Abstract
Rare cases of thrombosis with thrombocytopenia syndrome (TTS) have been reported after AZD1222. Anti-platelet factor-4 (PF4) antibodies were observed in patients following presentation of TTS, however it is unclear if AZD1222 was responsible for inducing production of anti-PF4. Paired samples (baseline and day-15) from a phase 3 trial of AZD1222 vs placebo were analyzed for anti-PF4 levels; 19/1727 (1.1%, AZD1222) vs 7/857 (0.8%, placebo) participants were anti-PF4-IgG-negative at baseline but had moderate Day-15 levels (P = 0.676) and 0/35 and 1/20 (5.0%) had moderate levels at baseline but high Day-15 levels. These data indicate that AZD1222 does not induce a clinically relevant general increase in anti-PF4 IgG.
Similar content being viewed by others
Introduction
Following the utilization of COVID-19 vaccines, rare cases of thrombosis with thrombocytopenia syndrome (TTS) have been reported1,2,3,4. Publications have noted that individuals experiencing TTS after receiving AZD1222 (ChAdOx1 nCoV-19), which has been reported primarily following the first dose and as generally occurring within 2 weeks post vaccination2,5, had elevated levels of antibodies targeting platelet factor 4 (PF4)1,2,3. While these studies point to a strong association between anti-PF4 IgG and TTS, they do not address whether AZD1222 causes a generalized increase in antibody levels post administration. To determine if vaccination with AZD1222 induces an increase in levels of anti-PF4 IgG, we analyzed paired serum samples collected prior to and 15 days after vaccination with AZD1222 or placebo from participants in a multicenter, randomized Phase 3 study6.
Results
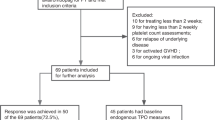
Serum samples were analyzed from 1777 participants who received AZD1222 and 888 who received placebo. Key demographics and clinical characteristics of these participants are summarized in Supplementary Table S1. None of the 2665 participants experienced TTS following administration of vaccine or placebo. Paired serum samples were available from 1762 participants who received AZD1222 and 877 who received placebo.
In the AZD1222 and placebo groups, respectively, 98.0% and 97.7% of baseline serum samples were classified as being negative for anti-PF4 IgG, as were 97.5% and 97.6% of Day 15 serum samples (Table 1). Optical density (OD) assay values were similar between the AZD1222 and placebo group at both baseline (median OD: 0.100 and 0.101, respectively; P = 0.4416) and Day 15 (median OD: 0.105 and 0.099, respectively; P = 0.0567) (Fig. 1). Overall, 96.9% of paired samples in both groups (AZD1222: 1708/1762; placebo: 850/877) were classified as negative at baseline and also negative at Day 15. There was a minimal increase in OD values in the AZD1222 arm from baseline to Day 15 (median OD: 0.100–0.105; P < 0.0001); however, these median values fall below the threshold for positivity, and there was no difference between the AZD1222 and placebo groups in the proportion of individuals shifting from negative at baseline to moderate or high at Day 15 (1.1% vs 0.8%; P = 0.676). At baseline, 2.0% and 2.3% of individuals in the AZD1222 and placebo groups, respectively, had samples positive for anti-PF4 IgG (OD > 0.4); all were classified as moderate. None of these individuals in the AZD1222 group increased to a high level of anti-PF4 IgG at Day 15 compared to 1 in the placebo group, while 10/35 (28.6%) and 6/20 (30.0%), respectively, decreased below the threshold for positivity.
Individual values and summary statistics for optical density from the anti-PF4 IgG assay prior to administration of AZD1222 or placebo and at Day 15. Baseline samples were collected on or before the first dose of AZD1222 or placebo, and Day 15 samples were collected between Day 8 and Day 22 (± 7 day window). The bottom and top edges of the boxes within each collection of optical density data points indicate the first and third quartiles, the lines inside the boxes are the medians, and the markers inside the boxes are the means. Any points > 1.5 × IQR from the box are considered outliers. Whiskers indicate the minimum and maximum optical density values after removing outliers. P-values comparing treatment arms at Baseline and at Day 15 are based on a 2-sample t-test. P-values comparing Baseline and Day 15 within a treatment arm are based on a paired t-test.
Discussion
These data fill a critical gap in our understanding of anti-PF4 IgG in individuals vaccinated with AZD1222. Our analysis demonstrates that AZD1222 did not result in an increased rate of detection of anti-PF4 IgG post-vaccination compared to placebo during the period of highest TTS risk. The majority of Day 15 samples that were classified as moderate were from participants with a moderate level of anti-PF4 IgG at baseline, and the only Day 15 sample classified as high was from a participant in the placebo group with a moderate level at baseline. The increase in median OD in the AZD1222 group from 0.100 at baseline to 0.105 at Day 15, although statistically significant, did not represent a clinically meaningful increase, with the median remaining well below the positive threshold. Our data are supported by findings from a previous analysis of a smaller cohort of patients who received AZD1222 or BNT162b2, which also reported low-titer anti-PF4/polyanion IgG post-vaccination primarily in individuals who were seropositive pre-vaccination10. A similarly low rate of anti-PF4/polyanion IgG post-vaccination was seen in a cross-sectional study of Thai healthcare workers who received AZD1222 or CoronaVac11; however, pre-vaccination data were not available. All positive sera had low titers, and none induced platelet aggregation11.
Additionally, our data indicate a ‘background’ prevalence rate of anti-PF4 IgG of 2.0–2.3%. This reflects the detection rates seen with other commercial immunoassays detecting IgG, IgA, and IgM in a healthy population12. The rate also reflects the low prevalence reported in the general population, which is estimated to be 3–8%13,14 but is dependent on population characteristics, as well as the very low prevalence of anti-PF4 IgG associated with heparin-induced thrombocytopenia in healthy individuals15. Our current understanding of anti-PF4 IgG levels in TTS is based on retrospective analyses following clinical presentation1,2,3,4, which have therefore been unable to assess antibody levels prior to syndrome manifestation. A limitation of our findings is that no TTS events were observed in this clinical trial of AZD12226, and therefore no TTS events are captured by this analysis. The identification of specific markers associated with the development of TTS is challenging due to the extremely rare frequency of TTS5.
In conclusion, these data support that vaccination with AZD1222 does not result in a generalized increase in anti-PF4 IgG levels in vaccine recipients.
Methods
Full study methodology has been reported previously6. Briefly, this phase 3, double-blind, placebo-controlled trial conducted in the United States, Chile, and Peru (ClinicalTrials.gov number NCT04516746; First posted 18/08/2020) assessed the efficacy, immunogenicity, and safety of two intramuscular doses of AZD1222 (N = 21,635) or saline placebo (N = 10,816; 2:1 randomization) given 4 weeks apart in participants aged ≥ 18 years who were at increased risk of SARS-CoV-2 infection. As part of an immunogenicity sub-study, serum samples were obtained from participants on days 1 (prior to first dose) and 15 (post first dose). The trial was conducted in accordance with the principles of the Declaration of Helsinki and the International Council for Harmonisation Good Clinical Practice guidelines. The trial protocol (available with the full text of the primary report6) and amendments were approved by the following independent ethics committees (IECs)/institutional review boards (IRBs): Universidad de Chile – Facultad de Medicina (covering 3 centers in Chile), El Comite Nacional Transitoria de Etica en Investigacion Para la Evaluacion y Supervision Etica de los Ensayos Clinicos de la Enfermedad (covering 3 centers in Peru), WCG IRB (covering 77 centers in the United States), Oregon Health & Science University, Sutter Health Institutional Review Board, The University of Vermont Committees on Human Subjects, The Ohio State Biomedical Sciences Institutional Review Board, and Columbia University. Prior to enrolment, all participants provided either written or secure electronic informed consent. The datasets generated and/or analyzed during the current study are not publicly available due to the study being ongoing at the time of analysis, but are available from the corresponding author on reasonable request.
For the purposes of this exploratory, post-hoc analysis, all participants in the immunogenicity sub-study with available serum samples obtained on both day 1 and day 15 were included, and their paired serum samples were assessed for anti-PF4 IgG using a validated IgG-specific PF4-polyvinylsulfate enzyme-linked immunosorbent assay (Immucor) similar to that previously used1. The assay output is an OD value that correlates with the level of anti-PF4 IgG. Serum samples were classified as being negative (OD ≤ 0.4, the threshold for a positive anti-PF4 IgG reading as defined by the manufacturer) for anti-PF4 IgG, or as having moderate (OD > 0.4 to ≤ 2.0) or high (OD > 2.0) levels of anti-PF4 IgG. This threshold for classification of high anti-PF4 IgG is based on the majority of TTS case reports having an OD of greater than 2.0 for anti-PF4 IgG, as highlighted in the American Society of Hematology TTS guidelines7, and the strong correlation between an OD of > 2.0 and heparin-induced thrombocytopenia8,9. The proportion of participants in whom the baseline (Day 1, pre-vaccination) serum sample was classified as negative and who had a Day 15 sample classified as moderate or high was compared between the AZD1222 and placebo groups using a Fisher’s Exact test; overall differences in median OD values were compared between baseline and Day 15 within each group and between the AZD1222 and placebo groups at baseline and Day 15 using t-tests.
Data availability
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
References
Greinacher, A. et al. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N. Engl. J. Med. 384, 2092–2101. https://doi.org/10.1056/NEJMoa2104840 (2021).
Pavord, S. et al. Clinical features of vaccine-induced immune thrombocytopenia and thrombosis. N. Engl. J. Med. 385, 1680–1689. https://doi.org/10.1056/NEJMoa2109908 (2021).
Schultz, N. H. et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N. Engl. J. Med. 384, 2124–2130. https://doi.org/10.1056/NEJMoa2104882 (2021).
See, I. et al. US Case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, March 2 to April 21, 2021. JAMA 325, 2448–2456. https://doi.org/10.1001/jama.2021.7517 (2021).
Bhuyan, P. et al. Very rare thrombosis with thrombocytopenia after second AZD1222 dose: A global safety database analysis. Lancet 398, 577–578. https://doi.org/10.1016/s0140-6736(21)01693-7 (2021).
Falsey, A. R. et al. Phase 3 safety and efficacy of AZD1222 (ChAdOx1 nCoV-19) Covid-19 vaccine. N. Engl. J. Med. 385, 2348–2360. https://doi.org/10.1056/NEJMoa2105290 (2021).
Bussel, J. B. et al. Thrombosis with thrombocytopenia syndrome (also termed Vaccine-induced Thrombotic Thrombocytopenia). Version 1.6. Last updated 12 August 2021. Available at: https://www.hematology.org/covid-19/vaccine-induced-immune-thrombotic-thrombocytopenia. Last accessed 13 Oct 2021.
Whitlatch, N. L., Kong, D. F., Metjian, A. D., Arepally, G. M. & Ortel, T. L. Validation of the high-dose heparin confirmatory step for the diagnosis of heparin-induced thrombocytopenia. Blood 116, 1761–1766. https://doi.org/10.1182/blood-2010-01-262659 (2010).
Pearson, M. A., Nadeau, C. & Blais, N. Correlation of ELISA optical density with clinical diagnosis of heparin-induced thrombocytopenia: A retrospective study of 104 patients with positive anti-PF4/heparin ELISA. Clin. Appl. Thromb. Hemost. 20, 349–354. https://doi.org/10.1177/1076029613513319 (2014).
Thiele, T. et al. Frequency of positive anti-PF4/polyanion antibody tests after COVID-19 vaccination with ChAdOx1 nCoV-19 and BNT162b2. Blood 138, 299–303. https://doi.org/10.1182/blood.2021012217 (2021).
Noikongdee, P. et al. Prevalence of anti-platelet factor 4/polyanionic antibodies after COVID-19 vaccination with ChAdOx1 nCoV-19 and CoronaVac in Thais. Res. Pract. Thromb. Haemost. 5, e12600. https://doi.org/10.1002/rth2.12600 (2021).
Arepally, G. M. & Hursting, M. J. Platelet factor 4/heparin antibody (IgG/M/A) in healthy subjects: A literature analysis of commercial immunoassay results. J. Thromb. Thrombolysis 26, 55–61. https://doi.org/10.1007/s11239-008-0217-y (2008).
Hursting, M. J. et al. Platelet factor 4/heparin antibodies in blood bank donors. Am. J. Clin. Pathol. 134, 774–780. https://doi.org/10.1309/AJCPG0MNR5NGKNFX (2010).
Greinacher, A. et al. Association of natural anti-platelet factor 4/heparin antibodies with periodontal disease. Blood 118, 1395–1401. https://doi.org/10.1182/blood-2011-03-342857 (2011).
Arepally, G. M. Heparin-induced thrombocytopenia. Blood 129, 2864–2872. https://doi.org/10.1182/blood-2016-11-709873 (2017).
Acknowledgements
This AZD1222 study has been funded in whole or in part by the US Government under Agreement No. W15QKN-20-9-1003, supported by the Office of the Assistant Secretary for Preparedness and Response, Biomedical Advanced Research and Development Authority (contract W15QKN-21-9-1003) and NIAID. The NIAID provides grant funding to the HIV Vaccine Trials Network (HVTN) Leadership and Operations Center (UM1 AI 68614HVTN), the Statistics and Data Management Center (UM1 AI 68635), the HVTN Laboratory Center (UM1 AI 68618), the HIV Prevention Trials Network Leadership and Operations Center (UM1 AI 68619), the AIDS Clinical Trials Group Leadership and Operations Center (UM1 AI 68636), and the Infectious Diseases Clinical Research Consortium leadership group 5 (UM1 AI148684-03). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH). The U.S. government is authorized to reproduce and distribute reprints for governmental purposes notwithstanding any copyright notation thereon. Medical writing and editing support for the development of this manuscript, under the direction and guidance of the authors, was provided by Steve Hill, PhD, of Ashfield MedComms, an Ashfield Health company, in accordance with Good Publication Practice (GPP3) guidelines (Ann Intern Med. 2015;163:461–4). This support was funded by AstraZeneca.
Funding
This article was funded by AstraZeneca, US Government, Agreement No. W15QKN-20-9-1003.
Author information
Authors and Affiliations
Contributions
Conceptualization and design: T.S.C., E.J.K., and S.N. Data acquisition, analysis, and interpretation: All authors. Statistical analysis: H.B. and B.J. Original draft: T.S.C. and E.J.K. Writing- review and editing: All authors. Critical revision of the manuscript for important intellectual content: All authors. Final approval of the completed version: all authors. Responsibility for the integrity of all the data and the accuracy of the data analysis: all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing non-financial interests but the following competing financial interests. TSC, EJK, SN, HB, BJ, and PB are employees of AstraZeneca. TSC, EJK, and SN hold or may hold stock in AstraZeneca. MES receives NIH/NIAID funding for trials of COVID-19 vaccines and for HIV/AIDS Clinical Trials Unit (CTU) (2UM1AI069470), as well as research grants from the Gates Foundation, Sanofi, Janssen, Merck, and Gilead to her institution. ARF receives research grants from Pfizer, Janssen, and Merck Sharpe and Dohme, and is a member of the Data Safety Monitoring Board for Novavax.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cohen, T.S., Kelly, E.J., Nylander, S. et al. Serum levels of anti-PF4 IgG after AZD1222 (ChAdOx1 nCoV-19) vaccination. Sci Rep 12, 7961 (2022). https://doi.org/10.1038/s41598-022-11623-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-11623-9
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.