Abstract
Although the prevalence of cognitive impairment and depression is higher in patients with atrial fibrillation (AF) than in the general population, the mechanism has not been fully examined and impact of catheter ablation (CA) of AF also remains unclear. Recently, the development of near-infrared spectroscopy (NIRS) has enabled noninvasive measurements of regional cerebral blood volume and brain activity, in terms of cerebral oxyhemoglobin in the cerebral cortex. We assessed brain activities by NIRS, depressive symptoms by the Center for Epidemiologic Studies Depression Scale (CES-D) and cognitive function by Mini-Mental State Examination (MMSE). We then compared the results between AF patients (paroxysmal AF n = 18 and persistent AF n = 14) and control subjects (n = 29). Next, we also followed up persistent AF patients who kept sinus rhythm at 3 months after CA (n = 8) and measured their brain activities using NIRS, CES-D and MMSE after CA to investigate the associations of changes in brain activities with changes in both CES-D and MMSE. Our results showed that (1) frontal and temporal brain activities were lower in patients with persistent AF than both in control subjects and paroxysmal AF patients (P < 0.01), (2) frontal and temporal brain activities were improved in more than half of the persistent AF patients who kept sinus rhythm at 3 months after CA, especially in those who presented impaired brain activity before CA, and (3) improvement of frontal brain activity was associated with improvement of CES-D (R = − 0.793, P = 0.019), whereas improvement of temporal brain activity was associated with improvement of MMSE (R = 0.749, P = 0.033). NIRS measurement showed reduced frontal and temporal brain activities in the persistent AF patients, CA improved frontal and temporal brain activities in some of these patients, and associated with improvement of depressive state and/or improvement of cognitive function.
Similar content being viewed by others
Introduction
The global burden of atrial fibrillation (AF) is currently on the rise, owing to aging of the society1,2. Dementia, mainly manifested as cognitive impairment, is being more common in the elderly population in a similar fashion to AF. Recently, many studies have shown that AF may be a risk factor for cognitive impairment independent of a stroke, suggesting that AF itself has additional adverse effects on the cognitive function3,4. In addition, it has recently been reported that catheter ablation (CA) of AF improves the cognitive function assessed by a brief cognitive screening tool (i.e. The Montreal Cognitive Assessment)5. On the other hand, Medi et al.6 reported that post-procedural neurocognitive dysfunction occurred in 13% to 20% of 90 AF patients after CA. In addition, AF patients have higher rates of depression than healthy subjects7. Depression to be more severe in patients with persistent AF than in those with paroxysmal AF8. Persistent AF patients were more likely to report more severe depressive state than paroxysmal AF patients8. Previous studies have found associations between depression and both increased symptom burden and increased mortality in AF patients, and between depressive symptoms and increased risk of AF recurrence after cardioversion9 and after CA10. Depression may be associated with dysregulation in the autonomic nervous system, particularly excessive sympathetic activity. Emotional stress has previously been reported to be associated with increased risk of arrhythmias11.
Although AF may increase the risk of cognitive impairment or depressive state through silent brain infarction12, brain hypoperfusion due to a reduced cardiac output13 or inflammation and platelet dysfunction14, the mechanism of cognitive impairment or depressive state caused by AF has not been fully examined, and direct imaging has not been performed. In addition, (1) cognitive improvement by CA is controversial, (2) changes in depressive state by CA have not been reported, and (3) direct imaging has not been performed to determine changes in brain perfusion.
Recently, the development of near-infrared spectroscopy (NIRS) has enabled noninvasive and bedside measurements of regional cerebral blood volume in terms of relative concentrations of oxyhemoglobin (oxy-Hb) and deoxyhemoglobin (deoxy-Hb), with a high time resolution. The concentrations of oxy-Hb and deoxy-Hb are assumed to reflect the regional cerebral blood volume15,16,17. In addition, oxy-Hb increases and deoxy-Hb decreases detected by NIRS have been shown to reflect cortical activation by simultaneous measurements with other methodologies, which demonstrate cerebral perfusion and is used as functional brain monitoring15. A positive correlation has been observed between oxy-Hb concentration measured by NIRS and blood-oxygen-level-dependent signal measured by functional magnetic resonance imaging (MRI)18,19. Furthermore, NIRS has recently been used to investigate the neurocognitive processes associated with neurological (e.g. Alzheimer’s disease, traumatic brain injury) and psychiatric disorders (e.g. depression, anxiety disorders)20. Compared to positron emission tomography, single photon emission computed tomography, and functional MRI, NIRS has the advantages of requiring minimal equipment and being easy to use.
In the present study, we aimed to (1) evaluate and compare frontal and temporal brain activities using NIRS in AF patients and control subjects, (2) evaluate changes in brain activities before and after CA in patients with persistent AF who kept sinus rhythm (SR) at 3 months after CA, and (3) determine the associations of changes in brain activity with changes in cognitive function and depressive symptoms.
Results
The comparisons of clinical features and psychological testing between the control subjects and AF patients are shown in Table 1. We found no significant difference in age, sex, prevalence of co-morbidity, left ventricular ejection fraction, B-type natriuretic peptide, hemoglobin, estimated glomerular filtration rate, percutaneous oxygen saturation, the Center for Epidemiologic Studies Depression Scale (CES-D) was used to evaluate depressive symptoms21, or the Mini-Mental State Examination (MMSE) was used to evaluate cognitive function22 between the two groups.
Figure 1 compares grand average of waveform changes in oxyhemoglobin concentrations during verbal fluency task (VFT) in the atrial fibrillation patients (red, n = 32) and control subjects (blue, n = 29). The horizontal axis represents time, and the vertical axis represents changes in mean oxy-Hb concentrations (mMmm) during VFT. Figure 2 shows a topographic map of the differences in mean oxy-Hb concentrations during VFT between the AF patients and control subjects. The mean oxy-Hb concentrations changes during VFT in the right temporal lobe (channels 24 and 45), left temporal lobe (channels 29 and 41) and frontal region (channels 45–47 and 49) were significantly lower in the AF patients than in the control subjects (P < 0.05).
Comparison of grand average of waveform changes in oxyhemoglobin concentrations during verbal fluency task in the atrial fibrillation patients (red, n = 32) and control subjects (blue, n = 29). The horizontal axis represents time, and the vertical axis represents changes in mean oxyhemoglobin concentrations (mMmm) during the verbal fluency task.
Topographic map of the differences in mean oxy-Hb concentration changes between the atrial fibrillation (AF) patients (n = 32) and control subjects (n = 29). The mean oxy-Hb concentrations in the right temporal lobe (channels 24 and 45), left temporal lobe (channels 29 and 41) and frontal region (channels 45–47 and 49) were significantly lower in the AF patients than in the control subjects (red circle, P < 0.05).
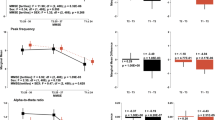
Next, we compared frontal and temporal brain activities (integral values of mean oxy-Hb concentrations in the frontal and temporal lobes) between the groups, and the results are presented in Fig. 3. Although frontal brain activity was significantly lower in the AF patients than in the control subjects (Fig. 3a), temporal brain activity did not differ between the groups (Fig. 3b). In the multiple regression analysis to determine brain activity confounding factors (Table 2), AF was independently associated with frontal brain activity (β = − 0.356, p = 0.004), but not with temporal brain activity. On the other hand, both frontal and temporal brain activities were lower in the persistent AF patients than in the control subjects and paroxysmal AF patients (Fig. 3c,d). Thus, frontal and temporal brain activities were impaired in the persistent AF patients, rather in the paroxysmal AF, which might have led to a depressive state and cognitive impairment in these patients.
Comparisons of frontal brain activity (integral values of mean oxy-Hb concentrations in the frontal region) and temporal brain activity (integral values of mean oxy-Hb concentrations in the temporal lobes) between the atrial fibrillation (AF) patients and control subjects. Although frontal brain activity was significantly lower in the AF patients than in the control subjects (a), temporal brain activity did not differ between the groups (b). However, both frontal and temporal brain activities were lower in the persistent AF patients than in the control subjects and paroxysmal AF patients (c,d). Data is presented as median and interquartile range (error bar).
Of 14 persistent AF patients, 11 patients underwent CA, among whom 8 patients (72.7%) kept SR at 3 months after CA. These 8 patients underwent follow-up examinations of NIRS, CES-D and MMSE 3 months after CA. As shown in Fig. 4, of these 8 patients, 5 patients (solid line, 62.5%) presented improved frontal brain activity, and 6 patients (solid line, 75.0%) presented improved temporal brain activity after CA. Almost of these patients, who presented brain activity as of less than 0 mMmm before CA, seemed to have impaired brain activity before CA and improve brain activity after CA. In addition (Fig. 5), there were significant correlations of changes in frontal brain activity with changes in CES-D (R = − 0.793, P = 0.019), but not changes in with MMSE, and there were significant correlations of changes in temporal brain activity with changes in MMSE (R = 0.749, P = 0.033), but not with changes in CES-D. Thus, (1) frontal and temporal brain activities were improved in some of the persistent AF patients who underwent CA and kept SR, and (2) improvement of frontal brain activity was associated with improvement of depressive state, and improvement of temporal brain activity was associated with improvement of cognitive function.
A solid line indicates a patient who showed improvement in brain activity, and a dotted line indicates a patient who showed no improvement. Changes in frontal and temporal brain activities before and after catheter ablation (CA) in the 8 patients with persistent atrial fibrillation (AF) who kept sinus rhythm after CA. Of these 8 patients, 5 (62.5%) presented improved frontal brain activity, and 6 (75.0%) presented improved temporal brain activity after CA.
Correlations between changes in brain activities and changes in CES-D and MMSE (n = 8). There were significant correlations of changes in frontal brain activity with changes in CES-D (R = − 0.793, P = 0.019), but not changes in with MMSE, and there were significant correlations of changes in temporal brain activity with changes in MMSE (R = 0.749, P = 0.033), but not with changes in CES-D.
Discussion
NIRS has recently been used to investigate not only the neurocognitive processes associated with neurological and psychiatric disorders20,23,24, but also neurological hemodynamic changes with cardiac arrest25, cardiopulmonary resuscitation26, cardiac surgery and cardiovascular anesthesia27,28. To the best of our knowledge, the current study is the first to evaluate both brain activity using NIRS, depressive state and cognitive function in AF patients. NIRS showed that (1) frontal and temporal brain activities were lower in the persistent AF patients, (2) frontal and temporal brain activities were improved in some of (over 50%) the persistent AF patients who underwent CA and kept SR at 3 months after CA, especially who presented impaired brain activity before CA, and (3) improvement of frontal brain activity was associated with improvement of depressive state (CES-D), whereas improvement of temporal brain activity was associated with cognitive function (MMSE).
Various AF-related pathologies, including low cardiac output, cerebral hypoperfusion, vascular inflammation, endothelial dysfunction, brain atrophy, thrombosis, genetics factors, and shared risk factors such as hypertension, sleep apnea, diabetes, and obesity, have been shown to affect cognitive function or depressive state29,30. Another potential, but unproven, mechanism is brain hypoperfusion following reduced stroke volume and cardiac output attributable to a beat-to-beat variability in the length of the cardiac cycle13,31. Moreover, cerebral microbleeds during anticoagulation can also contribute to an impairment of the cognitive function30. More severe depressive state was observed in the persistent AF patients than in the paroxysmal AF patients8. Reduced cerebral blood flow likely contributes to tissue changes, and thus, has the potential to change depressive symptom levels in AF patients. A decrease in the oxy-Hb concentration measured by NIRS reflects a decrease in frontal brain function in patients with depression or in a depressed state32, which may be further associated with cognitive impairment33. CA improves clinical symptoms of AF accompanied depressive symptom, can reduce the risk of left atrial thrombosis caused by atrial synchronicity and hemodynamic alternations, and can improve long-term cognitive function5. Change in cognitive function before and after the CA of AF, and improvement in the cognitive function 1 year after the CA were reported5. Restoring SR after CA improves both the systolic and diastolic function34 and may help the recovery from cerebral hypoperfusion and a cognitive impairment in patients with AF. On the contrary, Medi et al.6 reported that post-procedural neurocognitive dysfunction partly occurred in AF patients after CA. CA of AF improved neurocognitive function assessed by a brief cognitive screening tool (i.e. The Montreal Cognitive Assessment), especially in AF patients with pre-CA cognitive impairment5. This is concordant with the current study where frontal and temporal brain activities were improved in some of (over 50%) the persistent AF patients who kept SR at 3 months after CA, especially in those who presented impaired brain activity before CA.
Study limitations are as follows: First, as a prospective cohort study in a single center with a relatively small number of patients, the present results may not be representative of the general population. Second, since NIRS evaluates only a shallow layer of the brain, deep layers (e.g. hippocampus) or detailed regions could not be evaluated. Although functional MRI is used to accurately evaluate regional cerebral blood flow, high costs and a large-scale device interfere with easy examination. NIRS is superior to MRI for easy-to-repeat measurements. In this study, we evaluated NIRS findings only single measurement in 60 s during VFT. Since NIRS has advantages in high time resolution and easy-to-repeat measurements over MRI, repeated measurement NIRS with short time duration (e.g. every 5 s) may improve the quality of NIRS findings in future study. Third, because of artifacts, some NIRS signals in the temporal lobe could not be fully detected in some study subjects. NIRS signals during the VFT may be influenced by skin blood flow. Fourth, although we excluded the presence of carotid artery stenosis or cerebral infarction before CA, it may have affected cerebral oxy-Hb concentrations due to arteriosclerotic changes. Fifth, general condition may have affected the results of CES-D and MMSE. Sixth, associations between brain activities determined by NIRS and each score of psychological testing (e.g. depression and cognitive function) may not have been precisely examined. Therefore, since the present results should be viewed as preliminary, further prospective studies with a larger population and more frequent measurements of NIRS, CES-D and MMSE are needed.
Conclusion
NIRS measurement showed reduced frontal and temporal brain activities in the persistent AF patients, CA improved frontal and temporal brain activities in some of these patients, and associated with improvement of depressive state/ cognitive function.
Methods
Subjects and study protocol
This is a cross-sectional study with 29 age-matched control subjects and 32 AF patients (paroxysmal AF n = 18 and persistent AF n = 14) who came to Fukushima Medical University Hospital between June 2020 and October 2020. AF was diagnosed and classified as paroxysmal or persistent according to the duration of AF; paroxysmal, ≤ 7 days; persistent, > 7 days35. The study subjects underwent echocardiography, carotid artery ultrasonography, laboratory testing, psychological testing and NIRS. The verbal fluency task (VFT), which is commonly used with NIRS analysis, was used to test brain activity36,37,38. The control subjects had no past history of AF, heart failure or structural cardiac abnormalities detected by echocardiography. The study protocol was approved by the ethical committee of Fukushima Medical University (#823), and the investigation conforms to the principles outlined in the Declaration of Helsinki. All subjects provided written informed consent to participate in the study. Patients with carotid artery stenosis, cerebral infarction, dementia, and those receiving treatment for schizophrenia, depression, or bipolar disorder were excluded. Regarding the psychological testing, the Center for Epidemiologic Studies Depression Scale (CES-D) was used to evaluate depressive symptoms21, and the Mini-Mental State Examination (MMSE) was used to evaluate cognitive function22. We compared the findings of CES-D (depressive symptoms), MMSE (cognitive function) and NIRS (brain activities) between the AF patients and control subjects. Next, we also followed up persistent AF patients who kept SR at 3 months after CA and measured their brain activities using NIRS, CES-D and MMSE after CA to investigate the associations of changes in brain activities with changes in both CES-D and MMSE.
Echocardiography and carotid artery ultrasonography were performed blindly by experienced sonographers using standard techniques39,40. The left ventricular ejection fraction was calculated using Simpson’s method in a four-chamber view39,40. All measurements were performed using ultrasound systems (ACUSON Sequoia, Siemens Medical Solutions USA, Inc., Mountain View, CA, USA). Blood samples were obtained from all subjects at Fukushima Medical University Hospital. B-type natriuretic peptide levels were measured using a specific immunoradiometric assay (Shionoria BNP kit, Shionogi, Osaka, Japan)41.
Measurement of NIRS
In the current study, oxy-Hb, deoxy-Hb, and total hemoglobin were measured with a 52-channel NIRS machine (Hitachi ETG4000, Hitachi Medical Corp., Tokyo, Japan) using two wavelengths of near-infrared light (695 and 830 nm). Artifacts must be eliminated by having the subject sit in a chair, relax, and move as little as possible. The 52 channels were attached symmetrically around the prefrontal cortex42,43. The main measured channels were as follows: right temporal lobe (channels 1–3, 11–14, 22–24, 32–35, and 43–45), left temporal lobe (channels 8–10, 18–21, 29–31, 39–42, and 50–52) and frontal region (channels 25–28, 36–38, and 46–49). Since size of the personal head differed and measurement position in parietal region was easy to be mistaken, we did not use data from channels 4–7 and 15–1742,43. An increase in cerebral oxy-Hb concentration in response to the VFT is considered as a marker of brain activity43. Most of the lower and forward channels were placed along the line connecting T3-Fpz-T4, based on the international 10–20 system. This system allows prediction of the measurement sites on the brain surface with relatively high accuracy. The sampling rate of oxy-Hb concentration data was 0.1 s. The obtained data were analyzed using the integral mode: the mean oxy-Hb concentrations over the 10-s pre-task period and over the last 5 s of the post-task period were used as the baselines. Oxy-Hb concentrations were measured during a 10-s pre-task period, a 60-s VFT period, and a 55-s post-task baseline period. Linear fitting was applied to the data between these two baselines. The average oxy-Hb concentration during the 60-s VFT period was used for the analysis. A previously reported algorithm37 was used to automatically reject data with artifacts. Data are expressed as waveforms and topographic map. The intraclass correlation coefficient of the mean oxy-Hb concentration during the task period was calculated for the 52 channels. The single measure intraclass correlation coefficient was 0.5309, and the average measure intraclass correlation coefficient was 0.6936, which are both reliable, as previously reported44. NIRS analyses were performed using MATLAB R2011 (Math Works Inc., Natick, MA, USA), and Prism 6.0 software (GraphPad Software, Inc., San Diego, CA, USA). Changes in hemoglobin oxygenation occur in the subject undergoing the VFT.
Activation task, VFT
The VFT was used as an activating task during NIRS analysis as previously reported36,37,38. An outline of the VFT procedure is as follows37,38. The subject is first prompted by a voice saying, “Start /a/, /i/, /u/, /e/, /o/” to repeat the utterance “/a/, /i/, /u/, /e/, /o/” for 30 s. The baseline activity, which is recorded and is used to remove the effect of vocalization on brain activity from the data. The subject is next prompted by a voice to vocalize as many words as possible that start with a certain letter. This is done in three 20-s sets. The subject is verbally prompted to vocalize words starting with a certain letter to increase the difficulty of the task. The task is scored by recording the number of words uttered in every 20 s. Finally, the subject is prompted by a voice saying, “Stop /a/, /i/, /u/, /e/, /o/” to stop the task and repeat “/a/, /i/, /u/, /e/, /o/” for 70 s.
Catheter ablation for AF and follow-up
Detailed methods of CA and follow-up have been reported45,46,47. CA was performed for AF following the cessation of all antiarrhythmic drugs for over five half-lives. Circumferential pulmonary vein (PV) isolation was performed using a single-lasso technique while the patient was sedated with dexmedetomidine hydrochloride. A 7-Fr decapolar ring catheter (Lasso, Biosense Webster, Inc., Diamond Bar, CA, USA) and a 7.5-Fr irrigation catheter with a 3.5-mm distal electrode and real time contact force monitoring (ThermoCool SmartTouch SF, Biosense Webster, Inc., Diamond Bar, CA, USA) were inserted into the left atrium via the transseptal approach. After selective PV angiography, radiofrequency energy was delivered with a wide antral ablation line around the PVs, guided by a 3-dimensional mapping system (CARTO system, Biosense Webster, Inc., Diamond Bar, CA, USA). Radiofrequency power output was set at 45–50 W, and contact force was maintained between 10 and 15 g. After circumferential PV isolation, the elimination of the PV potential in each of the upper and lower PVs was confirmed. The endpoint of the PV isolation was defined as the creation of a bidirectional conduction block between the left atrium and PVs. Each patient was followed up at 1, 3, and 6 months after the CA, then every 3 months thereafter. At each visit, 12-lead electrocardiogram and 24-h Holter monitoring were performed. Recurrence was defined as documentation of atrial tachycardia or AF lasting > 30 s recorded in 12-lead ELECTROCARDIOGRAM or 24-h Holter monitoring. Administration of antiarrhythmic drugs after PV isolation depended on the operator’s decision.
Statistical analysis
Categorical variables are expressed as numbers and percentages. The chi-square test was used for comparisons of categorical variables and followed by Fisher’s exact test when/if appropriate. Normality was confirmed using the Shapiro–Wilk test in each group. Parametric variables are presented as mean ± SD, and non-parametric variables (e.g. BNP, and NIRS findings) are presented as a median and interquartile range. Parametric variables were compared using Student’s t-test, whereas non-parametric variables were compared using the Mann–Whitney U test. To compare continuous variables among the paroxysmal AF, persistent AF and control subjects, Kruskal–Wallis test was used. We performed regression analysis to determine brain activity confounding factors. Correlations of changes in brain activities with changes in CES-D and MMSE before and after CA were assessed using Spearman’s correlation analysis. A P value of < 0.05 was considered statistically significant for all comparisons. All analyses were performed using a statistical software package (SPSS ver. 24.0, IBM, Armonk, NY, USA).
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Shinohara, M. et al. Relationship between the nutritional status and safety and efficacy outcomes in atrial fibrillation patients aged 80 years and over receiving oral anticoagulants. J. Cardiol. 77, 147–153. https://doi.org/10.1016/j.jjcc.2020.10.008 (2021).
Kuroda, S. et al. Clinical impact of serial change in brain natriuretic peptide before and after catheter ablation in patients with atrial fibrillation and heart failure. J. Cardiol. 77, 517–524. https://doi.org/10.1016/j.jjcc.2020.11.011 (2021).
Kwok, C. S., Loke, Y. K., Hale, R., Potter, J. F. & Myint, P. K. Atrial fibrillation and incidence of dementia: A systematic review and meta-analysis. Neurology 76, 914–922. https://doi.org/10.1212/WNL.0b013e31820f2e38 (2011).
Santangeli, P. et al. Atrial fibrillation and the risk of incident dementia: A meta-analysis. Heart Rhythm 9, 1761–1768. https://doi.org/10.1016/j.hrthm.2012.07.026 (2012).
Jin, M. N. et al. Atrial fibrillation catheter ablation improves 1-year follow-up cognitive function, especially in patients with impaired cognitive function. Circ. Arrhythm Electrophysiol. 12, e007197. https://doi.org/10.1161/CIRCEP.119.007197 (2019).
Medi, C. et al. Subtle post-procedural cognitive dysfunction after atrial fibrillation ablation. J. Am. Coll. Cardiol. 62, 531–539. https://doi.org/10.1016/j.jacc.2013.03.073 (2013).
Ariansen, I., Dammen, T., Abdelnoor, M., Tveit, A. & Gjesdal, K. Mental health and sleep in permanent atrial fibrillation patients from the general population. Clin. Cardiol. 34, 327–331. https://doi.org/10.1002/clc.20883 (2011).
von Eisenhart Rothe, A. F. et al. Depression in paroxysmal and persistent atrial fibrillation patients: A cross-sectional comparison of patients enroled in two large clinical trials. Europace 16, 812–819. https://doi.org/10.1093/europace/eut361 (2014).
Lange, H. W. & Herrmann-Lingen, C. Depressive symptoms predict recurrence of atrial fibrillation after cardioversion. J. Psychosom. Res. 63, 509–513. https://doi.org/10.1016/j.jpsychores.2007.07.010 (2007).
Zhuo, C. et al. Depression and recurrence of atrial fibrillation after catheter ablation: A meta-analysis of cohort studies. J. Affect. Disord. 271, 27–32. https://doi.org/10.1016/j.jad.2020.03.118 (2020).
Ziegelstein, R. C. Acute emotional stress and cardiac arrhythmias. JAMA 298, 324–329. https://doi.org/10.1001/jama.298.3.324 (2007).
Gaita, F. et al. Prevalence of silent cerebral ischemia in paroxysmal and persistent atrial fibrillation and correlation with cognitive function. J. Am. Coll. Cardiol. 62, 1990–1997. https://doi.org/10.1016/j.jacc.2013.05.074 (2013).
Farina, E. et al. Neuropsychological deficits in asymptomatic atrial fibrillation. Acta Neurol. Scand. 96, 310–316. https://doi.org/10.1111/j.1600-0404.1997.tb00289.x (1997).
Lappegard, K. T. et al. Improved neurocognitive functions correlate with reduced inflammatory burden in atrial fibrillation patients treated with intensive cholesterol lowering therapy. J. Neuroinflamm. 10, 78. https://doi.org/10.1186/1742-2094-10-78 (2013).
Strangman, G., Boas, D. A. & Sutton, J. P. Non-invasive neuroimaging using near-infrared light. Biol. Psychiatry 52, 679–693 (2002).
Suto, T., Fukuda, M., Ito, M., Uehara, T. & Mikuni, M. Multichannel near-infrared spectroscopy in depression and schizophrenia: Cognitive brain activation study. Biol. Psychiatry 55, 501–511. https://doi.org/10.1016/j.biopsych.2003.09.008 (2004).
Miura, I. et al. Near-infrared spectroscopy and plasma homovanillic acid levels in bipolar disorder: A case report. Neuropsychiatr. Dis. Treat. 10, 507–511. https://doi.org/10.2147/NDT.S58302 (2014).
Strangman, G., Culver, J. P., Thompson, J. H. & Boas, D. A. A quantitative comparison of simultaneous BOLD fMRI and NIRS recordings during functional brain activation. Neuroimage 17, 719–731 (2002).
Sassaroli, A., Frederick, B. D., Tong, Y., Renshaw, P. F. & Fantini, S. Spatially weighted BOLD signal for comparison of functional magnetic resonance imaging and near-infrared imaging of the brain. Neuroimage 33, 505–514. https://doi.org/10.1016/j.neuroimage.2006.07.006 (2006).
Irani, F., Platek, S. M., Bunce, S., Ruocco, A. C. & Chute, D. Functional near infrared spectroscopy (fNIRS): An emerging neuroimaging technology with important applications for the study of brain disorders. Clin. Neuropsychol. 21, 9–37. https://doi.org/10.1080/13854040600910018 (2007).
Radloff, L. S. The CES-D scale: A self-report depression scale for research in the general population. Appl. Psychol. Meas. 1, 385–401 (1977).
Folstein, M. F., Folstein, S. E. & McHugh, P. R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 12, 189–198 (1975).
Yanagi, M. & Shirakawa, O. Application of near-infrared spectroscopy for understanding spontaneous brain activity during resting state in schizophrenia: A mini review. Front. Psychiatry 12, 704506. https://doi.org/10.3389/fpsyt.2021.704506 (2021).
Lee, Y. Q., Tay, G. W. N. & Ho, C. S. H. Clinical utility of functional near-infrared spectroscopy for assessment and prediction of suicidality: A systematic review. Front. Psychiatry 12, 716276. https://doi.org/10.3389/fpsyt.2021.716276 (2021).
Takegawa, R. et al. Near-infrared spectroscopy assessments of regional cerebral oxygen saturation for the prediction of clinical outcomes in patients with cardiac arrest: A review of clinical impact, evolution, and future directions. Front. Med. 7, 587930. https://doi.org/10.3389/fmed.2020.587930 (2020).
Kool, M. et al. Focused echocardiography, end-tidal carbon dioxide, arterial blood pressure or near-infrared spectroscopy monitoring during paediatric cardiopulmonary resuscitation: A scoping review. Resusc. Plus 6, 100109. https://doi.org/10.1016/j.resplu.2021.100109 (2021).
Yoshitani, K. et al. Guidelines for the use of cerebral oximetry by near-infrared spectroscopy in cardiovascular anesthesia: A report by the cerebrospinal Division of the Academic Committee of the Japanese Society of Cardiovascular Anesthesiologists (JSCVA). J. Anesth. 33, 167–196. https://doi.org/10.1007/s00540-019-02610-y (2019).
Zheng, F. et al. Cerebral near-infrared spectroscopy monitoring and neurologic outcomes in adult cardiac surgery patients: A systematic review. Anesth. Analg. 116, 663–676. https://doi.org/10.1213/ANE.0b013e318277a255 (2013).
Chopard, R. et al. Dementia and atrial fibrillation: Pathophysiological mechanisms and therapeutic implications. Am. J. Med. 131, 1408–1417. https://doi.org/10.1016/j.amjmed.2018.06.035 (2018).
Poels, M. M. et al. Cerebral microbleeds are associated with worse cognitive function: The Rotterdam Scan Study. Neurology 78, 326–333. https://doi.org/10.1212/WNL.0b013e3182452928 (2012).
Lavy, S. et al. Effect of chronic atrial fibrillation on regional cerebral blood flow. Stroke 11, 35–38. https://doi.org/10.1161/01.str.11.1.35 (1980).
Matsuo, K., Kato, N. & Kato, T. Decreased cerebral haemodynamic response to cognitive and physiological tasks in mood disorders as shown by near-infrared spectroscopy. Psychol. Med. 32, 1029–1037 (2002).
Castaneda, A. E., Tuulio-Henriksson, A., Marttunen, M., Suvisaari, J. & Lonnqvist, J. A review on cognitive impairments in depressive and anxiety disorders with a focus on young adults. J. Affect. Disord. 106, 1–27. https://doi.org/10.1016/j.jad.2007.06.006 (2008).
Cha, Y. M. et al. Success of ablation for atrial fibrillation in isolated left ventricular diastolic dysfunction: A comparison to systolic dysfunction and normal ventricular function. Circ. Arrhythm Electrophysiol. 4, 724–732. https://doi.org/10.1161/CIRCEP.110.960690 (2011).
Kirchhof, P. et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur. Heart J. 37, 2893–2962. https://doi.org/10.1093/eurheartj/ehw210 (2016).
Ichijo, Y. et al. Impaired frontal brain activity in patients with heart failure assessed by near-infrared spectroscopy. J. Am. Heart Assoc. 9, e014564. https://doi.org/10.1161/JAHA.119.014564 (2020).
Fujiwara, T. et al. Evaluation of brain activity using near-infrared spectroscopy in inflammatory bowel disease patients. Sci. Rep. 8, 402. https://doi.org/10.1038/s41598-017-18897-4 (2018).
Klumpp, H. & Deldin, P. Review of brain functioning in depression for semantic processing and verbal fluency. Int. J. Psychophysiol. 75, 77–85. https://doi.org/10.1016/j.ijpsycho.2009.10.003 (2010).
Sato, Y. et al. Subclinical hypothyroidism is associated with adverse prognosis in heart failure patients. Can. J. Cardiol. 34, 80–87. https://doi.org/10.1016/j.cjca.2017.10.021 (2018).
Yoshihisa, A. et al. Cardiovascular function and prognosis of patients with heart failure coexistent with chronic obstructive pulmonary disease. J. Cardiol. 64, 256–264. https://doi.org/10.1016/j.jjcc.2014.02.003 (2014).
Hotsuki, Y. et al. B-type natriuretic peptide is associated with post-discharge stroke in hospitalized patients with heart failure. ESC Heart Fail. 7, 2508–2515. https://doi.org/10.1002/ehf2.12818 (2020).
Takizawa, R. et al. Reduced frontopolar activation during verbal fluency task in schizophrenia: A multi-channel near-infrared spectroscopy study. Schizophr. Res. 99, 250–262. https://doi.org/10.1016/j.schres.2007.10.025 (2008).
Takizawa, R. et al. Neuroimaging-aided differential diagnosis of the depressive state. Neuroimage 85(Pt 1), 498–507. https://doi.org/10.1016/j.neuroimage.2013.05.126 (2014).
Kakimoto, Y. et al. Intrasubject reproducibility of prefrontal cortex activities during a verbal fluency task over two repeated sessions using multi-channel near-infrared spectroscopy. Psychiatry Clin. Neurosci. 63, 491–499. https://doi.org/10.1111/j.1440-1819.2009.01988.x (2009).
Kaneshiro, T. et al. Anatomical predisposing factors of transmural thermal injury after pulmonary vein isolation. Europace 20, 1122–1128. https://doi.org/10.1093/europace/eux185 (2018).
Kaneshiro, T. et al. Distinct forms of esophageal lesions after radiofrequency and cryoballoon pulmonary vein isolation. JACC Clin. Electrophysiol. 4, 1642–1643. https://doi.org/10.1016/j.jacep.2018.08.006 (2018).
Kaneshiro, T. et al. Characteristics of esophageal injury in ablation of atrial fibrillation using a high-power short-duration setting. Circ. Arrhythm. Electrophysiol. 13, e008602. https://doi.org/10.1161/CIRCEP.120.008602 (2020).
Author information
Authors and Affiliations
Contributions
A.Y., S.K., T.K., Y.I., T.M., S.Y., M.O., I.M., H.Y., and Y.T. contributed to the conception or design of the work. A.Y., S.K., T.K., Y.I., T.M., S.Y., M.O., I.M., H.Y., and Y.T. contributed to the acquisition, analysis, or interpretation of data for the work. A.Y., S.K., T.K., Y.I. and Y.T. drafted the manuscript. A.Y., S.K., T.K., Y.I., T.M., S.Y., M.O., I.M., H.Y., and Y.T. critically revised the manuscript. All gave final approval and agree to be accountable for all aspects of work ensuring integrity and accuracy.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yoshihisa, A., Kono, S., Kaneshiro, T. et al. Impaired brain activity in patients with persistent atrial fibrillation assessed by near-infrared spectroscopy and its changes after catheter ablation. Sci Rep 12, 7866 (2022). https://doi.org/10.1038/s41598-022-12097-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-12097-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.