Abstract
Data for Asian kidney transplants are very limited. We investigated the relative importance of prognostic markers in Asian kidney transplants by using Korean Organ Transplantation Registry (KOTRY) cohort. Prediction models were developed by data-driven variable selection approach. The relative importance of the selected predictors was measured by dominance analysis. A total of 4854 kidney transplant donor-recipient pairs were analyzed. Overall patient survival rates were 99.8%, 98.8%, and 91.8% at 1, 3, and 5 years, respectively. Death-censored graft survival rates were 98.4%, 97.0%, and 95.8% at 1, 3, and 5 years. Biopsy-proven acute rejection free survival rates were 90.1%, 87.4%, and 87.03% at 1, 3, and 5 years. The top 3 dominant predictors for recipient mortality within 1 year were recipient cardiovascular disease history, deceased donor, and recipient age. The dominant predictors for death-censored graft loss within 1 year were acute rejection, deceased donor, and desensitization. The dominant predictors to acute rejection within 1 year were donor age, HLA mismatched numbers, and desensitization. We presented clinical characteristics of patients enrolled in KOTRY during the last 5 years and investigated dominant predictors for early post-transplant outcomes, which would be useful for clinical decision-making based on quantitative measures.
Similar content being viewed by others
Introduction
The first kidney transplant in South Korea was conducted in 1969, and the procedure was popularized in the 1990s. The number of kidney transplants has been increasing since the 1980s. In past eras, most kidney transplant programs were based on living donations, and the deceased donor kidney transplant program operated in some centers1. After the introduction of brain death legislation and the establishment of regulatory agencies, deceased donor kidney transplant programs showed a decline in the early 2000s. However, with the effort to promote deceased organ donation and transparent allocation, deceased donor kidney transplants have rapidly increased, accounting for about 45% of total kidney transplants in recent years2,3,4. Accompanying this expansion of transplant volume, electronic claim databases became good resources for transplant research in South Korea, from which several good reports were produced overviewing Korean kidney transplants5,6. However, data based on claim reports or administrative databases generally lack many clinical details, and they should be supplemented by observational cohort or registry data.
In the transplant field, nationwide and international transplant registries have provided many valuable data resources and led to the development of a clinical science of transplantation7,8,9. The Korean Organ Transplantation Registry (KOTRY) has operated as an observational cohort of organ transplantation since 2012. In 2014, we reported the first nationwide retrospective data summary of 4,500 kidney transplant cases that had been performed from 2009 to 201210. Based on that project, a prospective observational cohort involving five different organ transplants (kidney, liver, heart, lung, and pancreas) started in 2014 under the same name (KOTRY)11. We provide a data summary of 5 years of enrollments of kidney transplant donor-recipient pairs in this paper. In addition, we investigated major dominant predictors of early kidney transplant outcomes including survival of patients and grafts, occurrence of acute rejection, and estimated glomerular filtration rate (eGFR) of transplanted grafts.
In terms of statistical modelling, most clinical epidemiological studies have used inferential methods based on the knowledge of experts and predefined hypotheses. On the contrary, the data-driven approach does not depend on prior hypotheses, which are usually used to build a prediction or prognostic model. Prognostic models in kidney transplants are an active area of research; however, the studies were scarce that compared the relative importance or the relative weight of clinical predictors for post-transplant outcomes12,13,14,15,16,17,18,19,20,21,22,23. In the present study, we compared the relative importance of clinical predictors based on a data-driven approach in addition to the 5 years outcome reporting of KOTRY.
Results
Descriptive baseline characteristics of Korean Organ Transplantation Registry (KOTRY)
In Table 1, baseline characteristics of kidney transplant recipients are described. Mean age of the recipients was 49.1 ± 11.5 years. In deceased donor kidney transplants, the mean age of recipients was higher (51.7 ± 10.6, p < 0.001). Females accounted for 40.6% of recipients. More male recipients received deceased donor kidneys. Mean body mass index (BMI) was 23.1 ± 3.6 kg/m2; mean systolic blood pressure before kidney transplant was 139.2 ± 20.8 mmHg. The proportion of current smokers was 8.6%. As comorbidities, diabetes were present in 29.8% and hypertension in 89.7% of recipients. The proportion of cardiovascular disease was 6.1%, which was higher in deceased donor kidney transplant recipients. History of malignancy was present in 6.6%. The most common cause of end-stage renal disease (ESRD) was chronic glomerulonephritis (33.3%), followed by diabetic nephropathy (23.5%). Hemodialysis was the most frequently used dialysis modality before transplant (70.9%). Preemptive transplantation was performed in 24.0% of living donor kidney transplants. Mean waiting time for deceased donor KT was 68.7 ± 38.0 months. Re-transplantation was done in 7.8% of cases. Mean number of HLA mismatches was 3.4 ± 1.8. Mean panel reactive antibody positivity percentage was 11.7 ± 24.3 in class I and 11.7 ± 24.8 in class II. As an induction agent, Basiliximab was used in 80.6% of kidney transplants, and ATG was used in 31.8% of deceased donor kidney transplants. Tacrolimus was the main calcineurin inhibitor (96.2%). Early steroid withdrawal was done in 2.0% of patients. Donor data was described as cases (Table 2). Mean age of donor cases was 47.2 ± 12.7 years. Females were more prevalent in living donors, and males were more prevalent in deceased donors. Diabetic donors accounted for 12.0% of deceased donors and 1.2% of living donors. Donors with hypertension accounted for 24.5% of deceased donor kidney transplants and 9.5% of living donor kidney transplants. Mean BMI of donors was 23.8 ± 3.4 kg/m2, and mean pre-transplant SBP of donors was 122.4 ± 17.2 mmHg. Proportion of smokers was 17.3% in living donors. Mean cold ischemic time was 289.0 ± 134.5 mins in deceased donors. Continuous renal replacement therapy was applied to 6.1% of deceased donors. Extracorporeal membrane oxygenator was applied to 2.5% of deceased donors.
Patient survival and cause of death
Overall patient survival rates were 99.8%, 98.8%, and 91.8% at 1, 3, and 5 years, respectively. Among living donor kidney transplant recipients, patient survival rates at 1, 3, and 5 years were 99.9%, 99.1%, and 96.6%, respectively. Among deceased donor kidney transplants, patient survival rates at 1, 3, and 5 years were 99.6%, 98.3%, 84.9%, respectively (Fig. 1a). The most common causes of death were infection (45.0%), followed by cardiovascular disease (10.0%), the latter occurring exclusively in deceased donor kidney transplants (Supplementary Table 1). Infection as the cause of death was defined regardless of microorganism.
Death-censored graft survival and cause of graft failure
Death-censored graft survival rates were 98.4%, 97.0%, and 95.8% at 1, 3, and 5 years, respectively. Among living donor kidney transplant recipients, death-censored graft survival rates were 99.0%, 97.6%, and 96.6% at 1, 3, and 5 years, respectively. Among deceased donor kidney transplants, death-censored graft survival rates were 97.5%, 96.0%, and 94.4% at 1, 3, and 5 years, respectively (Fig. 1b). Rejection (43.5%) was the most common cause of graft loss. Primary graft failure occurred in 11.1% of graft failures. BK virus nephropathy was the third most common cause of graft loss (5.6%) (Supplementary Table 2).
Indication of kidney biopsy and pathology outcomes
A total of 3,712 kidney biopsies were performed. Among them, 58.6% were protocol biopsies. The most common indication of for-cause biopsy was increased creatinine (37.2%) (Supplementary Table 3). Among for-cause biopsies, acute T-cell mediated rejection accounted for 25.6%, acute antibody mediated rejection for 13.9%, and borderline change for 21.1%. Recurrent glomerulonephritis occurred in 9.7% of cases and BK-virus-associated nephropathy in 7.9%. If protocol biopsies are included, the proportion of biopsy findings declined; however, the proportion of borderline change was similar (Table 3). Acute rejection free survival rates were 81.5%, 76.4%, and 75.1% at 1, 3, and 5 years, respectively. Among living donor kidney transplant recipients, acute rejection free survival rates were 81.8%, 76.7%, and 75.6% at 1, 3, and 5 years, respectively. Among deceased donor kidney transplants, acute rejection free survival rates were 80.9%, 75.9%, and 74.2% at 1, 3, and 5 years, respectively (Fig. 2a). Biopsy-proven acute rejection free survival rates were 90.1%, 87.4%, and 87.0% at 1, 3, and 5 years, respectively. Among living donor kidney transplant recipients, biopsy-proven acute rejection free survival rates were 90.1%, 87.4%, and 87.0% at 1, 3, and 5 years, respectively. Among deceased donor kidney transplant recipients, biopsy-proven acute rejection free survival rates were 90.0%, 87.3%, and 86.8% at 1, 3, and 5 years, respectively (Fig. 2b).
Dominant predictors for patient survival
To explore predictors for patient survival, we applied cross-validated LASSO, which resulted in all entered variables being selected at the optimum lambda. We interpret this due to sufficient n to predictors (not p > n condition), where LASSO might not show its strength in variable selection (Supplementary Table 4). Traditional backward stepwise selection showed reduced predictors from 19 to 11 variables at the threshold of p-value under 0.20 (Supplementary Table 5 and Table 4). To compare the relative importance of predictors, we chose 1-year patient survival as outcome and applied the dominance analysis method to the chosen predictors in previous backward stepwise selection. Cardiovascular disease history of recipient was the most dominant predictor for 1-year patient death, followed by deceased donor kidney transplant, recipient age, duration of dialysis, and diabetes of recipient (Table 4). Among cardiovascular disease history of recipients, all subcategories of cardiovascular disease except for arrhythmia history were significant predictors to post-transplant patient 1 year survival (Supplementary Table 6).
Dominant predictors for graft survival, post-transplant eGFR, and acute rejection
Dominant predictors for death-censored 1-year graft survival were determined at the base of backward stepwise selection. (Supplementary Table 7) To the dominance analysis for post-transplant graft outcome, acute rejection and BK-virus-associated nephropathy (BKVAN) were included in the predictors list input to the backward stepwise selection. Acute rejection within 1 year followed by deceased donor, desensitization, and BK virus-associated nephropathy (BKVAN) were the dominant predictors to 1-year graft survival (Table 5). Dominant predictors for post-transplant 1-year graft eGFR were donor age, followed by acute rejection within 1 year, BKVAN within 1 year, recipient BMI and HLA mismatch numbers (Table 6). To derive important predictors to acute rejection, cross-validated LASSO was applied again, however, still LASSO resulted in all entered variables being selected at the optimum lambda. (Supplementary Table 8) Dominant factors for acute rejection within 1 year were determined at the base of backward stepwise selection. (Supplementary Table 9) Donor age, followed by HLA mismatch, desensitization, recipient sex, and recipient age were the high-ranked dominant predictors to acute rejection within 1 year (Table 7). Coefficient path plot of LASSO variable selection to the aforementioned outcomes are depicted in Supplementary contents (Supplementary Fig. 1). Because elderly donor showed reduced odds ratio, we checked the nonlinearity of donor age in acute rejection, and found that among deceased donor kidney transplantation recipients, young deceased donor under 20 years old showed significant non-linear elevated rejection risk. (Supplementary Fig. 2) To avoid this local non-linearity, we restricted the donor age above 19 years old, and found the same top priority of donor age to the acute rejection within 1 year in dominance analysis. In this subgroup analysis, donor age showed significant elevated odds ratio (Odds ratio 1.019 (95%. C.I 1.012–1.026, p < 0.001, Supplementary Table 10). (Fig. 3) In addition, we estimated the best cutoff points of donor age for the classification of acute rejection within 1 year by Liu’s methods. Donor age of 48 years old was the best cutoff for the classification of acute rejection. Fig. 4 is a Kaplan-Meier curve of post-transplant acute rejection free survival, which showed lower rejection-free survival of kidney transplant patients who received elderly donors more than 48 years old. However, increment of log odds of post-transplant 1 year rejection started from the late 20s of donor age and become steeper after 60 years old in the non-linear logistic regression analysis (Fig. 5).
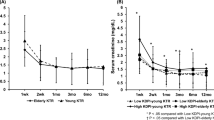
Hazard ratios of donor age to post-transplantation acute rejection within 1 year. (a) Three dimensional visualization of hazard ratio of donor age according to HLA mismatch numbers in overall study population (b) Two dimensional contour map of the hazard ratio of donor age according to HLA mismatch numbers in overall study population (c) Stratified hazard ratio of donor age in living donor kidney transplantation subpopulation (d) Stratified hazard ratio of donor age in deceased donor kidney transplantation subpopulation. Red line indicates logarithm of hazard ratio of donor age in HLA full match group. Each colored area indicates its 95% confidence interval. Green line indicates logarithm of hazard ratio of donor age in moderate HLA mismatch group (as representative, hazard ratio line of HLA mismatch number 3 is used). Blue line indicates logarithm of hazard ratio of donor age in high HLA mismatch group (hazard ratio line of HLA mismatch number 6 is used). All graphs are the results of multivariable regression analyses which included donor age, HLA mismatch numbers, desensitization, recipient sex, recipient age, donor hypertension, recipient blood pressure, deceased donor, duration of renal replacement therapy, ever smoking in recipients.
Visualization of log odds of acute rejection within 1 year according to different donor age (a) Visualization of log odds of acute rejection within 1 year according to different donor age in overall study population (b) Stratified log odds of acute rejection within 1 year according to different donor age in living donor kidney transplantation subpopulation (c) Stratified log odds of acute rejection within 1 year according to different donor age in deceased donor kidney transplantation subpopulation. Blue line indicates adjusted logarithm of odds of acute rejection within 1 year according to different donor age in each group. Each grey area indicates its 95% confidence interval. All graphs are the results of multivariable regression analyses which included donor age, HLA mismatch numbers, desensitization, recipient sex, recipient age, donor hypertension, recipient blood pressure, deceased donor, duration of renal replacement therapy, ever smoking in recipients.
Discussion
In the present study, we reported baseline characteristics and early outcomes based on the Korean Organ Transplantation Registry (KOTRY). In addition, we explored baseline predictors to early outcomes and reported dominant factors influencing patient and graft outcomes. Dominant factors for 1-year patient survival were found as predictors associated with recipient’s age or recipient’s comorbidities. Although infection was the most common cause of death, top dominant factor for 1-year patient survival was cardiovascular disease history. Aging might be intermediate process between infection as leading cause of death and cardiovascular history as leading predictor for patient 1 year mortality. For 1-year graft survival, dominant factors were predictors associated with immunologic risks and donor and recipient’s comorbidities. It was interesting to see that donor age was found to be the most dominant factor influencing graft function at 1 year, followed by post-transplant acute rejection and BKVAN.
When the KOTRY was launched, annual transplant numbers were 1,400. At the design stage of KOTRY, an annual enrollment of 1,200 cases was aimed at to cover more than 80% of total kidney transplants in South Korea. However, the recent rapid increase in kidney transplants has resulted in KOTRY covering about 50-60% of total kidney transplants in South Korea. Still, KOTRY projects compose the largest multi-center cohorts in this country. In KOTRY, clinical details that claim data cannot capture are important resources to future research. Another strength of KOTRY is its role as a biobank. Prospective sample collection will provide invaluable research resources.
The most common cause of ESRD in South Korea is diabetic nephropathy24, which is reflected in the high proportion of diabetes in KOTRY. A large proportion of glomerulonephritis as a cause of ESRD could represent the results of patient selection or accessibility to kidney transplantation. Another important feature of Korean kidney transplants is the high proportion of living donor kidney transplantation. We identified that among living donor kidney transplants, 24% were preemptive kidney transplants. Compared to the high proportion of preemptive transplants with living donor kidneys, a long waiting period among deceased donor kidney transplants is another feature of Korean kidney transplants. Aside from almost unanimous standard triple maintenance immunosuppressants, ATG induction was observed as a variation. The proportion of steroid withdrawal was 2% in this data. The most common cause of death was infection, followed by cardiovascular disease. Those causes of death are compatible with the predictors selected in the data-driven approach because recipient age and history of cardiovascular disease were selected as dominant predictors for 1-year mortality. The most common pre-transplant cardiovascular disease was ischemic heart disease in this study population. However, not only ischemic heart disease was significant predictors to post-transplant 1 year mortality, but other subcategories of cardiovascular disease were also significant predictors, which implies that holistic heart function itself is important for the early post-transplant mortality not limited to the presence or absence of coronary arterial occlusive disease.
Recent investigations of donor safety have concerned higher lifetime ESRD risk in young donors25. In terms of graft survival on the recipient side, it is interesting to see that selected predictors were donor characteristics such as donor age, donor hypertension, and donor diabetes. However, extension of this finding to long-term risk predictors needs cautious interpretation because non-modifiable donor factors could be exaggerated in early transplant outcomes. In terms of donor safety, marginal kidney function would also affect donors’ long-term outcomes; therefore, this data is evidence of the importance of proper donor selection.
It was interesting to see that donor age was the most dominant factor influencing acute rejection. Several publications pointed out the significance of donor age as a risk factor for acute rejection26,27; however, to the best of our knowledge, this is the first study to find that donor age is the most dominant factor influencing acute rejection in a quantitative comparison. Donor age has near-linear pattern of log odds increment, which might explain its dominancy in the regression-based dominance model. The best cutoff value which we can classify post-transplant acute rejection within 1 year with was 48 years old. However, it might be different from what we think as safety line of donor age, because the increment of log odds value is observed even in the late 20 s of donor age. In addition, the most profound increment of log odds of post-transplant rejection within 1 year from donor age was observed in the after 60 years old of donor age, especially among deceased donors. HLA incompatibility was the second most dominant predictor for acute rejection. This finding could be epidemiological evidence supporting the importance of passenger leukocyte and its memory, or the vulnerability of aged endothelial cells to ischemia reperfusion injury and damage-associated molecular pattern expression28,29. Desensitization was selected as an important predictor for acute rejection, which implies that although mitigation of immunological risk was performed by desensitization, residual risk still persisted. We anticipate that the details regarding desensitization will be investigated in future studies.
Dominant predictors to 1-year post-transplant recipient’s eGFR were donor age, acute rejection within 1 year, BKVAN within 1 year, and recipient BMI. We might interpret this as a mixture of kidney function and risk of rejection because donor age and body mass index can affect eGFR directly via muscle mass and intermediate outcomes including acute rejection, or that BKVAN directly represents the damaging process to the transplanted kidney. The importance of donor kidney-recipient weight gap was a well-known factor to post-transplant eGFR30,31. In this study, its importance to predicting post-transplant eGFR was high.
The limitations of the study are as follows. First, this project enrolled about 50% of total kidney transplant patients in South Korea. Informed consent was required; therefore, information bias might exist. For example, recipients with poor compliance could refuse study enrollment, and urgent transplants performed during weekends or late at night might not have been enrolled in this project. Second, dominance of predictors was based on variable selection in traditional stepwise regression, which is not completely independent as to the randomness of entering variables. We tried to overcome this limitation by applying regularized regression methods (LASSO), which were unsuccessful due to having a large number of cases compared to selected predictors. However, we think this quantitative comparison of the relative importance of variables is a significant contribution to the transplant field.
In conclusion, we presented clinical characteristics of patients enrolled in KOTRY during the past 5 years and investigated dominant predictors for early post-transplant outcomes by comparing relative contributions to the outcome prediction. The dominant predictors to recipient mortality within 1 year were deceased donor, recipient age, and recipient history of cardiovascular disease. The dominant predictors to death-censored graft loss within 1 year were deceased donor, desensitization, and donor hypertension. The dominant predictors of post-transplant 1-year recipient’s eGFR were donor age, acute rejection within 1 year, and BKVAN within 1 year. Finally, the dominant predictors to acute rejection within 1 year were donor age, HLA mismatches, and desensitization.
Methods
Study population
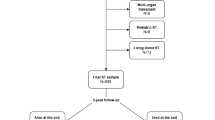
The Korean Organ Transplantation Registry (KOTRY) is a nationwide solid organ transplant cohort launched in 2014. The design and methods of KOTRY were described in detail in a previous report11. In brief, data on pretransplant evaluations, immunologic risks, induction and maintenance immunosuppressants, every kidney biopsy result, every treatment of acute rejection, graft function measured as eGFR, post-transplant cardiovascular events, post-transplant infection events, and the survival of patients and grafts were collected. As large-volume centers are participating in KOTRY, the numbers of organ transplantations performed in KOTRY-participating centers were 83% for kidney transplantation. However, because this nationwide cohort is based on patient’s informed consent and prospective follow up, KOTRY enrolls about 1,200 new kidney transplant cases per year, which reaches about 55% of annual total kidney transplantation in South Korea. Although some selection bias might exist for the patient enrollment, KOTRY data was shown to be compatible with the nationwide post-transplant hard outcome based on administrative claims32. For the details of post-transplantation outcome, KOTRY is the only source of Korean transplant recipient’s data in national scale. For this study, the dataset of patients who received kidney transplants from 2014 to 2018 was used. A total of 4,854 kidney transplant recipients were analyzed. Mean duration of follow-up was 36.4 ± 15.5 months.
Study objective, design, covariables, and statistical approach
We tried to derive a best prediction model for post-transplant outcomes (patient survival, graft survival, post-transplant recipient’s eGFR, and acute rejection) from baseline (pre-transplant) covariables. For the variable selection, we applied various approaches depending on the availability of existing methods and the character of the variables. For continuous measures, the Furnival-Wilson leaps-and-bound algorithm determined by Akaike’s information criteria was used for variable selection33,34. For the time to event outcomes and binary outcomes, least absolute shrinkage and selection operator (LASSO) or backward stepwise selection were used. LASSO is a statistical methodology for variable selection and penalization. During the coefficient shrinkage of LASSO methodology, some coefficient goes to exactly zero value, which resulted in variable selection. For the LASSO, chosen optimum lambda values were one standard error apart from the lambda value of minimal partial likelihood deviance at the iterative cross-validation35. A total of 19 covariate candidates for prediction model construction were as follows: recipient age, donor age, recipient sex, donor sex, recipient’s history of diabetes, recipient’s history of cardiovascular disease, recipient’s history of cancer, pre-transplant systolic blood pressure of recipient, pre-transplant body mass index of recipient, donor’s diabetes history, donor’s hypertension history, waiting time to kidney transplant, pre-transplant body mass index of donor, deceased donor, total number of human leukocyte antigen (HLA) mismatches, desensitization, anti-thymocyte globulin (ATG) as an induction agent, and smoking status of donor and recipient. When all covariables were entered into the prediction model for the death-censored graft loss, c-statistic was 0.671, which was comparable to previous prediction studies18.
After a model was built, we applied dominance analysis to rank the relative importance of each selected variable to target outcome36,37. Because dominance analysis could be applied to the generalized linear model, we applied dominance analysis for continuous outcomes (post-transplant eGFR) in the form of linear regression, or for binary outcomes (patient survival at 1 year, graft survival status at 1 year, and acute rejection within 1 year) in the form of logistic regression. Because panel reactive antibody were tested in 62.1% of recipients, we did not include panel reactive antibody from the previous tests. We separately conducted the same process as a sensitivity analysis including panel reactive antibody as one of the predictors, which made total dataset reduced to 3,019 donor-recipients pairs. Panel reactive antibody was dropped during the backward variable selection and did not remain in the dominance analysis to rank the relative importance. Continuous data are presented as mean with standard deviation. Categorical data are presented as count with percent. Cox regression for time to event data was performed under the proportional hazard assumption. Splines were applied to formalize non-linearity in the statistical models. For the optimal cutoff value estimation, Liu’s method was used38. Statistical analyses were performed using Stata software (version 16; StataCorp LP, College Station, TX) and R (version 3.6.3; R Foundation, Vienna, Austria).
Ethics approval and consent to participate
The study protocol was approved by the Seoul National University Hospital institutional review board (IRB No: H-1902-138-1014). Data analysis was done with de-identified datasets. Patient privacy was preserved in all instances, and the study methods complied with the tenets of the Declaration of Helsinki. All participants provided their written informed consent.
Data availability
The data that support the findings of this study are available from Korean Organ Transplantation Registry but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of Korean Organ Transplantation Registry.
References
Go, J. et al. A half-century 3000 cases of kidney transplant experiences in a single hospital: The longest registry in Korea. Transplant Proc. 51, 2559–2567 (2019).
Min, S. I. et al. Trends in deceased organ donation and utilization in Korea: 2000–2009. J Korean Med Sci 25, 1122–1127 (2010).
Kim, M.-G. et al. Operational and regulatory system requirements for pursuing self-sufficiency in deceased donor organ transplantation program in Korea. Korean J. Transpl. 24, 147–158 (2010).
Min, S. I. et al. To achieve national self-sufficiency: recent progresses in deceased donation in Korea. Transplantation 99, 765–770 (2015).
Oh, H. W. et al. Effect of institutional kidney transplantation case-volume on post-transplant graft failure: A retrospective cohort study. J. Korean Med. Sci. 34, e260 (2019).
Hong, S. K. et al. Long-term survival of 10,116 Korean live liver donors. Ann. Surg. 274, 375–382 (2021).
Opelz, G. et al. The collaborative transplant study registry. Transplant. Rev. (Orlando) 27, 43–45 (2013).
McDonald, S. P. & Russ, G. R. Australian registries-ANZDATA and ANZOD. Transplant. Rev. (Orlando) 27, 46–49 (2013).
Leppke, S. et al. Scientific Registry of Transplant Recipients: collecting, analyzing, and reporting data on transplantation in the United States. Transplant. Rev. (Orlando) 27, 50–56 (2013).
Ahn, C. et al. Initial report of the Korean Organ Transplant Registry: the first report of national kidney transplantation data. Transplant. Proc. 46, 425–430 (2014).
Yang, J. et al. Design and methods of the Korean Organ Transplantation Registry. Transplant. Direct 3, e191 (2017).
Foucher, Y. et al. A clinical scoring system highly predictive of long-term kidney graft survival. Kidney Int. 78, 1288–1294 (2010).
Clayton, P. A., McDonald, S. P., Snyder, J. J., Salkowski, N. & Chadban, S. J. External validation of the estimated posttransplant survival score for allocation of deceased donor kidneys in the United States. Am. J. Transplant. 14, 1922–1926 (2014).
Chapal, M. et al. A useful scoring system for the prediction and management of delayed graft function following kidney transplantation from cadaveric donors. Kidney Int. 86, 1130–1139 (2014).
Elbadri, A. et al. Factors affecting eGFR 5-year post-deceased donor renal transplant: analysis and predictive model. Ren. Fail. 37, 417–423 (2015).
Gonzales, M. M., Bentall, A., Kremers, W. K., Stegall, M. D. & Borrows, R. Predicting individual renal allograft outcomes using risk models with 1-year surveillance biopsy and alloantibody data. J. Am. Soc. Nephrol. 27, 3165–3174 (2016).
Patri, P. et al. Development and validation of a prognostic index for allograft outcome in kidney recipients with transplant glomerulopathy. Kidney Int. 89, 450–458 (2016).
Molnar, M. Z. et al. Predictive score for posttransplantation outcomes. Transplantation 101, 1353–1364 (2017).
Viglietti, D. et al. Dynamic prognostic score to predict kidney allograft survival in patients with antibody-mediated rejection. J. Am. Soc. Nephrol 29, 606–619 (2018).
Aubert, O. et al. Archetype analysis identifies distinct profiles in renal transplant recipients with transplant glomerulopathy associated with allograft survival. J. Am. Soc. Nephrol. 30, 625–639 (2019).
Loupy, A. et al. Prediction system for risk of allograft loss in patients receiving kidney transplants: International derivation and validation study. BMJ 366, l4923 (2019).
Mark, E., Goldsman, D., Gurbaxani, B., Keskinocak, P. & Sokol, J. Using machine learning and an ensemble of methods to predict kidney transplant survival. PLoS ONE 14, e0209068 (2019).
Udomkarnjananun, S. et al. The first Asian kidney transplantation prediction models for long-term patient and allograft survival. Transplantation 104, 1048–1057 (2020).
Jin, D. C. et al. Current characteristics of dialysis therapy in Korea: 2016 registry data focusing on diabetic patients. Kidney Res. Clin. Pract. 37, 20–29 (2018).
Muzaale, A. D. et al. Risk of end-stage renal disease following live kidney donation. JAMA 311, 579–586 (2014).
Tullius, S. G. & Milford, E. Kidney allocation and the aging immune response. N. Engl. J. Med. 364, 1369–1370 (2011).
Colvin, M. M., Smith, C. A., Tullius, S. G. & Goldstein, D. R. Aging and the immune response to organ transplantation. J. Clin. Invest. 127, 2523–2529 (2017).
Pratschke, J. et al. Donor hypertension increases graft immunogenicity and intensifies chronic changes in long-surviving renal allografts. Transplantation 77, 43–48 (2004).
Reutzel-Selke, A. et al. Donor age intensifies the early immune response after transplantation. Kidney Int. 71, 629–636 (2007).
Giral, M. et al. Kidney and recipient weight incompatibility reduces long-term graft survival. J. Am. Soc. Nephrol. 21, 1022–1029 (2010).
Hwang, J. K. et al. Does donor kidney to recipient body weight ratio influence long-term outcomes of living-donor kidney transplantation?. Transplant Proc. 44, 276–280 (2012).
Hong, S. K. et al. Outcomes of pediatric liver transplantation in Korea using two national registries. J. Clin. Med. 9, 3435–3448 (2020).
Furnival, G. M. & Wilson, R. W. Regressions by leaps and bounds. Technometrics 16, 499–511 (1974).
Lindsey, C. & Sheather, S. Best subsets variable selection in nonnormal regression models. Stand. Genomic Sci. 15, 1046–1059 (2015).
Tibshirani, R. The lasso method for variable selection in the Cox model. Stat. Med. 16, 385–395 (1997).
Wheeler, D. C. et al. Effects of cinacalcet on atherosclerotic and nonatherosclerotic cardiovascular events in patients receiving hemodialysis: The EValuation Of Cinacalcet HCl Therapy to Lower CardioVascular Events (EVOLVE) trial. J. Am. Heart Assoc. 3, e001363 (2014).
Luchman, J. N. Relative importance analysis with multicategory dependent variables: An extension and review of best practices. Organ. Res. Methods 17, 452–471 (2014).
Liu, X. Classification accuracy and cut point selection. Stat Med 31, 2676–2686 (2012).
Funding
This research was supported by a fund (2014-ER6301-00, 2014-ER6301-01, 2014-ER6301-02, 2017-ER6301-00, and 2017-ER6301-01) by Research of Korea Centers for Disease Control and Prevention and by a fund of Norvatis.
Author information
Authors and Affiliations
Consortia
Contributions
J.C.J., T.Y.K., H.R., C.A., J.Y. conceptualized the study design. J.C.J, T.Y.K., H.R., J.K., D.W.C., J.Y. analyzed statistical data. J.C.J., T.Y.K. wrote the main manuscript text, prepared all figures. All authors participated for the data collection and curation, and all authors reviewed the manuscript. Both J.C.J. and T.Y.K. contributed equally to this manuscript as co-first authors. Both C.A., and J.Y. contributed equally to this manuscript as co-corresponding authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jeong, J.C., Koo, T.Y., Ro, H. et al. Dominant predictors of early post-transplant outcomes based on the Korean Organ Transplantation Registry (KOTRY). Sci Rep 12, 8706 (2022). https://doi.org/10.1038/s41598-022-12302-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-12302-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.