Abstract
Direct eye contact is preferentially processed over averted gaze and has been shown to gain privileged access to conscious awareness during interocular suppression. This advantage might be driven by local features associated with direct gaze, such as the amount of visible sclera. Alternatively, a holistic representation of gaze direction, which depends on the integration of head and eye information, might drive the effects. Resolving this question is interesting because it speaks to whether the processing of higher-level social information in the visual system, such as facial characteristics that rely on holistic processing, is dependent on conscious awareness. The Wollaston Illusion is a visual illusion that allows researchers to manipulate perceived gaze direction while keeping local eye features constant. Here we used this illusion to elucidate the driving factor facilitating the direct gaze advantage during interocular suppression. Using continuous flash suppression, we rendered Wollaston faces with direct and averted gaze (initially) invisible. These faces conveyed different gaze directions but contained identical eye regions. Our results showed clear evidence for a direct gaze advantage with Wollaston faces, indicating that holistic representations of gaze direction may drive the direct gaze advantage during interocular suppression.
Similar content being viewed by others
Eye gaze is a crucial sensory cue essential for fluid social interactions1, facilitating our capacity to understand the intentions and focus of others2,3,4,5,6. The ability to detect another’s gaze direction is a critical factor in the development of theory of mind2,7 and is reported to be impaired in neuropsychiatric conditions such as autism spectrum disorder8 and schizophrenia9,10. Research has begun to uncover the perceptual and neural mechanisms that extract information about gaze direction from face stimuli, localised in part to higher-level visual pathways in the temporal cortex11,12,13.
A reported bias to detect faces with direct gaze is well-established14,15,16,17,18,19. This bias is thought to reflect a biological advantage for identifying predators in the environment1,20,21 but given that human eyes have unique morphological characteristics (e.g., an enlarged sclera to iris ratio22) it may have also evolved to facilitate communication with conspecifics.
Research suggests that the direct gaze bias is evident at preconscious stages of visual processing19,23,24,25,26,27. This research commonly employs continuous flash suppression (CFS28) in which a dynamic masking pattern presented to one eye temporarily suppresses awareness of a target stimulus presented to the other eye. Using this technique, researchers have shown that faces with direct gaze gain access to conscious awareness faster than faces with averted gaze, indicating preferential preconscious processing of direct gaze by the visual system. This finding is striking because it suggests that socially relevant facial cues can be processed in the human visual system in the absence of conscious awareness.
It is important to note that our perception of gaze direction does not rest solely on information gleaned from the eyes. Rather, the percept of gaze direction is an emergent, holistic property determined by integrating multiple physical attributes29,30,31,32. These attributes include cues from the eye region, such as the luminance distribution produced by the position of the darker pupil and iris compared to the lighter sclera1,33, as well as attributes of the head, such as the orientation of the nose and overall facial symmetry29,34. Also, the head and eye regions interact, such that changes in the surrounding head influence the perceived gaze direction conveyed by the eyes30. For example, when our friend’s pupil is in an averted position this can be a strong cue that they are not looking at us if their face is turned directly towards us. However, if our friend’s face is turned to the side, the same averted pupil position can indicate that they are looking towards us.
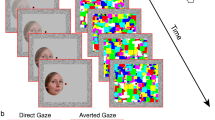
The visual integration of head and eye cues is best exemplified by the Wollaston Illusion35 (Fig. 1). Here, identical sets of eyes are superimposed onto heads facing different directions. Despite the faces containing identical eye regions, the surrounding context of the head drastically alters the perceived gaze direction. Wollaston stimuli are highly useful as they allow researchers to independently manipulate the perceived direction of gaze, while keeping the key low-level stimulus characteristics constant36,37,38. Using such stimuli, there is now evidence to show that the direct gaze advantage observed in visual search and attentional cueing tasks relies on the emergent property of perceived gaze direction, rather than the physical properties of the eye region per se36,38,39,40,41. Similarly, sensory adaptation to Wollaston faces produces perceptual aftereffects consistent with adaptation of higher-level representations of gaze direction that depend on the integration of head and eye features, rather than adaptation to local eye features alone37.
The Wollaston illusion, wherein identical sets of eyes (e.g., reflected in the iris position and amount of sclera visible) result in a different percept of gaze direction dependent upon the surrounding head orientation. The face images were generated with FaceGen Modeller 3.5., (https://facegen.com/).
While evidence suggests that the integration of head and eye cues occurs rapidly, it is unclear whether this form of visual processing requires conscious awareness. Similarly, we do not know whether the faster preconscious processing of faces with direct gaze observed under CFS is driven by the physical local properties of the eyes (e.g., differences in iris-sclera positioning) or the higher-level holistic representation of gaze direction that depends on the integration of head and eye information. In the current study, we investigated these questions by measuring the suppression of faces that elicit the Wollaston illusion using CFS. Specifically, we used Wollaston face stimuli that were matched across conditions in the features of their eye regions, but were perceived as looking in different directions (i.e., direct or averted gaze) due to the conjunction of head and eye features. In our design, we also controlled for differences in head features across conditions, described further in the “Methods”. We hypothesized that if integration of head and eye information occurs at a preconscious level of processing, then Wollaston faces perceived as looking directly at the observer will reach awareness significantly faster than Wollaston faces eliciting the percept of averted gaze, despite containing identical eye information. In contrast, if holistic processing of gaze direction requires conscious awareness, then no direct gaze advantage would be observed for our Wollaston stimuli given that they contain identical eye regions.
Methods
Ethical statement
The study was approved by the Western Sydney University Human Research Ethics Committee (H12571). All research was performed in accordance with relevant guidelines and regulations. Participants received course credit for their time and provided written and informed consent before participating.
Participants
Forty-eight participants (10 males, 38 females) between the ages of 18 to 64 (M 21.65, SD 7.40) were recruited through Western Sydney University’s research participation system. Four participants were not run on the main experiment after providing unreliable data from the Wollaston stimulus calibration (see below). From the remaining forty-four participants, seven participants were also removed as their data suggested a non-serious attempt or a failure to understand the task (i.e., > 90% missed trials, fast responses, incorrect localisations). Data from the remaining 37 participants (6 males, 31 females, age range 18–33; M 20.54, SD 3.48) were analysed. This sample size is similar to previous CFS research24,27,42, and is double that required to detect a main effect of gaze direction based on prior literature25 (e.g., d = 2.04). Participants had normal to corrected vision (as assessed by the Freiburg visual acuity test43) and were naïve to the purpose of the study.
Design
The current study utilised a within-subjects experimental design. Two within subject factors were manipulated: stimulus type (Standard/Wollaston) and gaze direction (Direct/Averted). The key dependent variable was stimulus suppression time (ms).
Apparatus and stimuli
A Dell Precision 3620 tower computer running MATLAB™ (MathWorks Ltd) was used to control the experimental tasks and to record participants responses. Stimuli were presented on a 24-inch VPixx monitor (1920 × 1080-pixel resolution, 120 Hz refresh rate) using Psychtoolbox44. Participants viewed stimuli from a distance of 57 cm with their head stabilised using a chinrest.
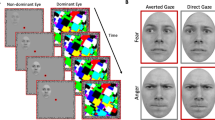
Stimuli were computer-generated faces presented on screen at 3.3° × 4.6°. 3D models of faces were created using FaceGen 3.5., then manipulated in the scene-based rendering program Blender 2.70. In Blender, we modelled the eyes as separate objects from the rest of the face, allowing head orientation and gaze direction to be precisely controlled. Eye deviations reported here are in viewer-centred coordinates. For example, when eyes are directed 0, they are looking directly at the viewer (i.e., the camera the image is rendered from). The experiment included two types of stimuli: Wollaston stimuli and Standard stimuli. Each stimulus type was presented with both direct and averted gaze, for both rightward and leftward oriented heads. Wollaston stimuli comprising the same base eye-region and head-region components (Fig. 2) were constructed following the method outlined by Palmer and Clifford37. This involved using Adobe Photoshop to merge eye regions and head regions from different images. Direct and averted gaze conditions were created by pairing either congruent or incongruent conjunctions of these base components. Congruent eye and head pairings (e.g. rightward eyes in a rightward head) resulted in face stimuli perceived as displaying averted gaze. Incongruent pairings of the same head and eye components (e.g. rightward eyes in a leftward head) resulted in face stimuli that were perceived as displaying direct gaze. The use of such stimuli allowed us to examine the processing of different gaze directions while keeping physical low-level properties of the stimuli matched overall across conditions (i.e., direct and averted conditions were each made up of the identical eyes directed left and right and the identical heads rotated left and right, but presented in different combinations).
Wollaston stimuli. (a) Eyes isolated from a frontal head were paired with heads angled either left or right. (b) Congruent pairings (e.g., leftwards-angled eyes in leftwards-angled head) tended to produce a sense of averted gaze. Incongruent pairings (e.g., leftwards-angled eyes in a rightwards-angled head) tended to produce a sense of gaze directed towards the viewer. When comparing between the congruent and incongruent pairings, the stimuli are comprised of identical base components, but presented in different combinations. Hence, features like iris position, visible sclera and head rotation cues are controlled across this comparison, despite the differences in perceived direction of gaze. The face images were generated with FaceGen Modeller 3.5., (https://facegen.com/).
Standard stimuli were created with the same face identities and head rotations used to create the Wollaston stimuli (Fig. 3). However, the eye regions were not identical across averted and direct conditions. Instead, analogous to previous research23,27,45, we allowed for low-level differences in visible-sclera to remain in the stimuli. Specifically, we used the same eye deviations employed in previous studies; faces with direct gaze had an eye deviation of 0° and averted gaze had an eye deviation of 20° or − 20°. The inclusion of these stimuli in our experiment allowed us to verify the presence of a direct gaze advantage with computerized faces.
Example of the Standard stimuli. These stimuli have gaze directed away from the viewer (a) or towards the viewer (b). The eyes in these faces differ in low-level physical cues to gaze direction (e.g., amount and position of visible sclera). The heads and eyes on the top row (a) are deviated at an angle of 20° relative to the viewer, whereas the bottom row contains stimuli with heads rotated at 20° and eyes deviated at an angle of 0° relative to the viewer. The face images were generated with FaceGen Modeller 3.5., (https://facegen.com/).
Six face identities were used for both the Wollaston and Standard stimuli. All stimuli were comprised of heads rotated laterally 20° leftwards or rightwards. Unique eye deviations were determined for each participant prior to running the CFS task (see Pre-test eye deviation calibration task below) to ensure the appropriate eye deviation was incorporated into the Wollaston stimuli to elicit the strongest illusion (i.e., where an incongruent head-eye pairing was subjectively perceived as direct and a congruent pairing was perceived as averted).
Procedure
Pre-test eye deviation calibration
Prior to the CFS experiment, each participant completed a calibration task to determine the optimal magnitude of eye deviation required to elicit the Wollaston illusion. Previous research has shown that there are considerable individual differences in gaze perception18,46,47,48. This step ensured that, for each participant, the stimuli used were subjectively perceived as averted gaze for congruent pairings of head and eye direction and perceived as direct gaze for incongruent pairings of head and eye direction. Following a similar approach to Palmer and Clifford37, participants viewed Wollaston stimuli and judged the perceived gaze direction from faces with eye deviations of 4°, 6°, 8°, 10° or 12°. Participants saw one stimulus at a time and were required to report whether they perceived the gaze direction as left, direct, or right. Stimuli were presented for 1 s in each trial, and participants completed 24 trials of each head/eye combination. The viewing distance, size and placement (2.2° to the left or right of fixation, presented randomly across trials but balanced across eye deviations) of stimuli were the same as that used in the subsequent CFS experiment34. On completion, data for each participant was visually inspected to find the angle of eye deviation associated with the cleanest pattern of direct percepts for incongruent head/eye combinations and averted percepts for congruent head/eye combinations. This eye deviation was then selected for that subject as the optimal gaze deviation and used to create their Wollaston stimuli for the CFS task. For example, if a participant’s optimal gaze direction was 8°, the deviations for the direct and averted Wollaston Stimuli was 8° or − 8°. Participants were precluded from performing the CFS task, if none of the tested eye deviations produced a consistent perceptual distinction between the congruent and incongruent head/eye combinations.
Continuous flash suppression (CFS)
We closely followed the CFS method used by Stein et al.27. Participants viewed a dichoptic display through a mirror stereoscope. Two red squares (11° × 11°) were presented on a uniform grey background. When viewed through the mirror stereoscope, the left eye saw only the left square and the right eye saw only the right square (confirmed prior to testing by asking participants to report their percept when viewing the stimulus monocularly). When binocularly fused, only one square was apparent to the participant. Fusion contours (width 0.8°) comprising black and white pixels helped to maintain binocular fusion throughout the task.
Figure 4 illustrates a single trial of CFS task. Each trial began with a 1 s presentation of the red squares, fusion contours, and a central fixation dot (0.5°). Next, a face was presented to the non-dominant eye (confirmed using the near convergence test49), initially presented at a contrast of 0% which increased linearly to 100% within a period of one second window. At the same time, a high contrast coloured mask flashing at 10 Hz was presented at full contrast to the participant’s dominant eye. This resulted in a temporary suppression of the face stimulus, rendering it invisible to the participant. Face stimuli were presented 2.2° to the left or right of the fixation with their centre being aligned vertically with the central dot. This location was randomly selected on any given trial but was balanced across conditions.
(a) A schematic of an example CFS trial. A high contrast flashing mask was presented to the participant’s dominant eye, while a face stimulus (here Standard Direct) was presented to the non-dominant eye. (b) A schematic example of a participant’s view during the task. The binocular presentation results in the temporary suppression of the face stimulus from conscious awareness. Participants are instructed to respond as quickly and as accurately as possible when any aspect of the face becomes visible.
Participants were required to maintain fixation throughout the experiment. They were instructed to indicate with the left or right arrow key whether the face appeared to the left or right of the fixation dot. They were instructed to respond as quickly as possible when any aspect of the face became visible and were not required to make judgements about the stimulus. Once a response was made, this initiated the next trial. A maximum trial length was 10 s. Response latencies greater than 8 s were excluded from the analysis, as at this stage of the trial the contrast of the flashing mask decreased linearly to 0% within a period of one second window. Trials with response latencies quicker than 300 ms were also removed, as these were likely to reflect a false response50,51. Participants completed a total of 576 trials wherein each of the 4 conditions (i.e., Wollaston Direct, Wollaston Averted, Standard Direct, Standard Averted) were presented 144 times in a completely randomised manner. A break was provided halfway through the task. Prior to testing, participants completed a number of practice trials to confirm visual fusion was achieved and that the task instructions were understood. Practice trials were not analysed. Only trials with correct responses were submitted to statistical analysis.
Statistics
Suppression times were calculated as the time taken to localise the face after it appeared at full contrast (i.e., a second after the start of the trial) and used as an index of stimulus potency in reaching conscious awareness. Each individual’s mean suppression times were normalised to their mean to account for individual differences and positive skew19,52 (i.e., mean suppression times for each condition were divided by the participant’s mean across all conditions). All statistical analyses were conducted on the normalised data, but Fig. 5a shows raw data in milliseconds to facilitate an intuitive interpretation of the results (also see Supplementary Information for analysis on raw suppression times).
Mean suppression times for each condition (a). Error bars denote ± standard error. *(p < 0.05). (b) Sample distributions of the direct effect (i.e., the extent to which faces with direct gaze are detected faster than faces with averted gaze) are reported as latency normalised mean differences in suppression times between averted and direct conditions for Wollaston stimuli and Standard stimuli. Values above zero indicate a direct gaze effect. Note, not all participants show the direct effect with Wollaston or Standard stimuli.
In addition to classical statistics, we report the results of a Bayesian repeated-measures ANOVA computed with default priors. We report Bayes factors for the inclusion of each effect in the ANOVA model (BFincl), which represent the likelihood of the data given the model(s) that include the effect relative to matched models that lack the effect. We also report Bayesian paired samples t-tests, computed with a default Cauchy prior scale of 0.707. We report one-tailed BF10 values for t-tests to quantify evidence for the alternative hypothesis over the null hypothesis. These analyses were conducted using JASP version 0.12.2 (JASP Team, 2020). The method for these analyses followed recommendations in Keysers et al.53.
Results
Perception of Wollaston images
To control for individual differences in direct gaze perception, the Wollaston stimuli shown during the CFS task were tailored to individual participants. For the majority of the 48 participants tested, the optimal magnitude of eye deviation to elicit the Wollaston illusion was either 6° or 8°. With these deviations, participants consistently judged the faces as exhibiting direct gaze for incongruent head/eye combinations (M 80% SD 15%) and averted gaze for congruent head/eye combinations (M 99% SD 14%). Four participants were precluded from the CFS experiment based on results from the pre-test eye deviation calibration task, as none of the tested eye deviations produced a consistent perceptual distinction between the congruent and incongruent head/eye combinations.
Continuous flash suppression
Data from seven participants were removed from the analysis due to a high proportion (i.e., > 90%) of invalid trials. For the remaining 37 participants, accuracy data is as follows: Wollaston direct (M 96.82%, SD 4.57%), Wollaston averted (M 97.35%, SD 4.40%), Standard direct (M 97.33%, SD 4.46%), Standard averted (M 97.76%, SD 4.40%). A 2 × 2 repeated measures ANOVA conducted on these data revealed a significant difference in accuracy between Wollaston and Standard stimuli (F(1, 36) = 4.776, p = 0.035, η2 = 0.038) with slightly more correct trials being observed with Standard stimuli. Accuracy also differed between averted and direct trials (F(1, 36) = 4.479, p = 0.041, η2 = 0.043), with slightly more averted trials being observed. Importantly, however, there was no interaction between gaze direction and stimulus type (F(1, 36) = 0.056, p = 0.815) that could pose a potential confound with our experimental design and interpretation of the suppression time results.
Normalised mean suppression times were calculated for Wollaston and Standard conditions for both direct and averted gaze stimuli for each participant. In testing whether the integration of head and eye information occurs at a preconscious level of processing, we sought evidence for a direct gaze advantage for both Wollaston and Standard face stimuli. We conducted a 2 × 2 repeated measures ANOVA with gaze direction and stimulus type as factors. We found no main effect for stimulus type, F(1, 36) = 2.170, p = 0.15, ηp2 = 0.057, BFincl = 0.42, indicating a good match in low-level properties between our Wollaston and Standard stimuli. Consistent with our hypothesis, our results revealed strong evidence for a main effect of gaze direction, F(1, 36) = 7.91, p = 0.008, ηp2 = 0.18, BFincl = 82.01. We found no evidence for a significant interaction, F(1, 36) = 0.308, p = 0.582, ηp2 = 0.002, BFincl = 0.27 (Fig. 5a), indicating that the direct gaze advantage is not significantly influenced by stimulus type. Post-hoc paired t-tests (one-sided) verified a difference in suppression times between direct and averted gaze with Standard stimuli, t(36) = 2.50, p = 0.009, d = 0.410, BF10 = 5.17. Consistent with prior research using real-face stimuli23,25,27, faces with direct gaze became visible significantly faster (M 1878 ms, SD 544) than averted gaze faces (M 1907 ms, SD 549). Similarly, we found a significant difference between direct (M 1886 ms, SD 536) and averted gaze conditions (M 1922 ms, SD 548) with Wollaston stimuli, t(36) = 2.07, p = 0.023, d = 0.340, BF10 = 2.31.
Because normality assumptions were violated in our data we also ran statistical analyses on latency normalised difference in suppression times between averted and direct for Wollaston stimuli and Standard stimuli54. For each participant and each stimulus type (Wollaston or Standard), we calculated the difference in mean suppression time between averted and direct gaze divided by the participants’ overall mean suppression time. One sample t-tests (one-sided) of these data showed a significant direct effect for both Wollaston (t(36) = 2.070, p = 0.023, d = 0.340, BF10 = 2.311, normalised mean suppression time difference = 0.021, 95% CI [0.06, ∞]) and Standard stimuli (t(36) = 2.491, p = 0.009, d = 0.410, BF10 = 5.170, normalised mean suppression time difference = 0.015, 95%, CI [0.125, ∞]. We plot sample cumulative distributions of the direct effect for Wollaston and Standard face stimuli (Fig. 5B).
In the current study, the difference in detection times between direct and averted gaze were around 29–36 ms on average. This is similar to at least one previous study that used CFS to investigate the direct gaze effect24, though some past studies have tended to find effects closer to ~ 0.5–1 s25,27. This may be related to suppression times being generally shorter across conditions in the current study compared to these previous studies. Shorter suppression times may have occurred in the current study due to the use of coloured rather than greyscale face stimuli. Moreover, it has been shown that similarity in visual properties between mask and target stimuli can influence suppression times55. The majority of previous studies reporting a direct gaze advantage with larger effect sizes use greyscale faces and colour masks. Our experiment deviated from this also, which could have led to the shorter suppression times observed in our study.
Discussion
The aim of the current study was to elucidate whether the integration of head and eye information occurs outside of conscious awareness. Using CFS, we tested whether the direct gaze advantage during interocular suppression25,26,27 is driven by the holistic representation of gaze direction that requires the integration of head and eye cues or whether it depends on local differences in the physical properties of the eyes. In particular, we used the Wollaston illusion, in which identical eye regions are perceived as looking in different directions depending on the orientation of the head (Fig. 1). We created stimuli with different conjunctions of head and eye features that result in different perceived directions of gaze, whilst maintaining identical physical eye region characteristics across direct and averted gaze conditions. We hypothesized that if integration of head and eye information occurs at a preconscious level, then the Wollaston conjunctions perceived as looking direct (i.e., incongruent head and eye deviations) would gain access to awareness over Wollaston faces perceived as looking away (i.e., congruent head and eye deviations), despite the identical eye features that these stimuli contained. We found clear evidence for a direct gaze advantage with Wollaston faces during CFS, suggesting the integration of head and eye information does not require conscious awareness. Moreover, our study suggests that the prioritised unconscious processing of direct gaze observed in previous studies might be driven more by a holistic representation of gaze direction rather than low-level morphological differences in eye regions.
It has previously been shown that humans are hyper-sensitive to low-level sensory signals that convey the attentional focus of our conspecifics16,45,56. For instance, it has been reported that young infants can differentiate direct and averted gaze by relying on physical information such as motion of the pupil or the contrast distribution within the eye socket57,58. Also, adult humans are sensitive to slight changes in the size, luminance or contrast of the sclera or the iris, which can significantly influence perceived gaze direction33,59,60,61. The results of our study, which removed these low-level differences between direct and averted gaze stimuli, suggest that such information is not necessary for driving the direct gaze advantage during interocular suppression. Instead, our data indicate that at unconscious stages of visual processing, the visual system may be sensitive to direct gaze as an integrated perceptual property, independent of the specific low-level features that signal it.
In the original study by Stein et al.27, low-level differences in eye regions were manipulated by inverting the contrast of the eyes (i.e., making the sclera dark and iris light). This manipulation retained local contrast differences but abolished the direct gaze advantage during interocular suppression27. This result could be taken as evidence for a reliance on low-level eye region characteristics being critical for the early unconscious processing of gaze direction. To the best of our knowledge, the current study is the first to assess the direct gaze advantage using Wollaston face stimuli. Our approach allowed the perceived direction of gaze to be disassociated from low-level stimulus characteristics. In particular, the Wollaston faces that we compared across were composed of identical eye regions and identical head regions, but presented in different combinations that resulted in different perceived directions of gaze. Thus, no differences in sclera shape and position were apparent in our stimuli. By using Wollaston stimuli we were able to test for a direct gaze advantage across stimuli that differed in the perceived direction of gaze (i.e., relying on holistic processing of the face), while matching all low-level morphological features in the eye region. Hence, our finding of a direct gaze advantage during CFS is consistent with studies showing that early fundamental mechanisms involved in the perception and automatic orienting to another’s gaze direction may rely on the holistic representation of gaze direction, rather than the local eye region per se30,36,37,38,39,40,41,62,63.
The detection of eye contact has been proposed to initially recruit a fast-subcortical pathway involving the superior colliculus, amygdala, and pulvinar27,64. This pathway is believed to be hardwired and to operate outside of conscious awareness65,66,67 and hence might underlie the processing of gaze direction during CFS28. While there is evidence that higher cortical areas such as the superior temporal sulcus (STS) carry information about gaze direction that is invariant to head and eye deviations, the contribution of subcortical areas to head and eye integration has been elusive. In the current study, we show evidence suggesting the integration of head and eye information occurs at an unconscious level of processing. Because CFS is thought to disrupt processing in cortex67,68 we therefore speculate that a subcortical locus may carry the integrated signal of head and eye information that leads to the prioritized and rapid detection of direct gaze. However, it should be noted that although our study assumes differences in suppression times to reflect differences in the unconscious processing that our stimuli receive while being suppressed, debates highlight the possibility that such differences may reflect disparities at very early conscious stages of processing41,66. Some b-CFS studies base their conclusions about unconscious processing on a comparison with a non-CFS control condition. In designing our experiment, we did not include such a control as we believe it lacks validity. For instance, it has been shown that such control conditions have rather poor sensitivity for measuring differences between conditions and fail to mimic the rather unique perceptual experience under CFS (see: Stein and Peelen, 2021)69. Namely, these control conditions differ from b-CFS conditions in subjective perceptual experience, target appearance, perceptual uncertainty, and target predictability. In a recent CFS study, Stein and Peelen69 advocate for use of an additional discrimination task to rule out conscious processes as a cause of suppression time differences. Their data showed that despite participants being unable to report the orientation of a detected face, there were differences between upright and inverted faces reflected in suppression times. We did not employ this type of control. However, future extensions of our work with this new detection-discrimination approach to CFS may provide additional support for the integration of head and eye information occurring outside of awareness. Importantly, though, the current results as they stand provide a clear indication that the integration of eyes and head information occurs rapidly and that detection of direct eye gaze (based on the integration of head and eyes) is prioritised by the visual system. This supports other research using the Wollaston illusion that shows a reflexive orienting response to Wollaston faces with direct gaze in infants, suggesting that the mechanism for integrating head and eye information is hardwired and automatic62.
In our study, the use of a calibration task verified that the Wollaston stimuli used in the CFS experiment were consistently perceived as looking in different directions (categorised as either direct or averted) when presented in the absence of a CFS mask. This is an important aspect of our design, as large individual differences in gaze perception have been reported in the literature46. In particular, gaze perception and the automatic orienting to direct gaze has been shown to be altered in conditions such as schizophrenia and autism spectrum disorder10,70,71,72,73. Future research employing our approach may help to elucidate whether these anomalies result from perturbations in the early integration of head and eye information. In addition, our data reveal that gaze direction may be represented holistically at an early preconscious stage of processing. Holistic processing is a distinct feature of facial processing74,75. However, research suggests that the integration of some individual features into a interdependent representation may involve a two-stage process requiring conscious awareness (e.g., internal and external face information76, emotional facial expression and gaze direction77). Thus, future research to determine the type of social information that is integrated rapidly at the initial preconscious stages of visual processing will provide important insight into the mechanisms of holistic processing and social perception.
References
Emery, N. J. The eyes have it: The neuroethology, function and evolution of social gaze. Neurosci. Biobehav. Rev. 24, 581–604 (2000).
Baron-Cohen, S., Campbell, R., Karmiloff-Smith, A., Grant, J. & Walker, J. Are children with autism blind to the mentalistic significance of the eyes?. Br. J. Dev. Psychol. 13, 379–398 (1995).
Frith, C. D. & Frith, U. Implicit and explicit processes in social cognition. Neuron 60, 503–510 (2008).
Hamilton, A. F. D. C. Gazing at me: The importance of social meaning in understanding direct-gaze cues. Philos. Trans. R. Soc. B Biol. Sci. 371, 20150080 (2016).
Kleinke, C. L. Gaze and eye contact: A research review. Psychol. Bull. 100, 78 (1986).
Shepherd, S. V. Following gaze: Gaze-following behavior as a window into social cognition. Front. Integr. Neurosci. 4, 5 (2010).
Brooks, R. & Meltzoff, A. N. Connecting the dots from infancy to childhood: A longitudinal study connecting gaze following, language, and explicit theory of mind. J. Exp. Child Psychol. 130, 67–78 (2015).
Helminen, T. M. et al. Atypical physiological orienting to direct gaze in low-functioning children with autism spectrum disorder. Autism Res. 10, 810–820 (2017).
Brüne, M. “Theory of mind” in schizophrenia: A review of the literature. Schizophr. Bull. 31, 21–42 (2005).
Hooker, C. & Park, S. You must be looking at me: The nature of gaze perception in schizophrenia patients. Cogn. Neuropsychiatry 10, 327–345 (2005).
Carlin, J. D. & Calder, A. J. The neural basis of eye gaze processing. Curr. Opin. Neurobiol. 23, 450–455 (2013).
Clifford, C. W. & Palmer, C. J. Adaptation to the direction of others’ gaze: A review. Front. Psychol. 9, 2165 (2018).
Nummenmaa, L. & Calder, A. J. Neural mechanisms of social attention. Trends Cogn. Sci. 13, 135–143 (2009).
Doi, H. & Shinohara, K. Task-irrelevant direct gaze facilitates visual search for deviant facial expression. Vis. Cogn. 21, 72–98 (2013).
Farroni, T., Csibra, G., Simion, F. & Johnson, M. H. Eye contact detection in humans from birth. Proc. Natl. Acad. Sci. 99, 9602–9605 (2002).
Hood, B., Willen, J. & Driver, J. Gaze perception triggers corresponding shifts of visual attention in young infants. Psychol. Sci. 9, 131–134 (1998).
Kesner, L. et al. Perception of direct vs. averted gaze in portrait paintings: An fMRI and eye-tracking study. Brain Cogn. 125, 88–99 (2018).
Senju, A., Kikuchi, Y., Hasegawa, T., Tojo, Y. & Osanai, H. Is anyone looking at me? Direct gaze detection in children with and without autism. Brain Cogn. 67, 127–139 (2008).
Stein, T., Hebart, M. N. & Sterzer, P. Breaking continuous flash suppression: A new measure of unconscious processing during interocular suppression?. Front. Hum. Neurosci. 5, 167 (2011).
Calder, A. J. et al. Separate coding of different gaze directions in the superior temporal sulcus and inferior parietal lobule. Curr. Biol. 17, 20–25 (2007).
Hietanen, J. K., Leppänen, J. M., Peltola, M. J., Linna-aho, K. & Ruuhiala, H. J. Seeing direct and averted gaze activates the approach–avoidance motivational brain systems. Neuropsychologia 46, 2423–2430 (2008).
Kobayashi, H. & Hashiya, K. The gaze that grooms: Contribution of social factors to the evolution of primate eye morphology. Evol. Hum. Behav. 32, 157–165 (2011).
Akechi, H. et al. Absence of preferential unconscious processing of eye contact in adolescents with autism spectrum disorder. Autism Res. 7, 590–597 (2014).
Chen, Y.-C. & Yeh, S.-L. Look into my eyes and I will see you: Unconscious processing of human gaze. Conscious. Cogn. 21, 1703–1710 (2012).
Seymour, K., Rhodes, G., Stein, T. & Langdon, R. Intact unconscious processing of eye contact in schizophrenia. Schizophr. Res. Cogn. 3, 15–19 (2016).
Yokoyama, T., Noguchi, Y. & Kita, S. Unconscious processing of direct gaze: Evidence from an ERP study. Neuropsychologia 51, 1161–1168 (2013).
Stein, T., Senju, A., Peelen, M. V. & Sterzer, P. Eye contact facilitates awareness of faces during interocular suppression. Cognition 119, 307–311 (2011).
Tsuchiya, N. & Koch, C. Continuous flash suppression reduces negative afterimages. Nat. Neurosci. 8, 1096–1101 (2005).
Langton, S. R., Honeyman, H. & Tessler, E. The influence of head contour and nose angle on the perception of eye-gaze direction. Percept. Psychophys. 66, 752–771 (2004).
Otsuka, Y., Mareschal, I., Calder, A. J. & Clifford, C. W. Dual-route model of the effect of head orientation on perceived gaze direction. J. Exp. Psychol. Hum. Percept. Perform. 40, 1425 (2014).
Van Belle, G., De Graef, P., Verfaillie, K., Rossion, B. & Lefèvre, P. Face inversion impairs holistic perception: Evidence from gaze-contingent stimulation. J. Vis. 10, 10–10 (2010).
Vrancken, L., Germeys, F. & Verfaillie, K. Holistic integration of gaze cues in visual face and body perception: Evidence from the composite design. J. Vis. 17, 24–24 (2017).
Ando, S. Luminance-induced shift in the apparent direction of gaze. Perception 31, 657–674 (2002).
Todorović, D. The effect of face eccentricity on the perception of gaze direction. Perception 38, 109–132 (2009).
Wollaston, W. H. In Abstracts of the Papers Printed in the Philosophical Transactions of the Royal Society of London. 214–215 (The Royal Society London).
Doi, H. & Ueda, K. Searching for a perceived stare in the crowd. Perception 36, 773–780 (2007).
Palmer, C. J. & Clifford, C. W. Adaptation to other people’s eye gaze reflects habituation of high-level perceptual representations. Cognition 180, 82–90 (2018).
Qian, Q., Song, M. & Shinomori, K. Gaze cueing as a function of perceived gaze direction. Jpn. Psychol. Res. 55, 264–272 (2013).
Gobbini, M. I. et al. Prioritized detection of personally familiar faces. PLoS One 8, e66620 (2013).
Colombatto, C., van Buren, B. & Scholl, B. J. Hidden intentions: Visual awareness prioritizes perceived attention even without eyes or faces. Cognition 217, 104901 (2021).
Axelrod, V. & Rees, G. Conscious awareness is required for holistic face processing. Conscious. Cogn. 27, 233–245 (2014).
Stein, T., Sterzer, P. & Peelen, M. V. Privileged detection of conspecifics: Evidence from inversion effects during continuous flash suppression. Cognition 125, 64–79 (2012).
Bach, M. The Freiburg Visual Acuity Test-automatic measurement of visual acuity. Optom. Vis. Sci. 73, 49–53 (1996).
Brainard, D. H. The psychophysics toolbox. Spat. Vis. 10, 433–436 (1997).
Yang, E., Zald, D. H. & Blake, R. Fearful expressions gain preferential access to awareness during continuous flash suppression. Emotion 7, 882 (2007).
Schulze, L., Renneberg, B. & Lobmaier, J. S. Gaze perception in social anxiety and social anxiety disorder. Front. Hum. Neurosci. 7, 872 (2013).
Harbort, J., Witthöft, M., Spiegel, J., Nick, K. & Hecht, H. The widening of the gaze cone in patients with social anxiety disorder and its normalization after CBT. Behav. Res. Ther. 51, 359–367 (2013).
Gamer, M., Hecht, H., Seipp, N. & Hiller, W. Who is looking at me? The cone of gaze widens in social phobia. Cogn. Emot. 25, 756–764 (2011).
Rice, M. L., Leske, D. A., Smestad, C. E. & Holmes, J. M. Results of ocular dominance testing depend on assessment method. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 12, 365–369 (2008).
Meador, K. & Loring, D. (AAN Enterprises, 2016).
Miller, J. Reaction time analysis with outlier exclusion: Bias varies with sample size. Q. J. Exp. Psychol. 43, 907–912 (1991).
Gayet, S., Van der Stigchel, S. & Paffen, C. L. Breaking continuous flash suppression: Competing for consciousness on the pre-semantic battlefield. Front. Psychol. 5, 460 (2014).
Keysers, C., Gazzola, V. & Wagenmakers, E.-J. Using Bayes factor hypothesis testing in neuroscience to establish evidence of absence. Nat. Neurosci. 23, 788–799 (2020).
Gayet, S. & Stein, T. Between-subject variability in the breaking continuous flash suppression paradigm: Potential causes, consequences, and solutions. Front. Psychol. 8, 437 (2017).
Han, S. E. & Alais, D. Strength of continuous flash suppression is optimal when target and masker modulation rates are matched. J. Vis. 18, 3–3 (2018).
Senju, A. & Csibra, G. Gaze following in human infants depends on communicative signals. Curr. Biol. 18, 668–671 (2008).
Farroni, T., Johnson, M. H., Brockbank, M. & Simion, F. Infants’ use of gaze direction to cue attention: The importance of perceived motion. Vis. Cogn. 7, 705–718 (2000).
Ricciardelli, P., Baylis, G. & Driver, J. The positive and negative of human expertise in gaze perception. Cognition 77, B1–B14 (2000).
Lee, D. H., Susskind, J. M. & Anderson, A. K. Social transmission of the sensory benefits of eye widening in fear expressions. Psychol. Sci. 24, 957–965 (2013).
Ricciardelli, P., Betta, E., Pruner, S. & Turatto, M. Is there a direct link between gaze perception and joint attention behaviours? Effects of gaze contrast polarity on oculomotor behaviour. Exp. Brain Res. 194, 347–357 (2009).
West, R. W. Perceived direction of gaze from eyes with dark vs. light irises. Optom. Vis. Sci. 88, 303–311 (2011).
Otsuka, Y., Ichikawa, H., Clifford, C. W., Kanazawa, S. & Yamaguchi, M. K. Wollaston’s effect in infants: Do infants integrate eye and head information in gaze perception?. J. Vis. 16, 4–4 (2016).
Stiel, B., Clifford, C. W. & Mareschal, I. Adaptation to vergent and averted eye gaze. J. Vis. 14, 15–15 (2014).
Senju, A. & Johnson, M. H. The eye contact effect: Mechanisms and development. Trends Cogn. Sci. 13, 127–134 (2009).
Burra, N., Mares, I. & Senju, A. The influence of top-down modulation on the processing of direct gaze. Wiley Interdiscip. Rev. Cogn. Sci. 10, e1500 (2019).
Jiang, Y. & He, S. Cortical responses to invisible faces: Dissociating subsystems for facial-information processing. Curr. Biol. 16, 2023–2029 (2006).
Jiang, Y. et al. Dynamics of processing invisible faces in the brain: Automatic neural encoding of facial expression information. Neuroimage 44, 1171–1177 (2009).
Pasley, B. N., Mayes, L. C. & Schultz, R. T. Subcortical discrimination of unperceived objects during binocular rivalry. Neuron 42, 163–172 (2004).
Stein, T. & Peelen, M. V. Dissociating conscious and unconscious influences on visual detection effects. Nat. Hum. Behav. 5, 612–624 (2021).
Lawson, R. P., Aylward, J., Roiser, J. P. & Rees, G. Adaptation of social and non-social cues to direction in adults with autism spectrum disorder and neurotypical adults with autistic traits. Dev. Cogn. Neurosci. 29, 108–116 (2018).
Pellicano, E., Rhodes, G. & Calder, A. J. Reduced gaze aftereffects are related to difficulties categorising gaze direction in children with autism. Neuropsychologia 51, 1504–1509 (2013).
Rosse, R. B., Kendrick, K., Wyatt, R. J., Isaac, A. & Deutsch, S. I. Gaze discrimination in patients with schizophrenia: preliminary report. Am. J. Psychiatry (1994).
Tso, I. F., Mui, M. L., Taylor, S. F. & Deldin, P. J. Eye-contact perception in schizophrenia: Relationship with symptoms and socioemotional functioning. J. Abnorm. Psychol. 121, 616 (2012).
Rossion, B. Picture-plane inversion leads to qualitative changes of face perception. Acta Physiol. (Oxf.) 128, 274–289 (2008).
Maurer, D., Le Grand, R. & Mondloch, C. J. The many faces of configural processing. Trends Cogn. Sci. 6, 255–260 (2002).
Logan, A. J., Gordon, G. E. & Loffler, G. From individual features to full faces: Combining aspects of face information. J. Vis. 19, 23–23 (2019).
Caruana, N., Inkley, C., El Zein, M. & Seymour, K. No influence of eye gaze on emotional face processing in the absence of conscious awareness. Sci. Rep. 9, 1–8 (2019).
Acknowledgements
We would like to thank all reviewers of this manuscript whose recommended changes helped enhance its quality.
Author information
Authors and Affiliations
Contributions
C.J. and K.S. designed the experiment. C.J. collected the data. C.J. and K.S. analysed the data. C.J. and K.S. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jackson, C.D., Seymour, K.K. Holistic processing of gaze cues during interocular suppression. Sci Rep 12, 7717 (2022). https://doi.org/10.1038/s41598-022-11927-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-11927-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.