Abstract
The symbiotic relationship between insects and gut microbes contributes to their fitness by serving immense range of functions viz. nutrition and digestion, detoxification, communication and reproduction etc. However, this relationship between insect and gut microbes varies from mutualistic to pathogenic. Gut microbes become pathogenic when the healthy normal microbial composition is perturbed leading to the death of insect host. Spodoptera litura (Fab.) is a polyphagous pest that causes significant damage to many agricultural crops. The management of this pest primarily depends upon chemical insecticides which have resulted in development of resistance. Thus in search for alternative strategies, culturable gut bacteria isolated from S. litura were screened for insecticidal potential. Among these Serratia marcescens and Enterococcus mundtii induced higher larval mortality in S. litura. The mortality rate increased from 32 to 58% due to S. marcescens at concentrations ranging from 2.6 × 108 to 5.2 × 109 cfu/ml and 26 to 52% in case of E. mundtii due to increase in concentration from 4.6 × 108 to 6.1 × 109 cfu/ml. Both the bacteria negatively affected the development, nutritional physiology and reproductive potential of insect. The results indicated a change in gut microbial composition as well as damage to the gut epithelial membrane. Invasion of gut bacteria into the haemocoel led to septicaemia and ultimately death of host insect. In conclusion both these gut bacteria may serve as potential biocontrol agents against S. litura.
Similar content being viewed by others
Introduction
Insects live in a symbiotic relationship with various microbes that play a crucial role in their diversification and evolutionary success1. These gut microbes serve an immense range of functions including provision of nutrients, digestion, protection from pathogens, detoxification of secondary plant metabolites, communication and reproduction1. Contribution of symbiotic microorganisms in decomposition of cellulose components of plant material has been well documented in termites and grasshoppers2,3. Buchnera aphidicola associated with aphids is known to fulfil the requirement of essential amino acids that are lacking in plant sap4,5. Similarly Pseudomonas species, a predominant member of gut microbiota of coffee berry borer, Hypothenemus hampei (Ferrari) help in detoxification of caffeine6. The gut microbial composition in Drosophila melanogaster (Meigen) determines the mating attractiveness, preferentially with individuals harbouring similar microbiota7,8. The interactions between hosts and their microbes can range from mutualistic to pathogenic9. The gut bacteria may become opportunistic pathogens at a particular time of challenge due to some physiological or environmental changes that triggers their virulence factor or due to perturbation in the gut microbial diversity10,11,12. Mason et al.9 reported that translocation of Enterococcus from midgut to haemocoel led to its pathogenic state in Manduca sexta (Linnaeus). Similarly the mutualistic or pathogenic nature of Photorhabdus luminescens depends on whether it lives in gut or hemolymph of host insect13. Enterobacter cloacae, a member of gut microflora of Spodoptera litura (Fab.), when fed orally to its host showed pathogenicity due to change in gut microbial diversity and abundance of E. cloacae14. Similarly Cakici et al.15 reported the insecticidal potential of Flavobacterium sp. and Klebsiella sp. isolated from Spodoptera littoralis (Boisduval) when tested against same insect host. Serratia marcescens isolated from larvae of hazelnut weevil Curculio dieckmanni (Faust) has also been documented to induce larval mortality in host insect16.
Lepidoptera is one of the most diverse and widespread order of class Insecta. The insects belonging to this order play an important role in ecosystem as pollinators and in the food chain. However, the larval stage of most of these insects is phytophagous and cause destruction to many agricultural plants. S. litura commonly known as tobacco caterpillar, is a polyphagous lepidopteran pest of many economically important crops such as cotton, soybean, groundnut, tobacco and vegetables17. The control of this pest mainly involves the application of chemical insecticides such as organophosphates, carbamates and synthetic pyrethroids18,19. However, many of these insecticides have been found to be ineffective due to development of resistance in this pest to different groups of insecticides19,20,21,22. Besides development of insecticide resistant populations of insects, the hazardous effects of synthetic insecticides on human health, environment and non-target organisms are also a matter of concern19,23,24. Therefore, there is need for alternative ecofriendly strategies for pest management.
The use of pathogenic microbes viz. fungi, bacteria, viruses and nematodes are gaining popularity as an alternative strategy to chemical insecticides. Due to their species specificity and environmental safety, these have been exploited to develop insecticide formulations. Among these, Bacillus thuringiensis (Bt) has been commercially used as bioinsecticide against insect pests belonging to Diptera, Coleoptera and Lepidoptera. However, reports on development of resistance in lepidopteran pests viz. Plutella xylostella (Linnaeus), Pectinophora gossypiella (Saunders), Spodoptera frugiperda (JE Smith) and Helicoverpa zea (Boddie) towards Bt insecticides has become a matter of concern25,26,27,28. The resistance to Bt insecticides necessitates the need to explore new niches as sources of novel microorganisms having insecticidal activity. In this respect, as a step towards finding potential candidates for biological control, the present study aimed to determine the pathogenicity of culturable gut microbes associated with S. litura infesting crops of this region.
Results
Screening bioassays
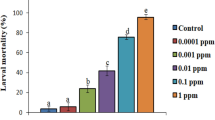
Screening of gut bacteria viz. S. gallinarum, B. safensis, E. mundtii, E. casseliflavus, S. sciuri, G. halophytocola, C. terpenotabidum, S. marcescens and P. brenneri for insecticidal activity against S. litura indicated significantly higher larval mortality (20–48%) in comparison to control (Fig. 1). Among the tested bacteria, E. mundtii and S. marcescens exhibited higher larval mortality i.e. 40% and 48%, thus both these bacteria were selected for detailed bioassay studies.
Dose–response experiments
Mortality and development period
Results presented in Table 1 depict a significant effect of S. marcescens and E. mundtii on survival and development of S. litura. Both the bacteria caused significantly higher larval mortality relative to control. The leaves treated with different concentrations of S. marcescens caused 32–58% mortality in S. litura larvae (F = 15.20**, p ≤ 0.05) (Fig. 2). The mortality rate increased in a concentration dependent manner. Similar results were obtained due to E. mundtii cell suspension that caused 26–52% mortality in S. litura larvae (F = 22.64**, p ≤ 0.05) (Fig. 2). The larval mortality started after 3rd day of treatment at higher concentrations (3.0 × 109 cfu/ml and 5.2 × 109 cfu/ml) of S. marcescens and continued till 13th day (Fig. 3). Maximum larval deaths were observed with cumulative mortality of 52% at 9th day of treatment. Similarly in case of E. mundtii, the larval mortality started after 3rd day of treatment at the highest concentration (6.1 × 109 cfu/ml) and continued for fifteen days (Fig. 4). The LC50 values for both the bacteria were calculated using Probit analysis, that came out to be 2.4 × 109 and 5.6 × 109 cfu/ml respectively for S. marcescens and E. mundtii. Relative to control, the infected larvae showed the symptoms of sluggishness, cessation of feeding and the dead larvae became black in colour, flaccid but with intact integument (Fig. 5a–c).
Influence of different concentrations of S. marcescens (C1 = 2.6 × 108 cfu/ml, C2 = 6.4 × 108 cfu/ml, C3 = 1.6 × 109 cfu/ml, C4 = 3.0 × 109 cfu/ml and C5 = 5.2 × 109 cfu/ml) and E. mundtii (C1 = 4.6 × 108 cfu/ml, C2 = 8.9 × 108 cfu/ml, C3 = 1.8 × 109 cfu/ml, C4 = 3.4 × 109 cfu/ml and C5 = 6.1 × 109 cfu/ml) on larval mortality of S. litura. Columns and bars represent the mean ± SE. Different letters above the columns represent significant differences at Tukey's test p ≤ 0.05.
Bacterial treatment also influenced the development of insect. The larval period tended to increase but significant effect was observed at higher concentrations (Table 1). At the highest concentration of S. marcescens, the larvae took 14.88 days to pupate in comparison to 12.06 days in control (F = 6.05, p ≤ 0.05). The pupal period was also affected at the highest concentration. The overall development period from larva to adult extended significantly at higher concentrations i.e. 3.0 × 109 and 5.2 × 109 cfu/ml where the insect took 23.28 and 24.98 days respectively in comparison to 20.96 days in control (F = 10.51, p ≤ 0.05) (Table 1).
Similar effects were observed due to E. mundtii where the larval period prolonged significantly by 2.59 to 4.03 days at higher concentrations i.e. 3.4 × 109 and 6.1 × 109 cfu/ml with respect to control (Table 1). Significant effect was also detected on pupal period that ultimately extended the total development period by 2.68 to 5.43 days at concentrations ranging between 1.8 × 109 to 6.1 × 109 cfu/ml in comparison to control.
Adult emergence and reproductive potential
Serratia marcescens treatment significantly decreased the adult emergence of S. litura at higher concentrations i.e. 3.0 × 109 and 5.2 × 109 cfu/ml (F = 15.47, p ≤ 0.05) (Table 1). Similarly adult emergence tended to decrease when the larvae were fed on cell suspension of E. mundtii, however, significant effect was recorded at higher concentrations (3.4 × 109 and 6.1 × 109 cfu/ml) where 76.60 to 71.20% adults emerged as compared to 91.06% in control (F = 6.15, p ≤ 0.05) (Table 1). The bacterial infection also caused morphological deformities in adults such as unequal and crumpled wings (Fig. 5d,e). Except for the lower concentrations, the percentage of morphologically deformed individuals was significantly higher in both the bacterial treatments (S. marcescens, F = 13.22, p ≤ 0.05; E. mundtii, F = 22.32, p ≤ 0.05) (Table 1).
The effects of bacterial suspensions were also detected on females which showed reduced longevity. Except for the lowest concentration of S. marcescens, the female longevity decreased significantly by 2.0 to 2.67 days in comparison to control (F = 7.15, p ≤ 0.05) (Fig. 6). Significant effect of S. marcescens was also observed on male longevity at the highest concentration (F = 4.40, p ≤ 0.05). In case of E. mundtii no significant inhibitory effects were detected on adult longevity except for the highest concentration in case of females (F = 5.97, p ≤ 0.05) (Fig. 6). The reproductive potential of females was significantly reduced at higher concentrations (3.0 × 109 and 5.2 × 109 cfu/ml) of S. marcescens where the female laid only 696.66 to 671.00 eggs throughout its life as compared to 866.66 eggs in control (F = 4.53, p ≤ 0.05) (Fig. 7). Similarly in case of E. mundtii, fecundity was found to be decreased significantly at the highest concentration. The bacterial infection further decreased the viability of eggs with significant effect at higher concentrations (S. marcescens, F = 16.35, p ≤ 0.05; E. mundtii, F = 17.11, p ≤ 0.05) (Fig. 8).
Influence of different concentrations of S. marcescens (C1 = 2.6 × 108 cfu/ml, C2 = 6.4 × 108 cfu/ml, C3 = 1.6 × 109 cfu/ml, C4 = 3.0 × 109 cfu/ml and C5 = 5.2 × 109 cfu/ml) and E. mundtii (C1 = 4.6 × 108 cfu/ml, C2 = 8.9 × 108 cfu/ml, C3 = 1.8 × 109 cfu/ml, C4 = 3.4 × 109 cfu/ml and C5 = 6.1 × 109 cfu/ml) on adult longevity of S. litura. Columns and bars represent the mean ± SE. Different letters above the columns represent significant differences at Tukey's test p ≤ 0.05.
Influence of different concentrations of S. marcescens (C1 = 2.6 × 108 cfu/ml, C2 = 6.4 × 108 cfu/ml, C3 = 1.6 × 109 cfu/ml, C4 = 3.0 × 109 cfu/ml and C5 = 5.2 × 109 cfu/ml) and E. mundtii (C1 = 4.6 × 108 cfu/ml, C2 = 8.9 × 108 cfu/ml, C3 = 1.8 × 109 cfu/ml, C4 = 3.4 × 109 cfu/ml and C5 = 6.1 × 109 cfu/ml) on fecundity of S. litura. Columns and bars represent the mean ± SE. Different letters above the columns represent significant differences at Tukey's test p ≤ 0.05.
Influence of different concentrations of S. marcescens (C1 = 2.6 × 108 cfu/ml, C2 = 6.4 × 108 cfu/ml, C3 = 1.6 × 109 cfu/ml, C4 = 3.0 × 109 cfu/ml and C5 = 5.2 × 109 cfu/ml) and E. mundtii (C1 = 4.6 × 108 cfu/ml, C2 = 8.9 × 108 cfu/ml, C3 = 1.8 × 109 cfu/ml, C4 = 3.4 × 109 cfu/ml and C5 = 6.1 × 109 cfu/ml) on egg hatching of S. litura. Columns and bars represent the mean ± SE. Different letters above the columns represent significant differences at Tukey's test p ≤ 0.05.
Effect of S. marcescens and E. mundtii on nutritional physiology
As is evident from Table 2, S. marcescens significantly influenced the nutritional indices of S. litura. The relative consumption rate of larvae feeding on bacteria treated leaves was significantly decreased which in turn led to decrease in relative growth rate of larvae at all the concentrations (RGR, F = 3.60, p ≤ 0.05; RCR, F = 17.12, p ≤ 0.05). However, no significant difference was found within the different concentrations of bacterial treatments. The ECI value also decreased from 1.82% in control to 1.10–1.00% due to bacterial infection (F = 8.75, p ≤ 0.05). A significant decrease in ECD was observed at 3.0 × 109 and 5.2 × 109 cfu/ml of S. marcescens cell suspension (F = 4.82, p ≤ 0.05). Except for the lowest concentration, a significant reduction was also detected in approximate digestibility (F = 5.44, p ≤ 0.05) (Table 2).
Similar effects of E. mundtii were observed on various nutritional parameters of S. litura (Table 2). There was a significant drop in relative consumption and growth rate of larvae. With respect to control, the values of RCR decreased by 57.06 to 59.98% with concomitant decrease of 33.33 to 42.42% in RGR at higher concentrations i.e. 1.8 × 109 cfu/ml and 6.1 × 109 cfu/ml (RGR, F = 5.53, p ≤ 0.05; RCR, F = 8.48, p ≤ 0.05). Similarly, the efficiency of conversion of ingested and digested food of larvae decreased significantly by 2.21 to 2.45 and 1.59 to 1.74 times respectively at higher concentrations (ECI, F = 3.44, p ≤ 0.05; ECD, F = 3.77, p ≤ 0.05) (Table 2). Significant negative impact of E. mundtii was also detected on approximate digestibility of food at concentrations ranging from 1.8 × 109 cfu/ml to 6.1 × 109 cfu/ml (F = 3.88, p ≤ 0.05) (Table 2).
Effect of S. marcescens and E. mundtii on gut microflora of S. litura
As is evident from Table 3 there is considerable difference in gut microbial composition of control and treated larvae. The gut microflora of control larvae consisted of E. mundtii, E. casseliflavus and A. hemolyticus with 7.4 × 106, 6.9 × 106 and 4.0 × 105 cfu/ml respectively. However, the treatment of larvae with S. marcescens led to change in bacterial abundance. There was increase in bacterial concentration of S. marcescens with 7.9 × 107 cfu/ml relative to other bacterial cultures i.e. E. mundtii and E. casseliflavus with 4.1 × 104 and 3.6 × 104 cfu/ml respectively (Table 3). Similarly the larvae infected with E. mundtii showed the dominance of E. mundtii with cfu count of 9.3 × 107 per ml in comparison to 5.6 × 104 cfu/ ml of E. casseliflavus. E. mundtii was observed in both the treated as well as control larvae, while A. hemolyticus was absent in the larvae treated with both the bacterial concentrations.
Histological analysis
Difference in the histology of gut of S. litura was observed among the control and treated larvae. The midgut cross-sections of larvae fed on cell suspensions of S. marcescens and E. mundtii showed damage of the midgut epithelial cells with vacuolization of the cytoplasm, brush border membrane and peritrophic membrane destruction (Fig. 9). However, the control larvae showed a well-preserved layer of epithelial cells, peritrophic membrane and muscular layer of the midgut.
Longitudinal section through the midgut of 4th instar S. litura larvae (a) control larva fed on untreated diet, (b) larva fed on leaves treated with S. marcescens, (c) larva fed on leaves treated with E. mundtii. PM Peritrophic membrane, EL Epithelial layer, ML Muscle layer, ELD Epithelial layer disruption, CV Cytoplasmic vacuolization, MLD Muscle layer disruption.
Presence of S. marcescens and E. mundtii in larval haemolymph
The growth of both the bacteria was observed in the hemolymph of infected larvae due to S. marcescens and E. mundtii infection, however, no growth was observed in case of control healthy larvae.
Discussion
Gut microbes play an important role in insects ranging from digestion, detoxification, communication and reproduction etc1. Besides their functional role the native gut microbes have also been reported to be pathogenic in insects14,29,30. In the present study, screening of culturable bacteria associated with larval and pupal stages of S. litura indicated higher mortality of host larvae due to S. marcescens and E. mundtii. The pathogenicity of S. marcescens and E. mundtii has earlier been reported in various lepidopteran insects viz. Bombyx mori (Linnaeus), Spodoptera exigua (Hubner), Galleria mellonella (Linnaeus), Lymantria dispar (Linnaeus), Malacosoma neustria (Linnaeus), Plodia interpunctella (Hubner) and Ephestia kuehniella (Zeller)30,31,32,33,34,35,36. Other strains of Enterococcus viz. Enterococcus faecalis and Enterococus faecium have earlier been documented to cause mortality in S. exigua and G. mellonella37,38,39.
The S. litura larvae infected with S. marcescens and E. mundtii showed the symptoms of lethargy, dark colouration of body, flaccid with intact integument which are typical symptoms of bacterial infection40. Likewise P. interpunctella and E. kuehniella infected with S. marcescens showed similar symptoms of infection32,34,36,41.
Histopathological studies conducted on S. litura infected with S. marcescens and E. mundtii indicated disruption of peritrophic membrane, damage to epithelial cells and cytoplasmic vacuolization which is similar to earlier report on S. litura due to bacterial infection of S. marcescens42. Peritrophic membrane acts as first line of defense in insects against microbial pathogens. Chitin is the main component of peritrophic membrane that lines the midgut epithelium43. There are reports documenting the production of toxins and hydrolytic enzymes such as hemolysins, chitinases, proteases, lipases and phospholipases from S. marcescens that contribute to its pathogenicity in insects42,44,45. The different type of chitinases viz. exochitinases, endochitinases and chitobiosidases damage the midgut peritrophic membrane that further help the bacterial invasion into the haemocoel42,46,47. S. marcescens and E. mundtii in our study were observed to grow in the hemolymph of the larvae indicating that the bacteria traversed the intestinal epithelial barrier. The bacterial invasion in hemolymph was also reported in S. litura, Helicoverpa armigera (Hubner) and M. sexta due to infection of Serratia and Enterococcus sp.9,42,48. The present study is in line with the earlier reports indicating that bacterial proliferation in hemolymph after crossing the intestinal barrier cause septicaemia which ultimately led to the death of its host49.
Serratia and Enterococcus have been known to be the normal flora of larvae, pupae and adults of lepidopteran insects50,51,52. These bacteria are generally found in low numbers in digestive tract and are not pathogenic. However, the bacteria may become pathogenic when the insect immune system gets weakened or due to alterations in gut microbial composition of insects12,53,54. Earlier studies revealed that perturbation of gut microbial composition led to the death of host insect12,14,55,56,57. Present study showed the difference in gut microbial composition of control and treated larvae. Serratia and Enterococcus have been found to increase in numbers in treated S. litura larvae with respect to control larvae. It is in line with the previous report on S. litura where the S. marcescens is able to colonize the midgut tissues after oral infection and there after the population increased as compared to control larvae42. E. mundtii found in low numbers in gut microflora of healthy larvae, however, increased number of bacterial colonies led to flacherie disease in B. mori larvae32. S. marcescens successfully inhabitated the gut by increasing its number and replacing the other gut associated beneficial microflora in H. armigera48. The infection due to S. marcescens and E. mundtii prolonged the development of S. litura which to similar to the reports on S. litura, H. armigera and Bactrocera dorsalis (Hendel) due to infection of S. marcescens, Enterobacter cloacae and Lactobacillus lactis14,46,48,58. Bacterial infection further affected the nutritional physiology of S. litura larvae. The significant decrease in growth rate of S. litura may be attributed to decreased relative consumption rate. The treated S. litura larvae also showed reduction in efficiency of conversion of ingested and digested food as well as approximate digestibility. Previous studies also revealed the inhibitory effects on nutritional physiology of S. litura and Cnaphalocrocis medinalis (Guenee) due to E. cloacae and B. thuringiensis infection14,59. Chandrasekaran et al.60 reported negative effect on nutritional physiology of S. litura due to extracellular chitinases produced from Bacillus subtilis. Destruction of peritrophic membrane and midgut epithelial cells observed during histopathological studies on S. litura may have impaired the digestive functions by interfering with digestive and protective enzymes activity as suggested by Zhang et al.16. The decrease in digestive function may further slow the growth of larvae. Reduction in adult emergence, fecundity and egg hatchability was also observed in the bacteria treated groups of S. litura larvae. S. marcescens was earlier reported to decrease the adult emergence and reproductive potential of S. litura46. These results indicate that S. marcescens and E. mundtii act as opportunistic pathogens which also exert growth inhibitory and toxic effects on S. litura.
Conclusion
Present study revealed the insecticidal potential of S. marcescens and E. mundtii. Both the bacterial isolates showed pathogenicity against second-instar larvae of S. litura. The ingestion of bacteria negatively affected the development and nutritional physiology of insect. Both the bacteria after successful establishment started degrading the gut wall and invaded the haemocoel thereby causing the death of the host. In conclusion these results indicate that S. marcescens and E. mundtii have a potential to be used as biocontrol agent against insect pests.
Materials and methods
Mass rearing of insect
The egg masses and larvae of S. litura were collected from cabbage and cauliflower fields around Amritsar (Punjab), India. The larvae were reared on fresh castor leaves. The culture was maintained in the laboratory at temperature and humidity conditions of 25 ± 2 °C and 65 ± 5% respectively as per the protocol of Datta et al.61. After maintaining the culture of S. litura for three generations in the laboratory, the newly hatched larvae were used for conducting experiments.
Bacterial isolation
The larvae and pupae of S. litura from third generation of laboratory culture were used for the isolation of culturable bacteria in the present study. Both larvae and pupae were sterilized with 70% (v/v) ethanol followed by washing with sterilized distilled water in order to remove the disinfectant. The larvae were dissected with sterilized micro scissors to remove the gut while the pupae were homogenised whole in 1.0 ml Phosphate Buffer Saline (PBS) solution (pH 7.0). The homogenised suspension was then serially diluted up to ten times and 100 µl of each diluted sample was then plated on Luria Bertani (LB) plates. The plates were incubated at 30 °C for 72 h for the observation of morphologically distinct colonies. The pure bacterial isolates were stored in 50% (w/v) glycerol at -80 °C. The identification of bacterial cultures was done by using various morphological, biochemical tests and molecular methods. On the basis of 16S rRNA gene sequencing the bacterial cultures were identified as Staphylococcus gallinarum (MW199124), Bacillus safensis (MW199274), Enterococcus mundtii (MW199120), Enterococcus casseliflavus (MW199276), Staphylococcus sciuri (MW199118), Glutamicibacter halophytocola (MW199121), Corynebacterium terpenotabidum (MW207679), Serratia marcescens (MW207987), and Pantoea brenneri (MW205745) (data submitted elsewhere).
Preparation of bacterial suspension
Bacterial isolates were inoculated into LB broth and incubated at 30 °C for 48 h. After incubation the cultures was centrifuged at 4000 rpm at 4 °C for 10 min to obtain the pellet. The pellet was dissolved in sterile PBS solution and the bacterial density was measured at optical density (OD600) and adjusted to 1.89 (1.8 × 109 cfu/ml approximately) and 10 ml of bacterial suspension was further used in bioassays as described by Eski et al.29 with some modifications.
Screening bioassays
Second instar larvae of S. litura were used for screening the insecticidal activity of isolated bacterial cultures. The larvae were randomly selected and kept in rearing vials. The castor leaves were surface sterilized with 5% (v/v) NaOCl and washed with distilled water. The surface sterilized leaves of approximately 10cm2 were treated by dipping in 10 ml of bacterial suspension and were used in bioassays as described by Eski et al.29 with some modifications. After air drying at room temperature the treated leaves were kept in rearing vials containing larvae. Control group was fed on leaves dipped in PBS buffer only. The screening experiment for each bacterial culture was replicated 5 times with 10 larvae per replication (n = 50). During experiment the temperature and humidity conditions were maintained at 25 ± 2 °C and 65 ± 5% respectively. The diet was changed regularly after every 48 h till pupation and larval mortality was recorded.
Dose response experiments
Based on higher larval mortality in S. litura due to S. marcescens and E. mundtii, both these cultures were used for dose response experiments. The concentration range for S. marcescens was, C1 = 2.6 × 108 cfu/ml, C2 = 6.4 × 108 cfu/ml, C3 = 1.6 × 109 cfu/ml, C4 = 3.0 × 109 cfu/ml and C5 = 5.2 × 109 cfu/ml. The different concentrations used for cell suspension of E. mundtii were, C1 = 4.6 × 108 cfu/ml, C2 = 8.9 × 108 cfu/ml, C3 = 1.8 × 109 cfu/ml, C4 = 3.4 × 109 cfu/ml and C5 = 6.1 × 109 cfu/ml (based on their OD600 values). The leaves dipped in PBS buffer only were fed to control group. The experiment was conducted in a similar manner as for screening bioassays. Observations were made daily on larval mortality, development period and adult emergence. The freshly emerged adults from all the treatments and control were transferred to oviposition jar in 2:1 ratio (2 females: 1 male) to observe the longevity and fecundity of adults. One oviposition jar represented one replicate and all the treatments were replicated thrice. Based on larval mortality data, lethal concentration (LC50) values for both the bacteria were determined by Probit analysis using the SPSS 20.0 statistical software.
Nutritional analysis
In order to investigate the effect of bacteria on nutritional physiology of S. litura, the larvae were fed on castor leaves treated with different concentrations of S. marcescens and E. mundtii as mentioned above. The second instar larvae starved for 3–4 h were weighed individually and released in rearing vials containing treated and control leaves of known weight. The experiment was performed on 50 larvae for each concentration of both the bacterial cultures following the procedure of Datta et al.61. After 72 h of feeding, observations were made on larval weight, residual diet and faecal matter and overall change in each variable was compared with the last recorded value. Relative growth (RGR) and consumption rates (RCR) were calculated as G/I (G = change in larval dry weight/day and I = initial larval dry weight) and C/I (C = change in diet dry weight/day and I = initial larval dry weight) respectively. Both were calculated as mg mg−1 day−1. Index of food conversion efficiency (ECI) was calculated as 100 × G/C; where G = dry weight gain of insect and C = dry weight of food consumed. Approximate digestibility (AD) and efficiency of conversion of digested food (ECD) were calculated as C − F/C × 100 (where C = change in diet dry weight/day and F = dry weight of frass/day) and G/C − F × 100 (where G = change in larval dry weight/day, C = change in diet dry weight/day and F = dry weight of frass/day, respectively. All the nutritional indices were calculated as per Farrar et al.62.
Determination of effect of S. marcescens and E. mundtii on gut microflora of S. litura
To determine the effect of oral infection of bacteria on gut microbial composition of S. litura, pure cultures of E. mundtii and S. marcescens were inoculated in LB media. Second instar larvae were fed on leaves treated with LC50 values of S. marcescens and E. mundtii. After 96 h of feeding on treated leaves, ten infected larvae showing the symptoms of slow growth, reduction in size, black pigmentation on integument and control larvae were dissected separately to remove the gut. These larval guts of both infected and control larvae were then homogenized separately in 1 ml 0.1 M phosphate buffer (pH 7.0). A serial dilution of homogenized suspension was performed up to ten times and 100 µl of each dilution was spread on Luria Bertani (LB) agar plates. The plates were incubated for 48 h at 30 °C for appearance of bacterial colonies. The cfu/ml of different bacteria was calculated by plate count method. Each morphotype was purified by further streaking on LB plates. The bacterial isolates obtained were identified by using various morphological, biochemical tests and molecular methods. Based on 16S rRNA gene sequencing these bacteria were identified as Enterococcus mundtii (MW199120), Enterococcus casseliflavus (MW199276), Serratia marcescens (MW207987) and Acinetobacter haemolyticus (MW199127).
Histological analysis
For histological studies the second instar larvae were fed on LC 50 values of S. marcescens and E. mundtii cell suspension. In case of control, larvae were fed on leaves dipped in PBS buffer only. The experimental conditions were maintained at 25 ± 2 °C and 65 ± 5% respectively temperature and humidity respectively. After 96 h, both treated and control larvae were dissected aseptically and the gut was preserved in 10% formalin until processing of tissue. After fixation, the material was washed with distilled water in a tube and 30–90% grades of alcohol were used for progressive dehydration of tissue. After dehydration, the tissue from both control and treated larvae was fixed in paraffin wax. Thin ribbons from blocks were prepared using the microtome after solidification of wax blocks. These thin ribbons having gut sections were placed on slides coated with very thin layer of Mayer’s egg albumin and kept on warm hot plate at 40-45ºC temperature for equal spreading of wax. Again tissue section placed on slide was passed through 30–90% grades of alcohol in ascending and descending order. Then permanent staining of slides was done using the methodology of Verma and Srivastava63. Permanent mounting of tissue on slide was done using the DPX and covered with coverslip. After staining and mounting, the slides were observed under the microscope (Evos XL Core) at magnification 400X for morphological changes in gut tissue.
Growth of bacteria in larval hemolymph
The second instar larvae were fed on LC50 values of S. marcescens and E. mundtii. After 96 h of bacterial treatment, 100 µl of hemolymph was collected from both infected as well as control larvae. The hemolymph was serially diluted and spread on LB agar plates with the help of spreader. Plates were incubated at 30 °C and observed after 48 h upto 72 h for the appearance of bacterial colonies.
Data analysis
To determine the differences among treated and control groups, the data on larval mortality, development period, adult emergence, adult deformities, reproductive potential and nutritional physiology were subjected to one way analysis of variance (ANOVA) followed by Tukey’s test at p ≥ 0.05. SPSS 20.0 software was used for statistical analysis.
Ethics declarations
This article does not contain any studies involving humans/animals/plants that need approval from ethical committee.
References
Engel, P. & Moran, N. A. The gut microbiota of insects-diversity in structure and function. FEMS Microbiol. Rev. 37(5), 699–735 (2013).
Ali, H. R., Hemeda, N. F. & Abdelaliem, Y. F. Symbiotic cellulolytic bacteria from the gut of the subterranean termite Psammotermes hypostoma Desneux and their role in cellulose digestion. AMB Express 9(1), 1–9 (2019).
Wang, J. M. et al. Diversity of the gut microbiome in three grasshopper species using 16S rRNA and determination of cellulose digestibility. PeerJ 8, e10194 (2020).
Baumann, P. Biology of bacteriocyte-associated endosymbionts of plant sap-sucking insects. Annu. Rev. Microbiol. 59, 155–189 (2005).
Douglas, A. E., Francois, C. L. M. J. & Minto, L. B. Facultative ‘secondary’bacterial symbionts and the nutrition of the pea aphid, Acyrthosiphon pisum. Physiol. Entomol. 31(3), 262–269 (2006).
Ceja-Navarro, J. A. et al. Gut microbiota mediate caffeine detoxification in the primary insect pest of coffee. Nat. Commun. 6(1), 1–9 (2015).
Sharon, G. et al. Commensal bacteria play a role in mating preference of Drosophila melanogaster. Proc. Natl. Acad. Sci. 107(46), 20051–20056 (2010).
Sharon, G., Segal, D., Zilber-Rosenberg, I. & Rosenberg, E. Symbiotic bacteria are responsible for diet-induced mating preference in Drosophila melanogaster, providing support for the hologenome concept of evolution. Gut Microbes 2(3), 190–192 (2011).
Mason, K. L. et al. From commensal to pathogen: translocation of Enterococcus faecalis from the midgut to the haemocoel of Manduca sexta. MBio 2(3), e00065-e111 (2011).
Haloi, K., Kalita, M. K., Nath, R. & Devi, D. Characterization and pathogenicity assessment of gut-associated microbes of muga silkworm Antheraea assamensis Helfer (Lepidoptera: Saturniidae). J. Invertebr. Pathol. 138, 73–85 (2016).
Ketola, T., Mikonranta, L., Laakso, J. & Mappes, J. Different food sources elicit fast changes to bacterial virulence. Biol. Lett. 12(1), 20150660 (2016).
Broderick, N. A. et al. Contributions of gut bacteria to Bacillus thuringiensis-induced mortality vary across a range of Lepidoptera. BMC Biol. 7(1), 1–9 (2009).
Ffrench-Constant, R. et al. Photorhabdus: Towards a functional genomic analysis of a symbiont and pathogen. FEMS Microbiol. Rev. 26(5), 433–456 (2003).
Thakur, A., Dhammi, P., Saini, H. S. & Kaur, S. Pathogenicity of bacteria isolated from gut of Spodoptera litura (Lepidoptera: Noctuidae) and fitness costs of insect associated with consumption of bacteria. J. Invertebr. Pathol. 127, 38–46 (2015).
Cakici, F. O., Sevim, A., Demirbag, Z. & Demir, I. Investigating internal bacteria of Spodoptera littoralis (Boisd.)(Lepidoptera: Noctuidae) larvae and some Bacillus strains as biocontrol agents. Turk. J. Agric. For. 38(1), 99–110 (2014).
Zhang, P., Zhao, Q., Ma, X. & Ma, L. Pathogenicity of Serratia marcescens to hazelnut weevil (Curculio dieckmanni). J. For. Res. 32(1), 409–417 (2021).
Ahmad, M., Ghaffar, A. & Rafiq, M. Host plants of leaf worm, Spodoptera litura (Fabricius) (Lepidoptera: Noctuidae) in Pakistan. Asian J. Agric. Biol. 1, 23–28 (2013).
Dudhbale, C., Surpam, A., Kothikar, R. & Koche, M. Bio-efficacy of chemical insecticides against Spodoptera litura infesting soybean. Am. J. Entomol. 1(1), 16–18 (2017).
Saleem, M., Hussain, D., Ghouse, G., Abbas, M. & Fisher, S. W. Monitoring of insecticide resistance in Spodoptera litura (Lepidoptera: Noctuidae) from four districts of Punjab, Pakistan to conventional and new chemistry insecticides. Crop Prot. 79, 177–184 (2016).
Ahmad, M., Sayyed, A. H., Saleem, M. A. & Ahmad, M. Evidence for field evolved resistance to newer insecticides in Spodoptera litura (Lepidoptera: Noctuidae) from Pakistan. Crop Prot. 27(10), 1367–1372 (2008).
Sang, S. et al. Cross-resistance and baseline susceptibility of Spodoptera litura (Fabricius) (Lepidoptera: Noctuidae) to cyantraniliprole in the south of China. Pest Manag. Sci. 72(5), 922–928 (2016).
Wang, X. et al. Insecticide resistance and enhanced cytochrome P450 monooxygenase activity in field populations of Spodoptera litura from Sichuan, China. Crop Prot. 106, 110–116 (2018).
Gandhi, K., Patil, R. H. & Srujana, Y. Field resistance of Spodoptera litura (Fab.) to conventional insecticides in India. Crop Prot. 88, 103–108 (2016).
Tong, H., Su, Q., Zhou, X. & Bai, L. Field resistance of Spodoptera litura (Lepidoptera: Noctuidae) to organophosphates, pyrethroids, carbamates and four newer chemistry insecticides in Hunan, China. J. Pest Sci. 86(3), 599–609 (2013).
Zago, H. B., Siqueira, H. A., Pereira, E. J., Picanço, M. C. & Barros, R. Resistance and behavioural response of Plutella xylostella (Lepidoptera: Plutellidae) populations to Bacillus thuringiensis formulations. Pest Manag. Sci. 70(3), 488–495 (2014).
Naik, V. C., Kumbhare, S., Kranthi, S., Satija, U. & Kranthi, K. R. Field-evolved resistance of pink bollworm, Pectinophora gossypiella (Saunders) (Lepidoptera: Gelechiidae), to transgenic Bacillus thuringiensis (Bt) cotton expressing crystal 1Ac (Cry1Ac) and Cry2Ab in India. Pest Manag. Sci. 74(11), 2544–2554 (2018).
Yang, F., Williams, J., Porter, P., Huang, F. & Kerns, D. L. F2 screen for resistance to Bacillus thuringiensis Vip3Aa51 protein in field populations of Spodoptera frugiperda (Lepidoptera: Noctuidae) from Texas, USA. Crop Prot. 126, 104915 (2019).
Yang, Y., Li, Y. & Wu, Y. Current status of insecticide resistance in Helicoverpa armigera after 15 years of Bt cotton planting in China. J. Econ. Entomol. 106(1), 375–381 (2013).
Eski, A., Demir, I., Güllü, M. & Demirbağ, Z. Biodiversity and pathogenicity of bacteria associated with the gut microbiota of beet armyworm, Spodoptera exigua Hübner (Lepidoptera: Noctuidae). Microb. Pathog. 121, 350–358 (2018).
Zhang, Y. et al. Isolation and identification of two Serratia marcescens strains from silkworm, Bombyx mori. Antonie Leeuwenhoek 113(9), 1313–1321 (2020).
Ishii, K., Adachi, T., Hara, T., Hamamoto, H. & Sekimizu, K. Identification of a Serratia marcescens virulence factor that promotes hemolymph bleeding in the silkworm, Bombyx mori. J. Invertebr. Pathol. 117, 61–67 (2014).
Cappellozza, S. et al. Identification of Enterococcus mundtii as a pathogenic agent involved in the “flacherie” disease in Bombyx mori L. larvae reared on artificial diet. J. Invertebr. Pathol. 106(3), 386–393 (2011).
De Mandal, S. et al. iTRAQ-based comparative proteomic analysis of larval midgut from the beet armyworm, Spodoptera exigua (Hübner)(Lepidoptera: Noctuidae) challenged with the entomopathogenic bacteria Serratia marcescens. Front. Physiol. 11, 442 (2020).
Tambong, J. T. et al. Molecular detection and analysis of a novel metalloprotease gene of entomopathogenic Serratia marcescens strains in infected Galleria mellonella. Can. J. Microbiol. 60(4), 203–209 (2014).
Ruiu, L. et al. Oral insecticidal activity of new bacterial isolates against insects in two orders. Biocontrol Sci. Technol. 27(7), 886–902 (2017).
Bidari, F., Shams-Bakhsh, M. & Mehrabadi, M. Isolation and characterization of a Serratia marcescens with insecticidal activity from Polyphylla olivieri (Col.: Scarabaeidae). J. Appl. Entomol. 142(1–2), 162–172 (2018).
Youngjin, P., Kim, K. & Kim, Y. A pathogenic bacterium, Enterococcus faecalis, to the beet armyworm, Spodoptera exigua. J. Asia Pac. Entomol. 5(2), 221–225 (2002).
Chibebe Junior, J. et al. Photodynamic and antibiotic therapy impair the pathogenesis of Enterococcus faecium in a whole animal insect model. PLoS ONE 8(2), e55926 (2013).
Park, S. Y., Kim, K. M., Lee, J. H., Seo, S. J. & Lee, I. H. Extracellular gelatinase of Enterococcus faecalis destroys a defense system in insect hemolymph and human serum. Infect. Immun. 75(4), 1861–1869 (2007).
Jurat-Fuentes, J. L., Jackson, T. A., Kaya, H. & Vera, F. Bacterial entomopathogens. Insect Pathol. 1, 265–349 (2012).
Tan, B., Jackson, T. A. & Hurst, M. R. Virulence of Serratia strains against Costelytra zealandica. Appl. Environ. Microbiol. 72(9), 6417–6418 (2006).
Aggarwal, C., Paul, S., Tripathi, V., Paul, B. & Khan, M. A. Characterization of putative virulence factors of Serratia marcescens strain SEN for pathogenesis in Spodoptera litura. J. Invertebr. Pathol. 143, 115–123 (2017).
Merzendorfer, H. & Zimoch, L. Chitin metabolism in insects: structure, function and regulation of chitin synthases and chitinases. J. Exp. Biol. 206(24), 4393–4412 (2003).
Chen, S., Blom, J. & Walker, E. D. Genomic, physiologic, and symbiotic characterization of Serratia marcescens strains isolated from the mosquito Anopheles stephensi. Front. Microbiol. 8, 1483 (2017).
Petersen, L. M. & Tisa, L. S. Molecular characterization of protease activity in Serratia sp. strain SCBI and its importance in cytotoxicity and virulence. J. Bacteriol. 196(22), 3923–3936 (2014).
Aggarwal, C., Paul, S., Tripathi, V., Paul, B. & Khan, M. A. Chitinolytic activity in Serratia marcescens (strain SEN) and potency against different larval instars of Spodoptera litura with effect of sublethal doses on insect development. Biol. Control. 60(5), 631–640 (2015).
Shimuta, K. et al. The hemolytic and cytolytic activities of Serratia marcescens phospholipase A (PhlA) depend on lysophospholipid production by PhlA. BMC Microbiol. 9(1), 1–10 (2009).
Mohan, M., Selvakumar, G., Sushil, S. N., Bhatt, J. C. & Gupta, H. S. Entomopathogenicity of endophytic Serratia marcescens strain SRM against larvae of Helicoverpa armigera (Noctuidae: Lepidoptera). World J. Microbiol. Biotechnol. 27(11), 2545–2551 (2011).
Sikorowski, P. P. & Lawrence, A. M. Transmission of Serratia marcescens (Enterobacteriaceae) in adult heliothis virescens (Lepidoptera: Noctuidae) laboratory colonies. Biol. Control. 12(1), 50–55 (1998).
González-Serrano, F. et al. The gut microbiota composition of the moth Brithys crini reflects insect metamorphosis. Microb. Ecol. 79(4), 960–970 (2020).
Lin, X. L., Kang, Z. W., Pan, Q. J. & Liu, T. X. Evaluation of five antibiotics on larval gut bacterial diversity of Plutella xylostella (Lepidoptera: Plutellidae). Insect Sci. 22(5), 619–628 (2015).
Chen, B. et al. Biodiversity and activity of the gut microbiota across the life history of the insect herbivore Spodoptera littoralis. Sci. Rep. 6(1), 1–14 (2016).
Sikorowski, P. P. & Lawrence, A. M. Microbial contamination and insect rearing. Am. Entomol. 40(4), 240–253 (1994).
Alverdy, J., Holbrook, C., Rocha, F., Seiden, L. & Licheng, R. Gut-derived sepsis occurs when the right pathogen with the right virulence genes meets the right host: Evidence for in vivo virulence expression in Pseudomonas aeruginosa. Ann. Surg. 232(4), 480 (2000).
Ryu, J. H. et al. Innate immune homeostasis by the homeobox gene caudal and commensal-gut mutualism in Drosophila. Sci. 319(5864), 777–782 (2008).
Robinson, C. J., Schloss, P., Ramos, Y., Raffa, K. & Handelsman, J. Robustness of the bacterial community in the cabbage white butterfly larval midgut. Microb. Ecol. 59(2), 199–211 (2010).
Vacheron, J. et al. T6SS contributes to gut microbiome invasion and killing of an herbivorous pest insect by plant-beneficial Pseudomonas protegens. ISME J. 13(5), 1318–1329 (2019).
Khaeso, K. et al. Assessing the effects of gut bacteria manipulation on the development of the oriental fruit fly, Bactrocera dorsalis (Diptera; Tephritidae). Symbiosis 74(2), 97–105 (2018).
Nathan, S. S., Chung, P. G. & Murugan, K. Effect of biopesticides applied separately or together on nutritional indices of the rice leaffolder Cnaphalocrocis medinalis. Phytoparasitica 33(2), 187 (2005).
Chandrasekaran, R. et al. Physiological effect of chitinase purified from Bacillus subtilis against the tobacco cutworm Spodoptera litura Fab. Pestic. Biochem. Physiol. 104(1), 65–71 (2012).
Datta, R., Kaur, A., Saraf, I., Singh, I. P. & Kaur, S. Effect of crude aextracts and purified compounds of Alpinia galanga on nutritional physiology of a polyphagous lepidopteran pest, Spodoptera litura (Fabricius). Ecotoxicol. Environ. Saf. 168, 324–329 (2019).
Farrar, R. R., Barbour, J. D. & Kennedy, G. G. Quantifying food consumption and growth in insects. Ann. Entomol. Soc. Am. 82(5), 593–598 (1989).
Verma, P. S. & Srivastava, P. C. Advanced Practical Zoology (S. Chand and company Ltd, 2012).
Acknowledgements
Financial assistance received from University Grants Commission (UGC) (UGC-Ref. No.: 484), Government of India, New Delhi, is duly acknowledged.
Author information
Authors and Affiliations
Contributions
S.K. and H.S.S. conceived and designed the experiments. S.D. performed the experiments, maintained the insect culture, analyzed the data and prepared the manuscript with the help of S.K. and H.S.S.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Devi, S., Saini, H.S. & Kaur, S. Assessing the pathogenicity of gut bacteria associated with tobacco caterpillar Spodoptera litura (Fab.). Sci Rep 12, 8257 (2022). https://doi.org/10.1038/s41598-022-12319-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-12319-w
This article is cited by
-
Effects of Gelsemium elegans extract on the red fire ant: disruption of peritrophic membrane integrity and alteration of gut microbial diversity, composition, and function
Journal of Pest Science (2024)
-
Safety Assessment of the Potential Probiotic Bacterium Limosilactobacillus fermentum J23 Using the Mexican Fruit Fly (Anastrepha ludens Loew, Diptera: Tephritidae) as a Novel In Vivo Model
Probiotics and Antimicrobial Proteins (2024)
-
Isolation and identification of bacteria from the invasive pest Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) and evaluation of their biocontrol potential
International Microbiology (2023)
-
Comparative Genomics of Pesticide-Degrading Enterococcus Symbionts of Spodoptera frugiperda (Lepidoptera: Noctuidae) Leads to the Identification of Two New Species and the Reappraisal of Insect-Associated Enterococcus Species
Microbial Ecology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.