Abstract
A proton magnetic resonance spectroscopy (MRS) technique was used to measure 13C enrichments of glutamate and glutamine in a 3.5 × 1.8 × 2 cm3 voxel placed in the dorsal anterior cingulate cortex of five healthy participants after oral administration of [U-13C]glucose. Strong pseudo singlets of glutamate and glutamine were induced to enhance the signal strength of glutamate and glutamine. This study demonstrated that 13C labeling of glutamate and glutamine can be measured with the high sensitivity and spatial resolution of 1H MRS using a proton-only MRS technique with standard commercial hardware. Furthermore, it is feasible to measure 13C labeling of glutamate and glutamine in limbic structures, which play major roles in behavioral and emotional responses and whose abnormalities are involved in many neuropsychiatric disorders.
Similar content being viewed by others
Introduction
Noninvasive in vivo detection of 13C labeling of glutamate (Glu) and glutamine (Gln) is a powerful tool for investigating Glu and Gln metabolism and neurotransmission in the brain1. Two types of 13C magnetic resonance spectroscopy (MRS) techniques have been widely used: direct 13C MRS and indirect 1H-[13C] MRS. Due to the much lower sensitivity of 13C nuclei, direct 13C MRS generally requires surface coils and very large tissue volume to achieve adequate signal-to-noise ratio (SNR). Hence, only the neocortex is accessible to direct 13C MRS experiments. The indirect 1H-[13C] MRS techniques make use of the 1H-13C coupling and difference spectroscopy to detect signals from protons bound to 13C2,3,4,5,6,7,8,9. Because of the larger gyromagnetic ratio of 1H compared to 13C, these indirect 13C detection techniques have higher sensitivity compared to the direct 13C detection techniques. Broadband magnetic resonance imaging (MRI) scanners equipped with heteronuclear capabilities are required to perform either direct 13C or indirect 1H-[13C] MRS. To enhance sensitivity and make heteronuclear nuclear Overhauser enhancement/decoupling feasible, the RF coil assembly needs to be high-efficiency non-volume 13C and 1H coils, which is a non-standard device and not commercially available. Because of these technical barriers, 13C MRS of human brain has been largely confined to only a few research groups with very limited clinical applications so far.
Attempts have been made to circumvent the hardware limitation of 13C MRS by measuring signal changes in short echo time (TE) 1H MRS spectra caused by incorporation of 13C labels into brain amino acids10. However, it has been difficult to reliably separate Glu and Gln in the crowded 1H MRS spectra. With the incorporation of 13C labels, the short TE 1H MRS spectra become even more complex. Due to these obstacles, 13C labeling of both Glu and Gln, the most important components of the glutamatergic system detectable by MRS, has been difficult to measure in the human brain using proton-only MRS methods.
Previously, our laboratory developed an MRS sequence for measuring Glu and Gln at 7 Tesla with minimized N-acetyl-aspartate (NAA) multiplet at 2.48 ppm using a J-suppression pulse11. This sequence was used to study the dynamic 13C labeling of Glu and Gln in the prefrontal cortex of healthy participants after intravenous infusion of [U-13C6]glucose11,12. However, the signal intensity of Gln at TE = 106 ms was low, hampering accurate detection of its 13C labeling.
Recently, our laboratory developed a 7 Tesla pulse sequence which can reliably measure Gln at TE = 56 ms with significantly improved sensitivity13,14. In this work, we demonstrate the feasibility of measuring 13C labeling of Glu and Gln using 1H MRS technique (TE = 56 ms) with a commercial proton-only head coil at 7 Tesla. Since this is a proton-only technique, no broadband capability or any custom-made hardware is necessary. In addition, because of the much higher sensitivity of proton MRS, we will also demonstrate for the first time, detection of 13C labeling of both Glu and Gln from an area in the limbic system. Specifically, using oral administration of 13C-labeled glucose, we will show that 13C labeling of Glu and Gln can be measured with high precision from the dorsal anterior cingulate cortex (dACC), a limbic region involved in cognition and motor control but is beyond the reach of conventional 13C MRS that relies on surface coils. It is hoped that the demonstration of measuring 13C-labeling of Glu and Gln with the high sensitivity and spatial resolution of proton MRS using commercial scanners and RF coils will generate interest in further improving MRS technology and greatly facilitate the adoption of 13C MRS strategies for probing energy metabolism and glutamatergic neurotransmission in clinical research.
Results
Figure 1 shows the calculated spectra of Glu, 13C satellites of Glu ([13C]Glu), Gln, 13C satellites of Gln ([13C]Gln), Asp, 13C satellites of Asp ([13C]Asp), GABA, and 13C satellites of GABA ([13C]GABA). As shown in Fig. 1, the spectra of Glu and Gln at TE = 56 ms are dominated by their respective H4 and H2 pseudo singlets. The 13C satellite signals of Glu H4 in the proton channel, [13C]Glu, are resulted from the large one-bond scalar coupling between the H4 pseudo singlet and the 13C label at C4. As both carbons of the acetyl CoA are 13C-labeled after administration of uniformly labeled glucose15, [4,5-13C]Glu was used as the starting point for spectral fitting of Glu 13C satellites in this study. Similarly, [4,5-13C]Gln and [3,4-13C]Asp were chosen as the starting points for fitting the 13C satellites of Gln H4 and Asp H3, respectively. The [4,5-13C]Glu, [4,5-13C]Gln and [3,4-13C]Asp spectra in Fig. 1 were generated using one-bound and long-range 1H-13C coupling constants reported in the literature16 except for the long-range 1H-13C coupling constants involving the carboxylic carbons which, to the best of our knowledge, were not available. Instead, the corresponding long-range 1H-13C coupling constants involving C4 (C3 for Asp) were used as their substitutes. The actual values of the long-range 1H-13C couplings used in the spectral model are not important as the spectral fitting program fits the in vivo spectra by adjusting the lineshape and linewidth of the 13C satellites to account for changes in B0 inhomogeneity and additional 1H-13C scalar couplings. Furthermore, although 31C label is transferred from glutamate C4 to GABA C2 during GABA formation, [2-13C]GABA was omitted in spectral fitting because the spectra of [2-13C]GABA and GABA are very weak compared to the resonance signals of Glu H4, Gln H4, and their 13C satellites, as shown in Fig. 1. Note that the 13C satellite spectra of Glu H4 and Gln H4 in Fig. 1 are highly asymmetrical and dominated by a single downfield satellite peak.
Numerically simulated spectra of Glu, 13C satellites of Glu ([13C]Glu), Gln, 13C satellites of Gln ([13C]Gln), Asp, 13C satellites of Asp ([13C]Asp), GABA, and 13C satellites of GABA ([13C]GABA) at TE = 56 ms. The corresponding concentration ratios were set to 10:10:3:3:3:3:1:1. The spectra were broadened to a singlet width of 8 Hz.
Time-course 1H spectra from the dACC of the five participants are displayed in Fig. 2. The spectra are highly consistent with the spectral patterns by the numerical simulations. The Glu H4 peak at 2.34 ppm dropped dramatically after oral administration of [U-13C]glucose and, correspondingly, the peak at 2.56 ppm significantly increased due to the rise of the downfield 13C satellite signals of Glu H4. Figure 3 displays the time-course spectra of a representative participant and corresponding fitted spectra of Glu, Gln, and their 13C satellites. In the fitted Gln time-course spectra, the drop in peak amplitude of Gln H4 after oral administration of [U-13C]glucose can be clearly seen. Meanwhile, the rise of the peak for Gln 13C satellites (vertically scaled up by a factor of 4) after oral administration of [U-13C]glucose is apparent. The spectra and corresponding fits for the pre-13C MRS scan and the last post-13C scan of the participant are displayed in Fig. 4 with good fitting results. The spline baseline obtained by fitting the pre-13C spectrum was labeled as baseline1. To account for the participant repositioning (see “Methods” section), a second much weaker baseline (baseline2) was used, which was determined when fitting the post-13C spectrum. The total baseline for the post-13C spectrum was the sum of baseline1 and baseline2.
Time-course 1H spectra from the dorsal anterior cingulate cortex (dACC) of five healthy participants. No linebroadening was applied to the in vivo spectra. The listed times are the mid-point times of the MRS scans. Voxel size = 3.5 × 1.8 × 2 cm3; TR = 2.2 s; TE = 56 ms; spectral width = 4000 Hz; number of data points = 1024; number of averages = 264 and total scan time = 10 min for the pre-13C spectra; number of averages = 132 and total scan time = 5 min for each individual post-13C spectrum. The time course acquisition was initiated after the participants drank the glucose solution, rested, and then re-entered the magnet.
In vivo spectra and corresponding fitted spectra for the pre-13C MRS scan and the last post-13C scan of the participant in Fig. 3. Baseline1 is the baseline in the pre-13C spectrum, which is a spline baseline with 13 knots. The baseline in the post-13C spectrum is baseline1 + baseline2, in which baseline2 is a much weaker spline baseline with 8 knots.
Figure 5 displays the plots of 13C enrichments of Glu C4 and Gln C4 vs. time after oral administration of [U-13C]glucose for all five participants. The behavior of the time course of 13C-labled Glu and Gln shown in Fig. 5 is consistent with them approaching maximum 13C labeling as expected from the time scale of cerebral Glu and Gln turnover1. Similar behavior was also observed in our previous direct 13C MRS experiments in the carboxylic/amide spectral region following oral administration of [U-13C]glucose17.
Metabolite ratios (/[tCr]) in the dACC of the five participants quantified from the pre-13C spectrum of each participant are given in Table 1. The results are highly consistent with our earlier 1H-only MRS study of the same brain region using the same pulse sequence13. Using the 12.6 mL (3.5 × 1.8 × 2 cm3) voxel size and 10 min scan time, the CRLB values for Glu and Gln were found to be 1.6 ± 0.2% for Glu and 3.2 ± 0.4% for Gln, indicating excellent precision. 13C enrichments of Glu C4 and Gln C4 for the five participants computed from the last two post-13C spectra of each participant, acquired at 113 ± 9 min after oral administration of [U-13C]glucose, were found to be 64 ± 5% for Glu and 40 ± 10% for Gln.
Supplementary Figure S1 online shows the time-course difference spectra of the participant in Fig. 3, which were obtained by subtracting the pre-13C spectrum from the post-13C spectra. Due to repositioning of the participant after oral administration of glucose, there were significant subtraction errors clearly visible at the NAA, tCr, and tCho singlet peaks.
Supplementary Figure S2 online displays the reprocessed time-course spectra of a participant in the previous study11,12, in which 1H spectra with TE = 106 ms were acquired from the prefrontal cortex of eight healthy participants after intravenous infusion of [U-13C6]glucose. In the fitted Gln time-course spectra, the Gln H4 peak is relatively weak. As a result, the gradual drop in peak amplitude of Gln H4 and the gradual rise in peak amplitude of Gln 13C satellites (vertically scaled up by a factor of 4) after intravenous infusion of [U-13C6]glucose are also very weak, which leads to relatively large errors in the computed 13C enrichment values of Gln C4. Supplementary Figure S3 online displays the plots of 13C enrichments of Glu and Gln vs. time after intravenous infusion of [U-13C]glucose for all eight healthy participants in the previous study. The 13C enrichment curves of Glu are relatively smooth and consistent with each other, indicating reliable measurement of 13C labeling of Glu. However, the 13C enrichment curves of Gln have large variations within each curve and between different participants. The end-point 13C enrichments of Glu and Gln for the eight participants computed from the last two post-13C spectra of each participant were found to be 51 ± 9% for Glu and 43 ± 16% for Gln. The last two post-13C spectra for the eight participants were acquired at 77 ± 9 min after intravenous infusion of [U-13C]glucose using the TE = 106 ms pulse sequence11. In comparison, a more reliable measurement of 13C-labeling of Gln was achieved using TE = 56 ms as evidenced by the higher Gln peaks in Fig. 3 and less scattered Gln 13C enrichment curves in Fig. 5.
Discussion
Here, we demonstrated the feasibility of using a proton-only MRS technique (TE = 56 ms) to measure 13C enrichments of Glu and Gln in the dACC of healthy participants after oral administration of [U-13C]glucose. Compared to the existing indirect 1H-[13C] MRS techniques that use 1H and 13C surface coils, this 1H MRS method can acquire MRS data from a voxel away from the neocortex using a standard 1H head coil.
This method used both the pre-13C and post-13C MRS data to compute 13C enrichments of Glu and Gln. Because the participants exited the scanner after the pre-13C scan for oral administration of glucose and reentered the scanner for acquisition of the post-13C spectra, the pre-13C and post-13C spectra generally had small differences in metabolite linewidth and lineshape, as well as in the spectral baseline. In the previous work (TE = 106 ms)12, the participants stayed in the magnet during the entire scan. Frequency shift, zero-order phase, and line-broadening of the pre-13C spectrum were adjusted to fit each post-13C spectrum before generating a difference spectrum. The 13C-labeled Glu and Gln concentrations were obtained by fitting the difference spectrum. In the current study, the pre-13C and post-13C spectra generally do not match very well due to repositioning of the participants after oral glucose administration outside the magnet. As shown by Supplementary Fig. S1, subtraction of the pre-13C spectrum from the post-13C spectra caused significant subtraction errors. A novel post-processing method was developed in this work. The metabolite concentration ratios (/[tCr] + 3[tCho]) of acetate (Ace), NAA, N-acetylaspartylglutamate (NAAG), GABA, Glu, Gln, glutathione (GSH), Asp, total creatine (tCr), total choline (tCho), taurine (Tau), myo-inositol (mI), and scyllo-inositol (sI) obtained by fitting the pre-13C spectrum were used as constraints when fitting the post-13C spectra. Meanwhile, the spline baseline obtained from the pre-13C spectrum was also used in fitting the post-13C spectrum, along with an additional much weaker baseline. This approach of using the prior information from the pre-13C spectrum in the fitting of each post-13C spectrum avoids spectrum subtraction and hence the resultant subtraction errors.
The turnover of NAA and GSH in brain is known to be much slower than that of Glu and Gln. Therefore, 13C labeling of NAA and GSH, in addition to GABA, was omitted from our spectral model. In this study, 13C labeling of Glu and Gln was extracted using information from both the decrease in the pseudo singlet signals and the increase in their 13C satellite signals. The relationship between the parent Glu H4 signal and its 13C satellites is established by full density matrix simulations. In Fig. 1, the 13C satellite signals are much weaker than their parent signals for the same concentration. Therefore, the contribution from the 13C satellites to spectral fitting results is less than the corresponding parent signals. Our current approach simplifies the spectral fitting process by omitting the 13C labeling of GABA and adjusting the lineshape and linewidth to account for changes in B0 inhomogeneity and additional 13C labels. An alternative and more sophisticated approach is to use the actual 1H-13C couplings to compute the lineshape and linewidth of all signals since the outcome of the entire process of 13C labeling of Glu and Gln is determined by very few kinetic parameters such as the tricarboxylic acid cycle rate and the Glu-Gln cycle rate. This more sophisticated approach will be developed to improve the current technique. The high sensitivity of the proton MRS method employed in this study also suggests that it may be possible to use the current strategy to measure the initial rates of 13C incorporation into Glu and Gln during intravenous infusion of 13C-labeled substrates such as [1-13C]glucose, [2-13C]acetate, [3-13C]lactate, or [2,4-13C2]β-hydroxybutyrate.
The data acquired from the previous study11,12 were reprocessed using the new post-processing method. However, the 13C enrichment value of Gln was still highly scattered due to the small Gln resonance signals at TE = 106 ms. Using the current sequence (TE = 56 ms), the Gln H4 peak is at least 61% higher than that of the previous sequence (TE = 106 ms)13. Therefore, a more precise measurement of the end-point 13C enrichment of Gln was achieved in this study, which is evidenced by the more consistent 13C enrichment curves of Gln.
Although the current study used a 7 Tesla scanner to resolve Glu and Gln H4 signals in the 1H MRS spectra, spectral resolution of Glu and Gln H4 signals at 3 Tesla is also achievable18. In principle, it is possible to use a similar strategy to measure 13C labeling of Glu C4 and possibly Gln C4 using 1H MRS on the more prevalent 3 Tesla scanners. Research along this direction is currently in progress in our laboratory.
Previous studies have demonstrated quantification of the Glu-Gln neurotransmitter cycling flux between neurons and astroglia using direct 13C MRS by measuring 13C labeling of Glu and Gln at isotopic steady state following administration of 13C-labeled acetate or by measuring dynamic turnover of Glu and Gln following administration of 13C-labeled glucose, lactate, or β-hydroxybutyrate1,19. Therefore, it is possible to use proton-only MRS techniques to quantify the Glu-Gln neurotransmitter cycling flux with much higher spatial resolution and from brain regions inaccessible to surface coils, e.g., from limbic structures which play a major role in many neuropsychiatric disorders. As shown by Fig. 5, the transient isotopic steady state was approached but not attained in this study. Future studies should measure the initial rates, or the entire time course, or delay the time window of sampling the turnover curves after oral glucose administration to capture the transient isotopic steady state.
In summary, a proton-only MRS technique that induces intense Glu and Gln H4 singlets at TE = 56 ms was used to measure 13C enrichments of Glu and Gln in the dACC of five healthy participants after oral administration of [U-13C]glucose. A novel post-processing method was developed, in which the metabolite ratios and spline baseline obtained from fitting the pre-13C spectrum were used in the fitting of the post-13C spectra to compute the 13C enrichments of Glu C4 and Gln C4. At 113 ± 9 min after oral administration of [U-13C]glucose, the end-point 13C enrichment of Glu C4 was found to be 64 ± 5% (n = 5) and that of Gln C4 was found to be 40 ± 10% (n = 5). This technique offers a novel option to study Glu neurotransmission in the human brain with the high sensitivity and spatial resolution of 1H MRS using standard commercial equipment. 13C labeling in brain regions inaccessible to surface coils can also be investigated using proton MRS.
Methods
Five healthy participants (two females, three males; age = 34 ± 12 years) were recruited for the study. Written informed consent was obtained from the participants before the study following the procedures approved by the Institutional Review Board (IRB) of the National Institute of Mental Health (NIMH; NCT00109174). The 13C enriched glucose solution was prepared by the National Institutes of Health (NIH) Clinical Center Pharmacy Department. All experimental protocols and methods were performed in accordance with the guidelines and regulations of NIH MRI Research Facility. Experiments were carried out on a Siemens Magnetom 7 Tesla scanner equipped with a 32-channel receiver head coil. The participants underwent overnight fasting before the MRS study.
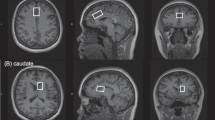
In each scan session, the participant was first scanned to acquire the pre-13C MRS data. T1-weighted magnetization prepared rapid gradient echo (MPRAGE) images were acquired with repetition time (TR) = 3 s, TE = 3.9 ms, matrix = 256 × 256 × 256, and resolution = 1 × 1 × 1 mm3. Based on the MPRAGE images, the MRS voxel with a size of 3.5 × 1.8 × 2 cm3 was placed in the dACC of the participant. The first- and second-order B0 shimming coefficients were adjusted, achieving water linewidths of 11.1 ± 0.4 Hz. The pre-13C MRS scan was subsequently performed using a previously described pulse sequence13. The main component of the pulse sequence was a point resolved spectroscopy sequence (PRESS) with a 10 ms truncated 180° Gaussian pulse applied at 2.12 ppm. The pulse sequence parameters were: TR = 2.2 s, TE = 56 ms, spectral width = 4000 Hz, number of data points = 1024, number of averages = 264, number of unsuppressed water signal averages = 2, and total scan time = 10 min.
After the pre-13C MRS scan was finished, the participant exited the scanner and was orally administered 20% w/w 99% enriched [U-13C]glucose solution at a dosage of 0.75 g [U-13C]glucose per kg of body weight following procedures described in our previous study of carbonic anhydrase-catalyzed 13C magnetization transfer and references therein17. After a rest period, the participant reentered the scanner. The MPRAGE images were repeated, based on which the MRS voxel was placed at the same location and with the same size as in the pre-13C MRS scan. Post-13C MRS scans were repeatedly performed, each lasted 5 min (number of averages = 132). B0 shimming coefficients were adjusted before each MRS scan.
The pre-13C MRS data were processed first and the process was similar to that of the previous work13. Briefly, the raw free induction decay (FID) data were reconstructed into the pre-13C spectrum after going through the necessary steps that include multi-channel data combination20, eddy current correction21, Bloch-Siegert phase shift correction22, frequency drift correction23, and Fourier transform. The reconstructed pre-13C spectrum was fitted in the range of 1.8–3.4 ppm by linear combination of numerically computed basis spectra of Ace, NAA, NAAG, GABA, Glu, Gln, GSH, Asp, tCr, tCho, Tau, mI, and sI, as well as a spline baseline with 13 knots. Chemical shifts and coupling constants were obtained from the literature for GABA24, GSH14, and the rest of the metabolites25. The fitting program was developed and improved in-house and was based on the Levenberg–Marquardt least square minimization algorithm. After the metabolite concentrations in arbitrary unit were obtained from the fitting, we computed the metabolite ratios, which were defined as the concentration of a metabolite divided by the sum of concentration of tCr and three times the concentration of tCho. The combined concentration, [tCr] + 3[tCho], weighs approximately equally the intensities of the tCr and tCho singlet peaks and is less prone to errors than using either [tCr] or [tCho] alone.
The post-13C spectra acquired after oral administration of [U-13C]glucose were reconstructed in the same way as the pre-13C spectrum. For the subsequent fitting process, additional basis spectra of 13C-labeled Glu, Gln, and Asp were simulated. Additional 13C chemical shifts and 1H-13C and 13C-13C coupling constants were obtained from Ref.16 with the exception of long-range 1H-13C couplings involving the carboxylic carbons. As shown in Fig. 1, contribution from GABA is minimal. Hence, 13C-labeled GABA was not included in spectral fitting. When fitting each post-13C spectrum, the metabolites ratios (/[tCr] + 3[tCho]) of Ace, NAA, NAAG, GABA, GSH, Asp, tCr, tCho, Tau, mI, and sI were fixed to the pre-13C values. However, the linewidths and lineshape of the metabolites in a post-13C spectrum were allowed to be different from those of the pre-13C spectrum because participant repositioning, B0 shimming, and additional 1H-13C couplings caused changes in the linewidths and lineshape. The sum of metabolite ratios of Glu and 13C-labeled Glu was constrained to be the same as the metabolite ratio of Glu obtained from the pre-13C spectrum. The same constraints were also applied to Gln and Asp. Because the spectral baseline in the post-13C spectrum was expected to be slightly different from the spectral baseline in the pre-13C spectrum due to participant repositioning and B0 shimming, the spectral baseline in each of the post-13C spectrum was approximated by the sum of the spline baseline in the pre-13C spectrum and another much weaker spline baseline with 8 knots.
After the metabolite concentrations were obtained by fitting each post-13C spectrum, the 13C enrichment of Glu C4 for the post-13C spectrum was computed as the ratio of the concentration of its 13C satellites to the total concentration of Glu.
For comparison purposes, the MRS data from the previous study11,12 were reprocessed using the new post-processing method as described above. The MRS data were acquired using the pulse sequence11 with TE = 106 ms from the prefrontal cortex of eight healthy participants (5 females and 3 males, age = 37 ± 8 years). The pulse sequence used the following parameters: voxel size = 2 × 2 × 2 cm3, TR = 2.5 s, TE = 106 ms, J-suppression pulse frequency = 4.38 ppm, J-suppression pulse flip angle = 90°, spectral width = 4000 Hz, number of data points = 2048, number of averages = 128, and total scan time = 5.5 min for each individual spectrum.
Data availability
Data are available upon request from the corresponding author Li An.
References
Rothman, D. L. et al. In vivo (13) C and (1) H-[(13) C] MRS studies of neuroenergetics and neurotransmitter cycling, applications to neurological and psychiatric disease and brain cancer. NMR Biomed. 32, e4172. https://doi.org/10.1002/nbm.4172 (2019).
Rothman, D. L. et al. H-1-observe C-13-decouple spectroscopic measurements of lactate and glutamate in the rat-brain in vivo. Proc. Natl. Acad. Sci. U. S. A. 82, 1633–1637. https://doi.org/10.1073/pnas.82.6.1633 (1985).
Petroff, O. A. C. et al. Cerebral lactate turnover after electroshock—In vivo measurements by H-1/C-13 magnetic-resonance spectroscopy. J. Cereb. Blood Flow Metab. 12, 1022–1029. https://doi.org/10.1038/jcbfm.1992.139 (1992).
Rothman, D. L. et al. H-1[C-13] Nmr measurements of [4-C-13]glutamate turnover in human brain. Proc. Natl. Acad. Sci. U. S. A. 89, 9603–9606. https://doi.org/10.1073/pnas.89.20.9603 (1992).
Mason, G. F. et al. Measurement of the tricarboxylic acid cycle rate in human grey and white matter in vivo by H-1-[C-13] magnetic resonance spectroscopy at 4.1 T. J. Cereb. Blood Flow Metab. 19, 1179–1188. https://doi.org/10.1097/00004647-199911000-00002 (1999).
Pan, J. W. et al. Spectroscopic imaging of glutamate C4 turnover in human brain. Magn. Reson. Med. 44, 673–679. https://doi.org/10.1002/1522-2594(200011)44:5%3c673::Aid-Mrm3%3e3.0.Co;2-L (2000).
Chen, W. et al. Study of tricarboxylic acid cycle flux changes in human visual cortex during hemifield visual stimulation using H-1-{C-13} MRS and fMRI. Magn. Reson. Med. 45, 349–355. https://doi.org/10.1002/1522-2594(200103)45:3%3c349::Aid-Mrm1045%3e3.0.Co;2-8 (2001).
Fitzpatrick, S. M., Hetherington, H. P., Behar, K. L. & Shulman, R. G. The flux from glucose to glutamate in the rat brain in vivo as determined by 1H-observed, 13C-edited NMR spectroscopy. J. Cereb. Blood Flow Metab. 10, 170–179. https://doi.org/10.1038/jcbfm.1990.32 (1990).
De Feyter, H. M. et al. Selective proton-observed, carbon-edited (selPOCE) MRS method for measurement of glutamate and glutamine C-13-labeling in the human frontal cortex. Magn. Reson. Med. 80, 11–20. https://doi.org/10.1002/mrm.27003 (2018).
Boumezbeur, F. et al. NMR measurement of brain oxidative metabolism in monkeys using C-13-labeled glucose without a C-13 radiofrequency channel. Magn. Reson. Med. 52, 33–40. https://doi.org/10.1002/mrm.20129 (2004).
An, L. et al. Detection of glutamate, glutamine, and glutathione by radiofrequency suppression and echo time optimization at 7 tesla. Magn. Reson. Med. 73, 451–458. https://doi.org/10.1002/mrm.25150 (2015).
An, L. et al. In Vivo Detection of 13C Labeling of Glutamate and Glutamine Using Proton MRS at 7 T. ISMRM 23rd Annual Meeting, 0207 (2015).
An, L., Araneta, M. F., Victorino, M. & Shen, J. Signal enhancement of glutamine and glutathione by single-step spectral editing. J. Magn. Reson. https://doi.org/10.1016/j.jmr.2020.106756 (2020).
Choi, C. H. et al. Improvement of resolution for brain coupled metabolites by optimized H-1 MRS at 7 T. NMR Biomed. 23, 1044–1052. https://doi.org/10.1002/Nbm.1529 (2010).
Li, S. Z. et al. In vivo detection of C-13 isotopomer turnover in the human brain by sequential infusion of C-13 labeled substrates. J. Magn. Reson. 218, 16–21. https://doi.org/10.1016/j.jmr.2012.03.012 (2012).
de Graaf, R. A. In Vivo NMR Spectroscopy—Principles and Techniques 2nd edn, 84–85 (Wiley, 2007).
Li, S. Z. et al. Determining the rate of carbonic anhydrase reaction in the human brain. Sci. Rep. UK https://doi.org/10.1038/s41598-018-20746-x (2018).
Zhang, Y. & Shen, J. Simultaneous quantification of glutamate and glutamine by J-modulated spectroscopy at 3 Tesla. Magn. Reson. Med. 76, 725–732. https://doi.org/10.1002/mrm.25922 (2016).
Shen, J. Modeling the glutamate–glutamine neurotransmitter cycle. Front. Neuroenerg. 5, 1. https://doi.org/10.3389/fnene.2013.00001 (2013).
An, L., van der Veen, J. W., Li, S. Z., Thomasson, D. M. & Shen, J. Combination of multichannel single-voxel MRS signals using generalized least squares. J. Magn. Reson. Imaging 37, 1445–1450. https://doi.org/10.1002/Jmri.23941 (2013).
Klose, U. In vivo proton spectroscopy in presence of Eddy currents. Magn. Reson. Med. 14, 26–30. https://doi.org/10.1002/mrm.1910140104 (1990).
An, L., Araneta, M. F., Johnson, C. & Shen, J. Simultaneous measurement of glutamate, glutamine, GABA, and glutathione by spectral editing without subtraction. Magn. Reson. Med. 80, 1776–1786. https://doi.org/10.1002/mrm.27172 (2018).
An, L., Araneta, M. F., Johnson, C. & Shen, J. Effects of carrier frequency mismatch on frequency-selective spectral editing. Magn. Reson. Mater. Phys. Biol. Med. 32, 237–246. https://doi.org/10.1007/s10334-018-0717-5 (2019).
Kaiser, L. G., Young, K., Off, D. J. M., Mueller, S. G. & Matson, G. B. A detailed analysis of localized J-difference GABA editing: Theoretical and experimental study at 4T. NMR Biomed. 21, 22–32. https://doi.org/10.1002/nbm.1150 (2008).
Govindaraju, V., Young, K. & Maudsley, A. A. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed. 13, 129–153. https://doi.org/10.1002/1099-1492(200005)13:3%3c129::Aid-Nbm619%3e3.3.Co;2-M (2000).
Acknowledgements
This study was supported by the Intramural Research Program of the National Institute of Mental Health, National Institutes of Health (IRP-NIMH-NIH). Annual Report Number: ZIA MH002803.
Funding
Open Access funding provided by the National Institutes of Health (NIH).
Author information
Authors and Affiliations
Contributions
J.S. and L.A. conceived and designed the experiments and prepared the manuscript. L.A. developed the pulse sequence and data processing software. L.A. and S.L. performed the experiments. S.L., M.F.A., and C.S.J. provided medical support. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
An, L., Li, S., Ferraris Araneta, M. et al. Detection of 13C labeling of glutamate and glutamine in human brain by proton magnetic resonance spectroscopy. Sci Rep 12, 8729 (2022). https://doi.org/10.1038/s41598-022-12654-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-12654-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.