Abstract
Radial and femoral artery catheterization is the most common procedure for monitoring patients with shock. However, a disagreement in mean arterial pressure (MAP) between the two sites has been reported. Hence, the aim of this study was to compare the MAP from the radial artery (MAPradial) with that of the femoral artery (MAPfemoral) in patients with refractory shock. A prospective study was conducted in the medical intensive care unit. The radial and femoral were simultaneously measured MAP in the patients every hour, for 24 h. In total, 706 paired data points were obtained from 32 patients. MAPradial strongly correlated with MAPfemoral (r = 0.89, p < 0.0001). However, overall MAPradial was significantly lower than MAPfemoral 7.6 mmHg. The bias between MAPradial and MAPfemoral was − 7.6 mmHg (95% limits of agreement (LOA), − 24.1 to 8.9). In the subgroup of patients with MAPradial < 65 mmHg, MAPradial moderately correlated with MAPfemoral (r = 0.63) and the bias was increased to − 13.0 mmHg (95% LOA, − 28.8 to 2.9). There were 414 (58.6%) measurements in which the MAP gradient between the two sites was > 5 mmHg. In conclusion, the radial artery significantly underestimated MAP compared with the femoral artery in patients with refractory shock.
Similar content being viewed by others
Introduction
Invasive arterial blood pressure measurement is essential for hemodynamic monitoring in patients with shock admitted to intensive care unit (ICU). Fluid and vasoactive administration are the fundamental management strategies in patients with shock. However, administration of more fluid and large doses of vasopressors are associated with an increased risk of death and organ failure in these shock patients1,2. Accurate blood pressure monitoring may lead to proper fluid management and decrease unnecessary vasoactive administration.
Ideally, measurement of central aortic pressures is the gold standard for blood pressure measurement; however, this procedure is invasive and unsuitable for routine clinical practice. Therefore, alternative arteries are used. The most frequently cannulated artery is the radial artery, because of ease of access and fewer complications3,4. The second most cannulated artery is the femoral artery. Theoretically, peripheral sites have greater systolic blood pressure (SBP) and lower diastolic blood pressure (DBP) than more central sites, due to the pulse amplification of pressure waves. However, mean arterial pressure (MAP) remains stable, regardless of the site of the arteries5.
Nevertheless, the difference in MAP between the radial and femoral arteries has been reported in several critically ill patients, such as cardiac surgery6,7, cardiopulmonary bypass8,9,10, liver transplantation11,12, and in septic shock patients13,14. Previous studies in patients with shock receiving high doses of norepinephrine found that MAP from the femoral artery (MAPfemoral) was higher than the radial artery (MAPradial), ranging from 4.3 to 15 mmHg and 62–75.4% of cases had a MAP gradient ≥ 5 mmHg13,14,15. In contrast some studies in critically ill adult patients16,17 and pediatric cardiac surgical patients18 reported good agreement between MAP from both sites and concluded that MAPradial was interchangeable with MAPfemoral and should be used for blood pressure monitoring in critically ill patients receiving high doses of vasopressors16,17.
Thus, definitive information about the gradient between MAPradial and MAPfemoral, as well as the best choice of blood pressure monitoring in patients with severe shock, peripheral or central arterial catheterization, remains controversial. If the MAPradial underestimates the MAPfemoral, it may result in an excess of fluid and vasopressor therapies. In addition, the factors associated with the radial-femoral MAP gradient have never been evaluated. The purpose of this study was to determine the correlation and agreement between simultaneous measurements of MAPradial and MAPfemoral in patients with refractory shock and to explore the clinical factors associated with the MAP gradient between the two sites.
Materials and methods
This prospective study was conducted in the medical ICU of a university-affiliated, tertiary referral center in Southern Thailand, from; May 2019 to October 2020. The study was approved by the Human Research Ethics Committee of Faculty of Medicine, Prince of Songkla University (REC: 62-008-14-4) and was registered in the Thai Clinical Trials Registry (TCTR20190603002) and was conducted under the ethical principles of the Declaration of Helsinki. Written informed consent was obtained from the next of kin of all the patients before inclusion to the study.
There is no definite consensus on the definition of refractory shock. However, in general, it can be summarized as; a shock that does not achieve hemodynamic targets, despite the use of high dose vasoactive agents19,20. In this study, we defined patients with refractory shock as those receiving an infusion of ≥ 0.5 µg/kg/min of norepinephrine equivalent (1 µg of epinephrine or 100 µg of dopamine, equivalent to 1 µg of norepinephrine)2,19,20. The inclusion criteria were as follows: (1) patients with shock who received at least four hours of norepinephrine equivalent ≥ 0.5 µg/kg/min and (2) patients with radial artery catheterization. The exclusion criteria were as follow: (1) contraindication to femoral arterial catheterization, including overlying skin infection, clinical history of severe peripheral vascular disease of the lower limbs, or critical limb ischemia; (2) patients with radial artery catheter malfunctioning, detected by the “fast-flush test” showing overdamping or underdamping of the pressure monitoring systems4; (3) use of intra-aortic balloon counterpulsation; and (4) post-cardiac surgery.
Blood pressure measurements
In our ICU, the radial artery was cannulated with a 20G 3.2-cm catheter (Terumo Surflo, Laguna, Philippines). For femoral catheterization, a 20 cm 16G single lumen catheter (Arrow International, Pennsylvania, USA) was used. The catheters were inserted into the femoral artery using the Seldinger technique under ultrasound-guidance21. The arterial catheters were connected to non-compliant pressure tubing and two pressure transducers (TruWave™, Edwards Lifesciences). Both transducers were placed at the same level (phlebostatic axis) and simultaneously zeroed to the atmospheric pressure. The pressure transducer systems were connected to a bedside hemodynamic monitor (Philips Intellivue MP70, Philips Medical Systems, Böeblingen, Germany). A fast-flush test was performed to confirm the adequacy of the frequency response and damping coefficient4.
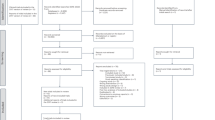
The following variables were recorded at inclusion were: age, sex, body weight and, height, Acute Physiology and Chronic Health Evaluation (APACHE) II score, Sequential Organ Failure Assessment (SOFA) score, type of shock, type and dose of vasopressor, and types and sites of infection. ICU mortality was also recorded. SBP, DBP, and MAP from two sites, doses of all the vasopressor provided were recorded after femoral catheterization for 10 min and thereafter, every one hour for 24 h by the ICU nurses (Fig. 1). Complications of arterial catheterization on both sites were observed during ICU stays.
Statistical analysis
The sample size of the study was calculated by assessing agreement between two methods of measurement by Bland–Altman method22. We expected the bias between MAPradial and MAPfemoral 4.5 mmHg14 and standard deviation of 6 mmHg and allowed maximum difference of 22 mmHg. Therefore, a sample size of 30 patients was calculated (alpha error of 5% and power of 20%).
Categorical variables are expressed as numbers and percentages. The distribution of variables for normality was tested using the Shapiro–Wilk test. Continuous variables are expressed as mean ± standard deviation (SD) or median and interquartile range (IQR) as appropriate.
The Pearson correlation coefficient was used to measure the strength of the linear association between MAPradial and MAPfemoral. The multilevel mixed-effect model was used to determine the statistical differences between MAPradial and MAPfemoral23,24. The multilevel model was constructed using the arterial site as a fixed effect with random intercepts for both patients and sequential measurement within the patient levels. Bland–Altman analysis with corrected multiple measurements was used to evaluate the agreement between MAP from both sites25. The bias and 95% limits of agreement (LOA) of the simultaneous measurements were calculated. Bias was defined as the mean difference between MAPradial and MAPfemoral. LOAs were calculated as the mean bias ± 2SD. We also performed a bias plot using the Taffé method26,27, defining MAPfemoral as a standard reference method, and MAPradial as a new method. Briefly, the bias plot shows the scatter plot of the two measurement methods versus the best linear unbiased prediction (BLUP) with the two regression lines added. Taffé uses an empirical Bayes method to compute the BLUP and uses the reference measurements for each subject to estimate their value26,27.
The clinically significant differences between MAPradial and MAPfemoral was defined as > 5 mmHg14,15,17. The correlation and agreement between MAP from two the sites were assessed in each subgroup of MAP levels and norepinephrine doses. Patients were separated to subgroup of MAPradial measurement < 65 or ≥ 65 mmHg. Regarding, norepinephrine dose group, patients were divided into two groups: those receiving maximum dose of norepinephrine < 1 or ≥ 1 µg/kg/min and those receiving maximum dose of norepinephrine < 0.5 or ≥ 0.5 µg/kg/min. Multilevel mixed-effect logistic regression analysis was used to determine the demographic or hemodynamic factors associated with a significant MAP gradient. Statistical significance was set at p-value < 0.05. All statistical analyses were performed using the Stata 15 software.
Results
There were 706 paired data obtained from 32 patients, with a mean of 22.1 ± 3.9 data sets per patient. The demographic, clinical characteristics and initial hemodynamic parameters of the patients are shown in Table 1. Septic shock was the most common type of shock in this study (28 patients, 87.5%), and 17 patients (60.7%) had community-acquired infections. Regarding the site of infection, 39.3% were respiratory tract infections, 17.9% were digestive tract and primary bacteremia, and 3.5% were urinary tract and dengue infections. Hemoculture was positive in 14 patients (50%). The most common organisms were Klebsiella pneumoniae (27.3%), Escherichia coli (18.2%) and Pseudomonas aeruginosa (13.6%). All patients required mechanical ventilator support, and ICU mortality rate was 66.7%.
All the patients received multiple vasoactive agents. Norepinephrine was administered to all patients, 78% and 9.4% of patients received epinephrine and dopamine, respectively. The dosages of each vasopressor are listed in Table 1.
MAPradial strongly correlated with MAPfemoral (r = 0.89, p < 0.0001) (Fig. 2). In the multilevel mixed-effect model, the overall mean for MAPradial and MAPfemoral were 71.2 (95% CI 67–75.3) and 78.8 (95% CI 74.6–82.9) mmHg, respectively. Thus, MAPradial was significantly lower than MAPfemoral by 7.6 mmHg (95% CI 7–8.2) (p < 0.0001) (Fig. 3). The overall mean bias between MAPradial and MAPfemoral was − 7.6 mmHg (95% LOA, − 24.1 to 8.9) (Fig. 4). The bias plot, as per the Taffé method, is shown in Fig. 5. The estimated bias (red dash-dot regression line) increased, with a decrease in the level of true MAP (BLUP of the x-axis). When MAP was around 100 mmHg, MAPfemoral and MAPradial provided similar values; however, the bias increased (MAPradial progressively lower than MAPfemoral) when MAP dropped from 100 to 60 mmHg.
Bias plot between mean arterial pressure measurement at the radial (MAPradial) and femoral artery (MAPfemoral). BLUP: best linear unbiased prediction, X = mean arterial pressure, y1 = mean arterial pressure from radial artery, y2 = mean arterial pressure from femoral artery. The second scale on the right shows the relationship between the estimated amount of bias and the predicted value \(\hat{x}_{i}\).
Subgroup analysis between patients with MAPradial < 65 mmHg (35.1% of measurements) and those with MAPradial ≥ 65 mmHg was performed. The MAP was markedly discrepant in cases of MAPradial < 65 mmHg; wherein, most of MAPfemoral were significantly higher than MAPradial. The correlation was fair in group of MAPradial < 65 mmHg (r = 0.63, p < 0.0001) (in the Supplementary file: Fig. S1), with bias of − 13.0 mmHg (95% LOA, − 28.8 to 2.9) (in the Supplementary file: Fig. S2). In contrast, there was also strong correlation if MAPradial ≥ 65 mmHg (r = 0.88, p < 0.0001) (in the Supplementary file: Fig. S3), with bias of only − 4.2 mmHg (95% LOA, − 17.1 to 8.7) (in the Supplementary file: Fig. S4). The bias in the group of patients with MAPradial < 65 mmHg was significantly higher than that in patients with MAPradial ≥ 65 mmHg (p < 0.0001).
In the subgroup of norepinephrine doses, we found a similar correlation and agreement between MAPradial and MAPfemoral in patients receiving norepinephrine lower than 0.5 µg/kg/min and those who received more (Table 2). However, the bias between MAPradial and MAPfemoral in the subgroup of patients requiring norepinephrine ≥ 1 ug/kg/min was significantly higher than that in the group of patients requiring norepinephrine < 1 µg/kg/min.
There were 414 (58.6%) measurements deviating from the MAP gradient by > 5 mmHg and 235 (33.3%) measurements deviated by > 10 mmHg. The multilevel mixed-effect logistic regression analysis revealed that patients with MAPradial < 65 mmHg (OR 2.34; 95% CI 1.13–4.84, p = 0.02) and body weight (OR 0.89; 95% CI 0.81–0.98, p = 0.01) were associated with significant MAP gradients.
The total complications rate were not statistically different between femoral and radial artery catheterization (25% vs. 12.5%, p = 0.22). Bleeding from the femoral arterial puncture site was observed in 6 (18.7%) patients. All of them were easily controlled and none of these patients needed blood transfusion or further intervention to stop bleeding. Two patients (6.7%) developed non-expand small groin hematoma after femoral catheter removal. In contrast, temporary occlusion at radial artery catheter was observed in 4 (12.5%) patients (detail in the Supplementary file: Table S1).
Discussion
Invasive blood pressure measurement is important for hemodynamic monitoring and for providing intensive care to patients with shock in the ICU. This prospective study, aimed to investigate the difference between radial and femoral MAP in refractory shock patients receiving high-dose vasopressor therapy. This study found that MAP monitoring at the radial artery significantly underestimated the central arterial pressure as estimated by the femoral artery. Nearly 60% of our refractory shock patients had significant radial-femoral MAP gradients, and MAPradial less than 65 mmHg was an independent risk factor associated with an increased MAP gradient.
Similar to previous studies, this study found disagreement between MAP obtained from the radial and femoral arteries in critically ill patients. Kim et al. demonstrated that the bias between MAPfemoral and MAPradial was 4.9 mmHg (95% LOA, − 6.9 to 17) in septic shock patients14. Previous study in 24 critically ill patients, in mixed ICU found that bias between femoral and radial MAP was 4.3 mmHg (95% LOA, − 3.4 to 11.9)15.
On the other hand, Mignini et al. proposed that measurement of MAP at radial or femoral arteries is clinically interchangeable in critically ill patients. They found that MAPfemoral was higher than MAPradial but was not statistically significant, with a mean bias of 3 ± 4 mmHg (95% LOA,16)16. A more recent study by Antal et al. also found that MAPfemoral and MAPradial had a good correlation and agreement in sepsis patients, with a bias of 1.4 ± 4.7 mmHg (95% LOA, 18.3). The difference between our results and those studies may be related to the different study populations and severity of vasopressors used. Patients with refractory shock in this study were diagnosed with septic shock and required a very high dose of vasopressor (equivalent dose of norepinephrine 0.85 µg/kg/min). However, in the study by Antal et al., only half of their study population was diagnosed with septic shock and they received a lower dose of norepinephrine (0.14 ± 0.17 µg/kg/min) compared to this study.
There were conflicting results regarding the vasopressor dose–effect on the radial-femoral MAP gradients. Previous studies have reported that clinically radial-femoral MAP gradients are commonly observed in patients receiving high doses of norepinephrine administration13,14. Kim et al. showed that septic shock patients receiving norepinephrine < 0.1 µg/kg/min had a bias between MAPfemoral and MAPradial of 3 mmHg (95% LOA, − 7.2 to 13.1); however, the large discrepancies between MAP were found in patients receiving norepinephrine ≥ 0.1 µg/kg/min, with a bias of up to 6.2 mmHg (95% LOA − 6.0 to 18.3). In contrast, other studies have found that norepinephrine dose was not associated with the MAP gradient. Mignini et al. demonstrated that the bias of MAPradial and MAPfemoral was not different between patients receiving high and low dose of vasoactive agents (high vasoactive dose defined as norepinephrine or epinephrine ≥ 0.1 µg/kg/min or dopamine ≥ 10 µg/kg/min). This is consistent with a study in sepsis patients showing that the norepinephrine dose did not the influence the radial-femoral MAP difference17. The results of this study support the statement that norepinephrine dose does not influence the radial-femoral MAP gradient.
This study found a high prevalence of clinically significant MAP gradients between the radial and femoral arteries in patients with refractory shock. This is similar to previous studies reporting that 62% of critically ill patients had a MAP difference of at least 5 mmHg and 27–29% of patients had a MAP gradient ≥ 10 mmHg14,15. Although, the factors associated with radial-femoral MAP gradients have been extensively investigated, the proper mechanism is still controversial and suggests multifactorial mechanisms for the development of pressure gradients. The radial-femoral MAP gradient may be caused by a decrease in vascular resistance at the level of the hand28,29, peripheral vasoconstriction30 or a decrease in arterial elastance in the radial artery8. Galluccio et al. determined the demographic or hemodynamic factors driving the radial-femoral MAP gradient in critically ill patients. However, they failed to identify any statistically significant associations, including vasopressor dose or any demographic or hemodynamic data15.
This study showed that patients with MAPradial < 65 mmHg had a moderate correlation and increased bias between radial and femoral MAP and were also associated with significant MAP gradients between both sites. These results suggest that, in patients with marginally maintained MAP, measured at the radial artery, femoral artery catheterization should be considered for accurate arterial blood pressure monitoring in patients with refractory shock31. Monitoring MAP at the femoral artery may avoid future fluid administration or increase of unnecessary vasopressor therapy.
The strength of this study was the use of new statistical analyze to determine bias in the repeated measurement study such as the, bias plot by the Taffé26 and the multilevel mixed-effect model23,24. However, our study had some limitations. First, most patient with refractory shock in this study had septic shock. Therefore, it may have limited generalizability to other types of shocks. Second, we selected patients with refractory shock who received high doses of vasopressors, which may limit extrapolation for patients with less severe shock. Lastly, the clinical impact of arterial sites on morbidities or mortality was not measure in this study. Therefore, a larger study is required to further investigate the impact of radial and femoral arterial blood pressure monitoring in patients with refractory shock for therapeutic management and patient outcomes.
Conclusions
The radial artery significantly underestimated MAP when compared with the femoral artery in patients with refractory shock. More than half of the patients had clinically significant MAP gradients. Patients with refractory shock with borderline blood pressure targets, from the radial artery site, should be considered for femoral artery catheterization to obtain accurate measurements of blood pressure.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Koonrangsesomboon, W. & Khwannimit, B. Impact of positive fluid balance on mortality and length of stay in septic shock patients. Indian J. Crit. Care Med. 19, 708–713 (2015).
Brown, S. M. et al. Survival after shock requiring high-dose vasopressor therapy. Chest 143, 664–671 (2013).
Scheer, B. V., Perel, A. & Pfeiffer, U. J. Clinical review: Complications and risk factors of peripheral arterial catheters used for haemodynamic monitoring in anaesthesia and intensive care medicine. Crit. Care. 6, 198–204 (2002).
Saugel, B., Kouz, K., Meidert, A. S., Schulte-Uentrop, L. & Romagnoli, S. How to measure blood pressure using an arterial catheter: A systematic 5-step approach. Crit. Care. 24, 1–10 (2020).
Lakhal, K. & Robert-Edan, V. Invasive monitoring of blood pressure: A radiant future for brachial artery as an alternative to radial artery catheterisation?. J. Thorac. Dis. 9, 4812 (2017).
Nakamura, Y. et al. Pressure difference between radial and femoral artery pressure in minimally invasive cardiac surgery using retrograde perfusion. Int. J. Artif. Organs. 41, 635–643 (2018).
Fuda, G. et al. Risk factors involved in central-to-radial arterial pressure gradient during cardiac surgery. Anesth. Analg. 122, 624–632 (2016).
Kanazawa, M., Fukuyama, H., Kinefuchi, Y., Takiguchi, M. & Suzuki, T. Relationship between aortic-to-radiad arterial pressure gradient after cardiopulmonary bypass and changes in arterial elasticity. Anesthesiology 99, 48–53 (2003).
Chauhan, S., Saxena, N., Mehrotra, S., Rao, B. H. & Sahu, M. Femoral artery pressures are more reliable than radial artery pressures on initiation of cardiopulmonary bypass. J. Cardiothorac. Vasc. Anesth. 14, 274–276 (2000).
Ahmad, R. A., Ahmad, S., Naveed, A. & Baig, M. A. R. Peripheral arterial blood pressure versus central arterial blood pressure monitoring in critically ill patients after Cardio-pulmonary Bypass. Pak. J. Med. Sci. 33, 310–314 (2017).
Lee, M. et al. Agreement between radial and femoral arterial blood pressure measurements during orthotopic liver transplantation. Crit. Care Resusc. 17, 101–107 (2015).
Arnal, D., Garutti, I., Perez-Pena, J., Olmedilla, L. & Tzenkov, I. G. Radial to femoral arterial blood pressure differences during liver transplantation. Anaesthesia 60, 766–771 (2005).
Dorman, T. et al. Radial artery pressure monitoring underestimates central arterial pressure during vasopressor therapy in critically ill surgical patients. Crit. Care Med. 26, 1646–1649 (1998).
Kim, W. Y. et al. Radial to femoral arterial blood pressure differences in septic shock patients receiving high-dose norepinephrine therapy. Shock 40, 527–531 (2013).
Galluccio, S. T., Chapman, M. J. & Finnis, M. E. Femoral-radial arterial pressure gradients in critically ill patients. Crit.. Care Resusc. 11, 34–38 (2009).
Mignini, M. A., Piacentini, E. & Dubin, A. Peripheral arterial blood pressure monitoring adequately tracks central arterial blood pressure in critically ill patients: An observational study. Crit. Care. 10, 1–5 (2006).
Antal, O., Stefanescu, E. & Hagau, N. Does norepinephrine infusion dose influence the femoral-to-radial mean arterial blood pressure gradient in patients with sepsis and septic shock?. Blood Press. Monit. 24, 74–77 (2019).
Cetin, S. et al. Radial mean arterial pressure reliably reflects femoral mean arterial pressure in uncomplicated pediatric cardiac surgery. J. Cardiothor. Vasc. Anesth. 28, 76–83 (2014).
Meresse, Z. et al. Vasopressors to treat refractory septic shock. Minerva Anestesiol. 86, 537–545 (2020).
Jentzer, J. C. et al. Management of refractory vasodilatory shock. Chest 154, 416–426 (2018).
Shiloh, A. L. & Eisen, L. A. Ultrasound-guided arterial catheterization: A narrative review. Intensive Care Med. 36, 214–221 (2010).
Lu, M. J. et al. Sample size for assessing agreement between two methods of measurement by Bland–Altman method. Int. J. Biosta. 12 (2016).
Carstensen, B., Simpson, J. & Gurrin, L. C. Statistical models for assessing agreement in method comparison studies with replicate measurements. Int. J. Biostat. 4, 16 (2008).
Hair, J. F. Jr. & Favero, L. P. Multilevel modeling for longitudinal data: Concepts and applications. RAUSP Manag. J. 54, 459–489 (2019).
Bland, J. M. & Altman, D. G. Agreement between methods of measurement with multiple observations per individual. J. Biopharm. Stat. 17, 571–582 (2007).
Taffe, P., Halfon, P. & Halfon, M. A new statistical methodology overcame the defects of the Bland–Altman method. J. Clin. Epidemiol. 124, 1–7 (2020).
Taffe, P. Effective plots to assess bias and precision in method comparison studies. Stat. Methods Med. Res. 27, 1650–1660 (2018).
Pauca, A. L. et al. Radial artery-to-aorta pressure difference after discontinuation of cardiopulmonary bypass. Anesthesiology 70, 935–941 (1989).
Hynson, J. M., Katz, J. A. & Mangano, D. T. On the accuracy of intra-arterial pressure measurement: The pressure gradient effect. Crit. Care Med. 26, 1623–1624 (1998).
Baba, T., Goto, T., Yoshitake, A. & Shibata, Y. Radial artery diameter decreases with increased femoral to radial arterial pressure gradient during cardiopulmonary bypass. Anesth. Analg. 85, 252–258 (1997).
Nandhabalan, P., Loannou, N., Meadows, C. & Wyncoll, D. Refractory septic shock: Our pragmatic approach. Crit. Care. 22, 1–5 (2018).
Acknowledgements
This study was supported by a research grant of the Faculty of Medicine, Prince of Songkla University (Grant No. 62-008-14-4). We also thank Sarayut Lucien Geater from the Department of Internal Medicine, Faculty of Medicine, Prince of Songkla University for parts of the statistical analysis. We would also like to acknowledge the International Affairs Department, Faculty of Medicine, Prince of Songkla University, for their help in editing the English of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors took part in the conceptualization and methodology planning of the study. B.K. analyzed of the study data. The original draft was prepared by H.W. and reviewed by B.K. All authors contributed to the article and approved the submitted and publish version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wisanusattra, H., Khwannimit, B. Agreements between mean arterial pressure from radial and femoral artery measurements in refractory shock patients. Sci Rep 12, 8825 (2022). https://doi.org/10.1038/s41598-022-12975-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-12975-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.