205–1211. https://doi.org/10.15585/mmwr.mm6544a2 (2016).
Adhikari, B., Kahende, J., Malarcher, A., Pechacek, T. & Tong, V. Smoking-attributable mortality, years of potential life lost, and productivity losses. Oncol. Times 31, 40–43 (2009).
Meyer, H. E., Tverdal, A. & Falch, J. A. Risk factors for hip fracture in middle-aged Norwegian women and men. Am. J. Epidemiol. 137, 1203–1211. https://doi.org/10.1093/oxfordjournals.aje.a116622 (1993).
Murray, R. The Role of Smoking in the Progressive Decline of the Body’s Major Systems (Public Health England, 2014).
Ward, K. D. & Klesges, R. C. A meta-analysis of the effects of cigarette smoking on bone mineral density. Calcif. Tissue Int. 68, 259–270. https://doi.org/10.1007/BF02390832 (2001).
Lee, Y. K. et al. Mortality after vertebral fracture in Korea: analysis of the national claim registry. Osteoporos. Int. J. Establ. Result Coop. Between Eur. Found. Osteoporos. Natl. Osteoporos. Found. USA 23, 1859–1865. https://doi.org/10.1007/s00198-011-1833-5 (2012).
Kannegaard, P. N., van der Mark, S., Eiken, P. & Abrahamsen, B. Excess mortality in men compared with women following a hip fracture. National analysis of comedications, comorbidity and survival. Age Ageing 39, 203–209. https://doi.org/10.1093/ageing/afp221 (2010).
Wu, Z.-J., Zhao, P., Liu, B. & Yuan, Z.-C. Effect of cigarette smoking on risk of hip fracture in men: a meta-analysis of 14 prospective cohort studies. PLoS One 11, e0168990–e0168990. https://doi.org/10.1371/journal.pone.0168990 (2016).
Lobo, E. et al. Gender differences in the incidence of and risk factors for hip fracture: A 16-year longitudinal study in a southern European population. Maturitas 97, 38–43. https://doi.org/10.1016/j.maturitas.2016.12.009 (2017).
Prieto-Alhambra, D. et al. Smoking and alcohol intake but not muscle strength in young men increase fracture risk at middle age: a cohort study linked to the Swedish national patient registry. J. Bone Miner. Res. 35, 498–504. https://doi.org/10.1002/jbmr.3917 (2020).
Cho, I. Y. et al. Effects of smoking habit change on hospitalized fractures: a retrospective cohort study in a male population. Arch. Osteoporos. 15, 29. https://doi.org/10.1007/s11657-020-0686-y (2020).
Domiciano, D. S. et al. Incidence and risk factors for osteoporotic non-vertebral fracture in low-income community-dwelling elderly: a population-based prospective cohort study in Brazil. The São Paulo ageing and health (SPAH) study. Osteoporos. Int. J. Establ. Result. Coop. Between Eur. Found. Osteoporos. Natl. Osteoporos. Found. USA 32, 747–757. https://doi.org/10.1007/s00198-020-05669-6 (2021).
Hadaegh, F. et al. Sex-specific incidence rates and risk factors for fracture: a 16-year follow-up from the Tehran lipid and glucose study. Bone 146, 115869. https://doi.org/10.1016/j.bone.2021.115869 (2021).
Preyer, O. et al. Serum uric acid is associated with incident hip fractures in women and men - results from a large Austrian population-based cohort study. Maturitas https://doi.org/10.1016/j.maturitas.2021.03.005 (2021).
Kanis, J. A., Johnell, O., Oden, A., Johansson, H. & McCloskey, E. FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos. Int. J. Establ. Result Coop. Between Eur. Found. Osteoporos. Natl. Osteoporos. Found. USA 19, 385–397. https://doi.org/10.1007/s00198-007-0543-5 (2008).
Stroup, D. F. et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA 283, 2008–2012. https://doi.org/10.1001/jama.283.15.2008 (2000).
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G. & PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS medicine 6(7), e1000097. https://doi.org/10.1371/journal.pmed.1000097 (2009).
Wells G, S. B., O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-analyses., <http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp> (2000).
Puisto, V. et al. Vertebral fracture and cause-specific mortality: a prospective population study of 3,210 men and 3,730 women with 30 years of follow-up. Eur. Spine J. 20, 2181–2186. https://doi.org/10.1007/s00586-011-1852-0 (2011).
DerSimonian, R. & Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 7, 177–188. https://doi.org/10.1016/0197-2456(86)90046-2 (1986).
Cochran, W. G. The combination of estimates from different experiments. Biometrics 10, 101–129. https://doi.org/10.2307/3001666 (1954).
Higgins, J. P. T., Thompson, S. G., Deeks, J. J. & Altman, D. G. Measuring inconsistency in meta-analyses. BMJ 327, 557. https://doi.org/10.1136/bmj.327.7414.557 (2003).
Baujat, B., Mahé, C., Pignon, J. P. & Hill, C. A graphical method for exploring heterogeneity in meta-analyses: application to a meta-analysis of 65 trials. Stat. Med. 21, 2641–2652. https://doi.org/10.1002/sim.1221 (2002).
Hemenway, D., Azrael, D. R., Rimm, E. B., Feskanich, D. & Willett, W. C. Risk factors for hip fracture in US men aged 40 through 75 years. Am. J. Public Health 84, 1843–1845. https://doi.org/10.2105/ajph.84.11.1843 (1994).
Hemenway, D., Azrael, D. R., Rimm, E. B., Feskanich, D. & Willett, W. C. Risk factors for wrist fracture: effect of age, cigarettes, alcohol, body height, relative weight, and handedness on the risk for distal forearm fractures in men. Am. J. Epidemiol. 140, 361–367. https://doi.org/10.1093/oxfordjournals.aje.a117258 (1994).
Nguyen, T. V., Eisman, J. A., Kelly, P. J. & Sambrook, P. N. Risk factors for osteoporotic fractures in elderly men. Am. J. Epidemiol. 144, 255–263. https://doi.org/10.1093/oxfordjournals.aje.a008920 (1996).
De Laet, C. E. et al. Hip fracture prediction in elderly men and women: validation in the Rotterdam study. J. Bone Miner. Res. 13, 1587–1593. https://doi.org/10.1359/jbmr.1998.13.10.1587 (1998).
Felsenberg, D. et al. Incidence of vertebral fracture in europe: results from the European prospective osteoporosis study (EPOS). J. Bone Miner. Res. 17, 716–724. https://doi.org/10.1359/jbmr.2002.17.4.716 (2002).
Kanis, J. A. et al. Smoking and fracture risk: a meta-analysis. Osteoporos. Int. J. Establ. Result Coop. Between Eur. Found. Osteoporos. Natl. Osteoporos. Found. USA 16, 155–162. https://doi.org/10.1007/s00198-004-1640-3 (2005).
Nguyen, T. V., Center, J. R., Sambrook, P. N. & Eisman, J. A. Risk factors for proximal humerus, forearm, and wrist fractures in elderly men and women: the dubbo osteoporosis epidemiology study. Am. J. Epidemiol. 153, 587–595. https://doi.org/10.1093/aje/153.6.587 (2001).
Roy, D. K. et al. Determinants of incident vertebral fracture in men and women: results from the European prospective osteoporosis study (EPOS). Osteoporos. Int. J. Establ. Result Coop. Between Eur. Found. Osteoporos. Natl. Osteoporos. Found. USA 14, 19–26. https://doi.org/10.1007/s00198-002-1317-8 (2003).
van der Klift, M. et al. Risk factors for incident vertebral fractures in men and women: the Rotterdam study. J. Bone Miner. Res. 19, 1172–1180. https://doi.org/10.1359/jbmr.040215 (2004).
Forsén, L. et al. Ex-smokers and risk of hip fracture. Am. J. Public Health 88, 1481–1483. https://doi.org/10.2105/ajph.88.10.1481 (1998).
Mussolino, M. E., Looker, A. C., Madans, J. H., Langlois, J. A. & Orwoll, E. S. Risk factors for hip fracture in white men: the NHANES I epidemiologic follow-up study. J. Bone Miner. Res. 13, 918–924. https://doi.org/10.1359/jbmr.1998.13.6.918 (1998).
Høidrup, S. et al. Tobacco smoking and risk of hip fracture in men and women. Int. J. Epidemiol. 29, 253–259. https://doi.org/10.1093/ije/29.2.253 (2000).
Olofsson, H. et al. Smoking and the risk of fracture in older men. J Bone Miner. Res. 20, 1208–1215. https://doi.org/10.1359/jbmr.050208 (2005).
Holmberg, A. H. et al. Risk factors for fragility fracture in middle age. A prospective population-based study of 33,000 men and women. Osteoporos. Int. J. Establ. Result Coop. Between Eur. Found. Osteoporos. Natl. Osteoporos. Found. USA 17, 1065–1077. https://doi.org/10.1007/s00198-006-0137-7 (2006).
White, S. C. et al. Risk factors for fractures in older men and women: The Leisure World Cohort Study. Gend Med 3, 110–123. https://doi.org/10.1016/s1550-8579(06)80200-7 (2006).
Hippisley-Cox, J. & Coupland, C. Predicting risk of osteoporotic fracture in men and women in England and Wales: prospective derivation and validation of QFractureScores. BMJ 339, b4229. https://doi.org/10.1136/bmj.b4229 (2009).
Koh, W.-P. et al. Gender-specific associations between soy and risk of hip fracture in the Singapore Chinese health study. Am. J. Epidemiol. 170, 901–909. https://doi.org/10.1093/aje/kwp220 (2009).
Moayyeri, A. et al. Estimation of absolute fracture risk among middle-aged and older men and women: the EPIC-Norfolk population cohort study. Eur. J. Epidemiol. 24, 259–266. https://doi.org/10.1007/s10654-009-9337-8 (2009).
Stolee, P., Poss, J., Cook, R. J., Byrne, K. & Hirdes, J. P. Risk factors for hip fracture in older home care clients. J. Gerontol. A Biol. Sci. Med. Sci. 64, 403–410. https://doi.org/10.1093/gerona/gln035 (2009).
Jutberger, H. et al. Smoking predicts incident fractures in elderly men: Mr OS Sweden. J. Bone Miner. Res. 25, 1010–1016. https://doi.org/10.1359/jbmr.091112 (2010).
Trimpou, P., Landin-Wilhelmsen, K., Odén, A., Rosengren, A. & Wilhelmsen, L. Male risk factors for hip fracture-a 30-year follow-up study in 7,495 men. Osteoporos. Int. J. Establ. Result Coop. Between Eur. Found. Osteoporos. Natl. Osteoporos. Found. USA 21, 409–416. https://doi.org/10.1007/s00198-009-0961-7 (2010).
Ma, C. C. et al. Risk factors for fractures among Japanese-American men: the Honolulu heart program and Honolulu-Asia aging study. Arch. Osteoporos. 6, 197–207. https://doi.org/10.1007/s11657-011-0068-6 (2011).
El Maghraoui, A. et al. Vertebral fracture assessment in asymptomatic men and its impact on management. Bone 50, 853–857. https://doi.org/10.1016/j.bone.2011.12.018 (2012).
Øyen, J. et al. Smoking and body fat mass in relation to bone mineral density and hip fracture: the Hordaland Health study. PLoS ONE 9, e92882–e92882. https://doi.org/10.1371/journal.pone.0092882 (2014).
Cauley, J. A. et al. Risk factors for hip fracture in older men: the osteoporotic fractures in men study (MrOS). J. Bone Miner. Res. 31, 1810–1819. https://doi.org/10.1002/jbmr.2836 (2016).
Brot, C., Rye Jørgensen, N. & Helmer Sørensen, O. The influence of smoking on vitamin D status and calcium metabolism. Eur. J. Clin. Nutr. 53, 920–926. https://doi.org/10.1038/sj.ejcn.1600870 (1999).
Fletcher, P. C. & Hirdes, J. P. Risk factor for accidental injuries within senior citizens’ homes: analysis of the Canadian survey on ageing and independence. J. Gerontol. Nurs. 31, 49–57. https://doi.org/10.3928/0098-9134-20050201-10 (2005).
Berger, C. et al. Association between change in BMD and fragility fracture in women and men. J. Bone Miner. Res. 24, 361–370. https://doi.org/10.1359/jbmr.081004 (2009).
Cusano, N. E. Skeletal effects of smoking. Curr. Osteoporos. Rep. 13, 302–309. https://doi.org/10.1007/s11914-015-0278-8 (2015).
Forsén, L. et al. Interaction between current smoking, leanness, and physical inactivity in the prediction of hip fracture. J. Bone Miner. Res. 9, 1671–1678. https://doi.org/10.1002/jbmr.5650091102 (1994).
Paganini-Hill, A., Chao, A., Ross, R. K. & Henderson, B. E. Exercise and other factors in the prevention of hip fracture: the Leisure World study. Epidemiology 2, 16–25. https://doi.org/10.1097/00001648-199101000-00004 (1991).
Borenstein, M., Hedges, L. V., Higgins, J. P. T. & Rothstein, H. R. in Introduction to Meta-Analysis pp. 51–55 (2009).
Szulc, P. et al. Increased bone resorption in moderate smokers with low body weight: the Minos study. J. Clin. Endocrinol. Metab. 87, 666–674. https://doi.org/10.1210/jcem.87.2.8232 (2002).
Laird, E., Ward, M., McSorley, E., Strain, J. J. & Wallace, J. Vitamin D and bone health: potential mechanisms. Nutrients 2, 693–724. https://doi.org/10.3390/nu2070693 (2010).
Author information
Authors and Affiliations
Contributions
Conceptualization, Q.W.; methodology, Y.X. and Q.W.; software, Y.X.; validation, Y.X., Y.B. and Q.W.; formal analysis, Y.X., Y.B., and M.W.; investigation, Y.X.; resources, Q.W.; data curation, Y.X.; writing—original draft preparation, Y.X.; writing—review and editing, Y.X. and Q.W.; visualization, Y.X.; supervision, Q.W.; project administration, Y.X. and Q.W.; All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xu, Y., Bao, Y., Wang, M. et al. Smoking and fracture risk in men: a meta-analysis of cohort studies, using both frequentist and Bayesian approaches. Sci Rep 12, 9270 (2022). https://doi.org/10.1038/s41598-022-13356-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-13356-1
This article is cited by
-
The development, incidence and treatment trends of trochanteric fractures in Germany: a cohort study
Journal of Orthopaedic Surgery and Research (2023)
-
Epidemiology of forearm fractures in women and men in Norway 2008–2019
Osteoporosis International (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.