Abstract
This paper describes the synthesis and evaluation of lead compounds with a new chemical skeleton that is not found in conventional antimicrobial agents. The biologically attractive cyclopentenoid (+)-hygrophorone B12, isolated from the fruiting bodies of Hygrophorus abieticola, and its analogues were synthesized in a longer linear sequence of twelve steps, starting from a cyclopentenone derivative. This synthesis involved the following crucial steps: (i) oximation of a ketone to stabilize the requisite aldehyde to install a side chain and (ii) coupling of an aldehyde with a side chain to assemble the desired hygrophorone. Then, the antimicrobial activity of these hygrophorones towards clinically relevant bacterial pathogens was evaluated. The results showed that hygrophorone B12 and its analogues are especially effective in preventing the proliferation of gram-positive bacteria. In addition, it was found that some structural features such as the presence of the enone moiety as well as the carbon–carbon triple bond on the hydrocarbon chain were pivotal to increase the antimicrobial activity of hygrophorone B. This study is expected to support the development of novel antimicrobial agents by flexibly synthesizing hygrophorone B analogues with a carbon five-membered ring skeleton from the common intermediate.
Similar content being viewed by others
Introduction
Various antibiotics and other antimicrobial agents have been developed and they have saved uncountable numbers of lives. However, the threat of antimicrobial resistance (AMR) has recently arisen and must be addressed urgently1,2. Even though the problem of morbidity and mortality associated with AMR is not new, AMR has recently been increasing at a significant rate due to bacteria that have acquired resistance to multiple groups of antimicrobial agents2. Moreover, the decline in the development and marketing of new antimicrobial agents worsens the situation. O'Neill’s report predicts that with the current increasing trend of AMR, an estimated 10 million lives per year may be lost to AMR-related disease by 20503. To avoid this worst-case scenario, it is necessary to develop new kinds of antimicrobial agents with chemical skeletons and/or antimicrobial mechanisms different from those of conventional drugs.
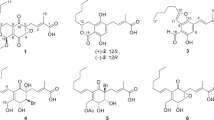
Molecules with a cyclopentenone framework are Michael acceptors for various cellular nucleophiles due to their highly reactive α,β-unsaturated carbonyl moiety4,5. Thus, the five-membered carbon ring framework of cyclopentenone is often used as the core of building blocks to synthesize natural products and related compounds6,7. Additionally, highly oxygenated cyclopentenoids are known to be effective as promising antimicrobials5. Recently, we have reported the synthesis of pentenomycin I (1; Fig. 1), which was isolated from a cultured strain of Streptomyces eurythermus8, and its analogues9. In addition, we tested their antimicrobial activity and determined some of the structural factors that are important for antimicrobial activity. According to the results of this evaluation, their antimicrobial activity was moderate and unsuitable for pharmaceutical lead compounds. Therefore, advancing investigations into highly effective antimicrobial cyclopentenoids would undoubtedly be valuable for pharmaceutical development. Between 2004 and 2017, the group of Arnold has reported the isolation and structural elucidation of new cyclopentenoids, which they named hygrophorones, derived from various Hygrophorus species10,11,12,13,14. Isolated natural products are highly substituted 2-cyclopentenones with two hydroxy groups at asymmetric centers C-4 and C-5 and a hydrocarbon chain that contains an additional hydroxylated asymmetric center bonded at C-5. The structures of representative hygrophorones are shown in Fig. 1. Hygrophorones A–D (2–8) consist of a 2-cyclopenten-1-one skeleton substituted with hydroxy or acetoxy groups at the C-4 and C-5 positions and an oxidized long hydrocarbon chain attached to C-5. The structures and the stereochemistry of hygrophorones have been determined via extensive spectroscopic studies, including 2D NMR spectroscopy experiments. Furthermore, 4,6-diacetylhygrophorone A12 (3) showed potent antimicrobial activity against several bacterial species in the sub- to low-micromolar range (MIC = 0.25–8 µg/mL)15. Interestingly, 3, which contains a long hydrocarbon chain, showed significantly higher antimicrobial activity than 18,9, which possesses an enone structure without an alkyl chain. Therefore, we hypothesize that other hygrophorones such as B type show high antibacterial activity. According to our survey of the literature, antimicrobial susceptibility tests of other hygrophorones toward bacterial-pathogen-caused human infectious diseases have not yet been reported, albeit in 2021 Westermann’s group disclosed the fungicidal activity of hygrophorone B12 (4) and its 6-deoxy analogue against plant pathogens16. Consequently, the development of efficient and flexible methods for the synthesis of the hygrophorone family, and the elucidation of their structure–activity relationship are desirable and useful from the viewpoint of medicinal/pharmaceutical chemistry.
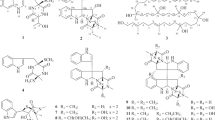
Due to their unique structural features, hygrophorones have been drawing increasing attention and become a target for total synthesis. Numerous efforts have been devoted to the total synthesis of this class of natural products, and so far, three methods have been reported for the total synthesis of hygrophorone-B-type compounds and hygrophorone A12 (2)17,18,19. Westermann et al. reported the asymmetric total synthesis of hygrophorone A12 (2) and B12 (4) using a biomimetic strategy, in which the stereo-controlled dihydroxylation of fatty acid ester 9 is the key step (9 → 10). (+)-2 and (−)-4 were obtained using AD-mix-alpha, and the enantiomers (−)-2 and (+)-4 using AD-mix-beta (Scheme 1a)17. Rao et al. achieved the stereoselective synthesis of (−)-6-epi-hygrophorone B14 (6) from D-mannose (11) using a stereoselective Grignard reaction for the insertion of the hydrocarbon chain, the Bernet–Vasella protocol (11 → 12), and a ring-closing metathesis (Scheme 1b)18. More recently, the enantioselective syntheses of (−)-Hygrophorone A12 (2) and (+)-Hygrophorone B12 (4) from 13 has been achieved by Gholap et al. (Scheme 1c)19. The common intermediate 15 was prepared starting from 13 by a Grignard reaction for the insertion of the carbon chain, followed by a Barton–McCombie reaction (13 → 14), and finally a diastereoselective intramolecular aldol reaction (14 → 15) . The deprotection of acetonide in 15 produced (–)-Hygrophorone A12 (2). In addition, Hygrophorone B12 (4) was obtained by epimerization at C-4 via the Mitsunobu inversion. All strategies are excellent methods for the stereoselective synthesis of the targeted hygrophorones. However, in order to synthesize hygrophorone analogues, an arbitrary carbon chain needs to be present from the beginning, or needs to be inserted at the earliest stage of the synthetic plan. In addition, there are so far no reports on the evaluation of the antimicrobial activity of such B-type hygrophorones with side chains of different length. Therefore, a new modular synthetic strategy for the preparation of a variety of hygrophorone analogues starting from a common intermediate would be useful to investigate the structure–activity relationship for hygrophorones. In this study, we focus on hygrophorone B12 (4) and we describe the enantioselective total synthesis of 4 and its analogues from cyclopentenone, as well as the evaluation of their antimicrobial activity.
Results and discussion
The outline of our synthetic plan is shown in Scheme 2. The most important component of our plan is the use of a key intermediate, i.e., formyl enone 18, which is suitably functionalized and oxidized. The production of a wide variety of hygrophorone analogues from 18 could theoretically be achieved via a convenient and general method. It is assumed that a simple sequence could be proposed to insert any hydrocarbon chains and aromatic groups using organometallic reagents (18 → 17). After the insertion of the hydrocarbon chain, conversion into the hygrophorone skeleton would be accomplished by a stereoselective dihydroxylation followed by the formation of an enone moiety (17 → 16 → 4). The key intermediate 18 would be obtained from optically active cyclopentenone 20 via 19.
The synthesis of key intermediate 18 is shown in Scheme 3. The starting material, cyclopentenone 20, was obtained from D-glucose (21) according to published procedures20,21. First, 22 was synthesized from 20 by the selective protection of hydroxyl group by catalyst-free method using tert-butyldimethylsilylchloride (TBSCl) and triethylamine9, and then 19 was obtained by treatment with triisopropylsilyl trifluoromethanesulfonate (TIPSOTf) and 2,6-lutidine, followed by the regioselective desilylation of primary TBS under mild conditions using scandium (III) triflate as a catalyst (19 → 23). We next investigated the oxidation to obtain the desired formyl enone 18 (see the Table in Scheme 3). We examined oxidation methods to transform the primary hydroxyl group into a formyl group; however, 18 was not obtained under any of the attempted conditions. When the direct oxidation of the C2-hydroxymethyl group of 23 into a formyl group was attempted, the substrate decomposed, maybe due to the instability of the resulting enone with a formyl group at the C-2 position, which is highly electron-deficient. Therefore, it was crucial to control the electron density of the enone moiety by conversion of the ketone into an oxime. The electron-donating features of the oxime are able to reduce the electron deficiency of the enone moiety. O-(tert-butyldimethylsilyl)hydroxylamine (TBSONH2), which enabled easy access to oxime,“from the resulting O-silyl oxime” was used. The cleavage of oxime has been reported under a wide variety of conditions, and we expected that it would enable the conversion of the relatively hard enone–oxime into a ketone22,23,24,25,26,27. So, 23 was converted to 24 using TBSONH2, which was prepared from TBSCl and hydroxylamine28, with an acidic catalyst in the presence of anhydrous magnesium sulfate as water scavenger29. The resulting compound 24 was selectively obtained as E steroisomer avoiding the steric hindrance between the TBSO group and the hydroxymethyl group at the C-2 position of the enone. In the subsequent step, the Dess–Martin oxidation of the crude product 24 gave aldehyde 25 in 79% yield over two steps (Scheme 4). With key intermediate 25 in hand, we proceeded with the synthesis of (+)-4 through the insertion of the hydrocarbon chain followed by the suitable ring modifications to obtain hygrophorone skeleton (Scheme 5). The coupling reaction of 25 with the hydrocarbon chain to construct the desired carbon skeleton of (+)-4 was investigated intensively. Initial attempts with commercially available dodecylmagnesium bromide (C12H25MgBr) in tetrahydrofuran (THF) at − 78 to − 20 °C30 resulted in the formation of 24 as the major product, while compound 26 was barely produced. We assumed that this result is due to the reduction of the β-hydride via a six-membered-ring transition state between the formyl group of 25 and C12H25MgBr as a hydride source, similar to the Meerwein–Ponndorf–Verley (MPV) protocol (for details, see the Supporting Information, Scheme S1)31,32,33,34,35. To test this hypothesis, we used an unsaturated hydrocarbon chain, 1-dodecyne, without a hydride in the beta position. The organolithium obtained in situ from the treatment of 1-dodecyne with n-BuLi was allowed to react with intermediate 25 at − 40 °C to give 27 in moderate yield (57%) without forming 24. Moreover, 27 was obtained in satisfactory yield (92%) as an inseparable diastereomeric mixture (α/β = 1:1) with regard to the C-6 position by using 2-methyl-THF, a less hygroscopic solvent than THF (for details, see the Supporting Information, Table S1). The scandium-catalyzed treatment of 27 under mild reaction conditions removed the TBS group to produce the oxime 28, and a subsequent reductive deoximation by titanium (III) chloride in the presence of ammonium acetate36 gave 29 and epi-29 as a mixture separable via column chromatography on silica gel. Compound 30 was prepared as a single stereoisomer from 29 by treatment with an osmium catalyst and N-methylmorpholine oxide at 70 °C; the formation of the osmate ester intermediate was stereoselective due to the steric hindrance of the TIPS group at the C-4 position. The catalytic hydrogenation of 30 over 10% Pd/C was performed in methanol at room temperature. Finally, the desilylation of the resulting intermediate and the subsequent elimination of the hydroxyl group to construct the enone moiety were efficiently achieved through treatment with 1 M aqueous HCl in ethanol at 90 °C37, affording the targeted (+)-hygrophorone B12 (4). The experimental evidences (1H and 13C NMR spectra and high-resolution mass spectrum) of synthetic (+)-4 were identical to those of natural hygrophorone B12 (4). The optical rotation of synthetic (+)-4 ([α]D25 = + 23.0; c = 0.10 in MeOH) matched that reported for natural 4 ([α]D27 = + 20.7; c = 0.135, MeOH)17. Furthermore, we prepared two analogues of 4 starting from key intermediate 30. Alkyne 31 was synthesized through direct treatment of 30 with 1 M aqueous HCl in ethanol at 90 °C, while the reduced analogue 32 was obtained by the Pd catalyzed hydrogenation of the endocyclic double bond of (+)-4.
The antimicrobial activity of the synthesized (+)-hygrophorone B12 (4) and its analogues 31 and 32 were evaluated to gauge their potency. Antimicrobial susceptibility testing was performed with the broth dilution method according to the Clinical and Laboratory Standards Institute (CLSI) 2020 guidelines9,38. Initially, the compounds were tested with six bacterial species (Staphylococcus aureus, Enterococcus faecium, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Acinetobacter baumannii) that represent the most common pathogens in clinical settings. Gentamicin was used as a positive control in this assay, and the minimum inhibitory concentration (MIC) values are summarized in Table 1. (+)-4 and 31 showed antimicrobial activity toward five of the bacterial species, excluding P. aeruginosa. In particular, they were significantly more effective against E. faecium than gentamicin, while the antimicrobial activity of 32 was remarkably lower against many species. These results indicate that the expression of antimicrobial activity depends on the α,β-unsaturated carbonyl framework in the hygrophorone B class of compounds. Moreover, the antimicrobial activities of (+)-4 and 31 were investigated against six antimicrobial-resistant (AMR) bacteria isolates (methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus faecium, carbapenem-resistant Escherichia coli, carbapenem-resistant Enterobacterales, multidrug-resistant Pseudomonas aeruginosa, and multidrug- resistant Acinetobacter baumannii) (Table 2). Intriguingly, (+)-4 and 31 were notably effective in suppressing the growth of methicillin-resistant S. aureus (MRSA) and vancomycin-resistant E. faecium (VRE). Moreover, 31 with a carbon–carbon triple bond in its hydrocarbon chain suppressed the growth of multidrug-resistant A. baumannii (MDRA) compared to (+)-4. However, these compounds were not effective against carbapenem-resistant Enterobacterales (E. coli or K. pneumoniae) (CRE) or multidrug-resistant P. aeruginosa (MDRP). The aforementioned results show that hygrophorone-B-type compounds including 4 and 31 can be expected to exhibit higher antimicrobial activity against specific bacterial species, especially gram-positive bacteria. Detailed antimicrobial activity data, including MIC values for hygrophorone B12 (4) and its analogues 31/32 against bacterial pathogens causing human infectious diseases, have not been reported previously in the literature, while the MIC value of hygrophorone-A-type compound (3) has been reported elsewhere15. The results of our evaluation suggest that their chemical structure (e.g., the presence of triple bonds or enone moiety) have a substantial influence on the microbial activity of hygrophorones. Therefore, our strategy for a new synthesis of hygrophorone-B-type compounds from key intermediate 25 with a suitable side chain is useful. These findings demonstrated that hygrophorone B is an accessible lead compound for designing novel antimicrobial drugs.
Conclusion
In conclusion, we have achieved the enantioselective total synthesis of (+)-hygrophorone B12 (4) and its analogues 31 and 32 in 4.5–4.8% overall yield over a linear sequence of 10–12 steps starting from cyclopentenone 20, which can be obtained from d-glucose (21). The main advantage of our synthetic method is expected to contribute the provision of a wide variety of hygrophorone B analogues starting from the common key intermediate 25 via the insertion of various hydrocarbon chains or aryl groups. As ongoing research, we are investigating other side chains including aryl groups that can be introduced, and studying toward the synthesis of other analogues. The antimicrobial evaluation of (+)-4 and its analogues revealed their potency, and the structure—activity relationship of hygrophorone-B-type compounds was disclosed. Moreover, the synthesized hygrophorones are highly effective in preventing the proliferation of chemical-sensitive bacteria and AMR bacteria (MRSA, VRE, and MDRA), especially gram-positive bacteria. These results can be expected to be useful for the design and development of new antimicrobial agents. Further studies concerning the synthesis of other hygrophorone B analogues and the evaluation of their antimicrobial activity, as well as an investigation of their side effects on human normal cells, are currently in progress in our group. In the near future, the bacterial intracellular target(s) that interact with hygrophorones will be revealed in detail, and we will provide accurate information on the mechanism of the expression of their activity.
Methods
Instruments
Optical rotations were recorded on an Anton Paar MCP-100. Nuclear magnetic resonance (NMR) spectra were recorded on a Bruker AVANCE–400 III and AVANCE-600 III spectrometer and calibrated using residual undeuterated solvent as an internal reference (CDCl3 at δ 7.26 ppm for 1H, δ 77.16 for 13C NMR). High–resolution mass spectrometry (HR–MS) was performed using a Bruker MicrOTOF–Q II–S1 using electrospray ionization (ESI) technique.
General organic synthetic methods
Reactions were monitored by analytical thin layer chromatography (TLC) carried out on 0.25 mm E. Merck silica gel plates (60 F254). Visualization of the developed chromatogram was performed by UV absorbance and aqueous cerium ammonium molybdate. Flash chromatography was performed on Kanto Chemical silica gel 60 N (spherical, neutral, 40–50 µm) with the indicated solvent systems.
Materials
Compound 20 (> 99% ee) and pyridinium p-toluenesulfonate (PPTS) stored in the freezer were used. trimethylamine (Et3N), 2,6-lutidine, anhydrous magnesium sulfate (MgSO4), ethylenediamine, ammonium acetate (NH4OAc), titanium(III) chloride (20% aqueous solution), potassium osmate(VI) dihydrate (K2OsO2(OH)2), palladium on carbon (10%, Pd/C), ethanol (EtOH), methanol (MeOH) and anhydrous solvents for organic synthesis, including CH2Cl2, tetrahydrofuran (THF) and acetonitrile (MeCN) were purchased from FUJIFILM Wako Pure Chemical Co. Triisopropylsilyl trifluoromethanesulfonate (TIPSOTf), scandium(III) trifluoromethanesulfonate (Sc(OTf)3) and 1-dodecyne were purchased from Tokyo Chemical Industry Co. n-butyllithium solution and methyllithium solution were purchased Kanto Chemical Co. tert-Butyldimethylsilyl chloride (TBSCl), dodecylmagnesium bromide solution (C12H25MgBr), N-methylmorpholine oxide (NMO), Gentamicin sulfate, anhydrous 2-methyl-THF for organic synthesis and phenylmagnesium bromide solution (PhMgBr) were purchased from Sigma-Aldrich Co. Gram–positive and Gram–negative bacterial reference strains, including Stapylococcus aureus ATCC 29,213, Enterococcus faecium ATCC 35,667, Escherichia coli DH5 α, Klebsiella pneumoniae ATCC 10,031, Pseudomonas aeruginosa PAO1, and Acinetobacter baumannii ATCC 17,978, S. aureus MRY04-1385, E. faecium MRY05-0006, E. coli MRY13-0331, K. pneumoniae MRY12-0017, P. aeruginosa MRY09-1249, A. baumannii MRY12-0277 were purchased from the American Type Culture Collection. Silica gel plates (60F–254) for thin layer chromatography were purchased from Merck. Silica gel 60 N (230–400 mesh) for flash chromatography was purchased from Kanto Chemical. All reagents were used without further purification. All reactions were carried out in flame–dried glassware under a nitrogen atmosphere with dry solvents. Unless stated otherwise, commercial grade reagents were used without further purification.
Experimental procedures
Synthesis of 22. TBSCl (5.24 g, 23.2 mmol) and Et3N (6.5 mL, 46.2 mmol) were added to a solution of cyclopentenone 2020,21 (2.97 g, 23.2 mmol) in THF (77 mL). The reaction mixture was stirred at room temperature. After 24 h, the resulting mixture was quenched with saturated aqueous NH4Cl. The resulting mixture was extracted with EtOAc (2 × 100 mL). The combined extracts were washed with brine then dried with MgSO4. Concentration in vacuo afforded a residue, which was purified by column chromatography (hexane/EtOAc 3:1 → 1:1) to give 22 (4.86 g, 86%) as a colorless-pale yellow oil. TLC (Hexane:EtOAc, 1:3 v/v): Rf = 0.73; [α]D20 = + 11.2 (c = 1.0 in CHCl3); 1H NMR (400 MHz, CDCl3): δ 0.080 (s, 3H), 0.085 (s, 3H), 0.92 (s, 9H), 1.91 (d, J = 5.2 Hz, 1H), 2.37 (dd, J = 2.0, 18.8 Hz, 1H), 2.86 (dd, J = 6.0, 18.8 Hz, 1H), 4.37–4.38 (m, 2H), 4.99 (brs, 1H), 7.37–7.38 (m, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ − 5.35, − 5.32, 18.4, 26.0 (3C), 45.8, 58.0, 68.9, 148.4, 155.9, 204.8 ppm; HR–MS (ESI–TOF): m/z calcd. for C12H23O3Si ([M + H]+), 243.1411; found, 243.1415.
Synthesis of 19. TIPSOTf (1.80 mL, 6.72 mmol) was added to a stirred solution of 22 (1.36 g, 5.61 mmol) and 2,6-lutidine (0.97 mL, 8.42 mmol) in CH2Cl2 (19 mL) at room temperature under argon atmosphere. After 1.5 h, the resulting mixture was quenched with saturated aqueous NH4Cl. The resulting mixture was extracted with EtOAc (2 × 100 mL), and the extracts were washed with brine then dried with MgSO4. Concentration under vacuum afforded a residue, which was purified by column chromatography (hexane/EtOAc 25:1 → 15:1) to give 19 (2.23 g, 99%) as a colorless oil. TLC (Hexane:EtOAc, 2:1 v/v): Rf = 0.90; [α]D20 = + 17.5 (c = 1.0 in CHCl3); 1H NMR (400 MHz, CDCl3) δ 0.078 (s, 3H), 0.085 (s, 3H), 0.92 (s, 9H), 1.06–1.12 (m, 21H), 2.39 (dd, J = 2.0, 18.2 Hz, 1H), 2.81 (dd, J = 5.8, 18.2 Hz, 1H), 4.37 (t, J = 2.0 Hz, 2H), 4.99–5.03 (m, 2H), 7.32 (q, J = 2.2, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ − 5.31, − 5.30, 12.2 (3C), 18.04 (4C), 18.05 (3C), 18.4 (2C), 25.9, 46.8, 58.0, 69.2, 147.2, 157.2, 205.1 ppm; HR–MS (ESI–TOF) : m/z C21H43O3Si2 ([M + H]+) calcd. for 399.2745, found 399.2741.
Synthesis of 23. Sc(OTf)3 (173 mg, 0.352 mmol) and H2O (2.5 mL, 140 mmol) were added to a stirred solution of 19 (2.80 g, 7.04 mmol) in MeCN (70 mL) at room temperature, and the mixture was stirred at same temperature. After 5.5 h, the reaction mixture was quenched with saturated aqueous NaHCO3. The resulting mixture was extracted with EtOAc (2 × 100 mL). The combined extracts were washed with brine then dried with MgSO4. Concentration under vacuum afforded a residue, which was purified by column chromatography (hexane/EtOAc 4:1 → 2:1) to give 23 (1.53 g, 77%) as a colorless oil. TLC (Hexane:EtOAc, 4:1 v/v): Rf = 0.30; [α]D20 = + 29.7 (c = 1.0 in CHCl3); 1H NMR (400 MHz, CDCl3) δ 1.05–1.16 (m, 21H), 2.11–2.15 (m, 1H), 2.39 (dd, J = 2.1, 18.3 Hz, 1H), 2.83 (dd, J = 6.0, 18.6 Hz, 1H), 4.34–4.45 (m, 2H), 5.02–5.05 (m, 1H), 7.31–7.32 (m, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ 12.2 (3C), 18.03 (4C), 18.06 (2C), 46.5, 57.6, 69.3, 145.5, 157.7, 206.1 ppm; HR–MS (ESI–TOF) : m/z C15H28O3SiNa ([M + Na]+) calcd. for 307.1700, found 307.1697.
Synthesis of 25. TBSONH228 (775 mg, 5.26 mmol) and MgSO4 (169 mg, 1.40 mmol) were added to a stirred solution of 23 (1.0 g, 3.51 mmol) and PPTS (88 mg, 0.351 mmol) in toluene (3.5 mL) at room temperature, and the mixture was stirred at 100 °C. After 1.5 h, the reaction mixture was quenched with saturated aqueous NaHCO3. The resulting mixture was extracted with EtOAc (2 × 15 mL). The combined extracts were washed with brine then dried with MgSO4. Concentration under vacuum afforded a crude residue 24 (1.98 g), which was immediately used without any purification. crude residue 24: 1H NMR (400 MHz, CDCl3) δ 0.15 (d, J = 13.5 Hz, 3H), 0.18 (d, J = 12.9 Hz, 3H), 0.93 (s, 9H), 1.07–1.14 (m, 21H), 2.51 (dd, J = 2.2, 18.4 Hz, 1H), 2.73 (brs,1H), 3.15 (dd, J = 6.5, 18.4 Hz, 1H), 4.37 (dd, J = 6.5, 12.7 Hz, 1H), 4.45 (dd, J = 5.2, 14.2 Hz, 1H), 4.95–4.98 (1H, m), 6.30–6.31 (m, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ − 5.1 (2C), 12.2 (3C), 18.1 (2C), 18.4 (4C), 26.2 (4C), 37.4, 59.2, 72.6, 141.8, 141.9, 167.3 ppm.
Dess-Martin periodinane (DMP) (1.78 g, 4.21 mmol) was added to a solution of a crude residue 24 in CH2Cl2 (18 mL) at room temperature, and the mixture was stirred at same temperature. After 0.5 h, the reaction mixture was quenched with aqueous NaHCO3 and Na2S2O3. The resulting mixture was extracted with CHCl3 (2 × 30 mL). The combined extracts were washed with brine then dried with MgSO4. Concentration under vacuum afforded a residue, which was purified by column chromatography (hexane/EtOAc 30:1) to give 25 (1.14 g, 79%, 2 steps) as a pale-yellow oil. TLC (Hexane:EtOAc, 6:1 v/v): Rf = 0.65; [α]D20 = + 113.1 (c = 0.51 in CHCl3); 1H NMR (400 MHz, CDCl3) δ 0.190 (s, 3H), 0.195 (s, 3H), 0.95 (s, 9H), 1.05–1.17 (m, 21H), 2.64 (dd, J = 2.9, 18.3 Hz, 1H), 3.26 (dd, J = 6.9, 18.5 Hz, 1H), 5.04–5.07 (m, 1H), 7.12–7.13 (d, J = 2.9 Hz 1H), 10.0 (s, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ − 5.1 (2C), 12.2 (3C), 18.1 (4C), 18.4 (2C), 26.2 (4C), 37.6, 72.3, 138.9, 152.1, 163.8, 188.0 ppm; HR–MS (ESI–TOF) : m/z C21H42O3NSi2 ([M + H]+) calcd. for 412.2698, found 412.2703.
Synthesis of 27. n-BuLi (1.59 M in n-hexane, 1.14 mL, 1.82 mmol) was dropwised to a solution of 1-dodecyne (0.39 mL, 1.82 mmol) in 2-methyl-THF (6 mL) at − 20 °C under argon atmosphere, and the mixture was stirred at same temperature. After 0.5 h, a solution of aldehyde 25 (500 mg, 1.21 mmol) in 2-methyl-THF (4 mL) was added to the resulting solution, and the mixture was stirred further an hour at same temperature. the reaction mixture was quenched with saturated aqueous NH4Cl. The resulting mixture was extracted with EtOAc (2 × 20 mL). The combined extracts were washed with brine then dried with MgSO4. Concentration under vacuum afforded a residue, which was purified by column chromatography (hexane/EtOAc 40:1) to give 27 (644 mg, 92%) as a pale-yellow syrup. TLC (Hexane:EtOAc, 6:1 v/v): Rf = 0.65; 1H NMR (400 MHz, CDCl3) δ 0.168–0.17 (6H), 0.195 (s, 3H), 0.87 (t, J = 6.9 Hz, 3H), 0.93–0.94 (9H), 1.05–1.14 (m, 21H), 1.26–1.43 (m, 14H), 1.48–1.51 (m, 2H), 2.21–2.28 (m, 2H), 2.54 (dd, J = 2.2, 18.4 Hz, 1H), 3.15–3.22 (m, 1H), 3.63 (d, J = 5.3 Hz, 0.5H), 3.82 (d, J = 5.3 Hz, 0.5H), 4.94–4.98 (m, 1H), 5.19–5.20 (m, 0.5H), 5.28–5.29 (m, 0.5H), 6.45–6.46 (m, 0.5H), 6.49–6.50 (m, 0.5H) ppm; 13C NMR (100 MHz, CDCl3) δ − 5.2, 12.1, 12.2, 14.3, 18.1, 18.4, 18.89, 18.9, 22.8, 26.18, 22.19, 28.7, 29.1, 29.3, 29.5, 29.7, 29.8, 32.0, 37.7, 37.8, 59.5, 59.6, 72.21, 72.24, 77.7, 77.9, 86.9, 87.0, 141.7, 141.8, 143.2, 143.6, 166.2, 166.5 ppm; HR–MS (ESI–TOF) : m/z C33H64O3NSi2 ([M + H]+) calcd. for 578.4419, found 578.4416.
Synthesis of 28. Sc(OTf)3 (12.8 mg, 0.0259 mmol) and H2O (0.19 mL, 10.4 mmol) were added to a stirred solution of 27 (300 mg, 0.519 mmol) in MeCN (5.2 mL) at room temperature, and the mixture was stirred at same temperature. After an hour, the reaction mixture was quenched with saturated aqueous NaHCO3. The resulting mixture was extracted with CHCl3 (2 × 20 mL). The combined extracts were washed with brine then dried with MgSO4. Concentration under vacuum afforded a residue, which was purified by column chromatography (hexane/EtOAc 6:1 → 4:1) to give 28 (217 mg, 91%) as a pale-yellow syrup. TLC (Hexane:EtOAc, 4:1 v/v): Rf = 0.45; 1H NMR (400 MHz, CDCl3) δ 0.88 (t, J = 7.0 Hz, 3H), 1.05–1.17 (m, 21H), 1.22–1.39 (m, 14H), 1.49–1.54 (m, 2H), 2.23–2.28 (m, 2H), 2.59 (dd, J = 2.3, 18.3 Hz, 1H), 3.15 (brs, 0.5H), 3.18–3.25 (m, 1H), 3.36 (brs, 0.5H), 4.34–4.45 (m, 2H), 4.97–5.02 (m, 1H), 5.20 (s, 0.5H), 5.27 (s, 0.5H), 6.53–6.54 (m, 0.5H), 6.55–6.56 (m 0.5H), 7.18 (brs, 0.5H), 7.22 (brs, 0.5H) ppm; 13C NMR (100 Mz, CDCl3) δ 12.2, 14.3, 18.1, 18.9, 19.0, 22.8, 28.7, 29.1, 29.3, 29.5, 29.7, 29.8, 32.0, 37.3, 58.7, 58.9, 72.2, 72.3, 77.7, 77.9, 87.3, 141.5, 141.6, 162.6, 162.9 ppm; HR–MS (ESI–TOF) : m/z C27H50O3NSi ([M + H]+) calcd. for 464.3554, found 464.3547.
Synthesis of 29/epi-29. A solution of NH4OAc (830 mg, 10.8 mmol) in H2O (1.5 mL) and TiCl3 (20% aq., 2.66 mL, 3.45 mmol) were added to a stirred solution of 28 (200 mg, 0.431 mmol) in THF (5.1 mL) at room temperature, and the mixture was stirred at 60 °C. After 30 min, the reaction mixture was quenched with H2O. The resulting mixture was extracted with EtOAc (2 × 20 mL). The combined extracts were washed with saturated aqueous NaHCO3 and brine then dried with MgSO4. Concentration under vacuum afforded a residue, which was purified by column chromatography (hexane/EtOAc 13:1 → 10:1) to give 29 (39.9 mg, 41%) and epi-29 (32.8 mg, 34%) as a pale-yellow viscous oil.
29; TLC (Hexane:EtOAc, 3:1 v/v): Rf = 0.55; [α]D20 = + 12.0 (c = 1.0 in CHCl3); 1H NMR (400 MHz, CDCl3) δ 0.89 (t, J = 6.8 Hz, 3H), 1.05–1.19 (m, 21H), 1.27–1.40 (m, 14H), 1.50–1.57 (m, 2H), 2.26 (td, J = 2.0, 7.1 Hz, 2H), 2.46 (dd, J = 2.1, 18.3, 1H), 2.85–2.91 (m, 2H), 3.36 (brs, 0.5H), 5.03–5.06 (m, 1H), 5.21–5.22 (m, 1H), 7.43 (q, J = 3.4 Hz, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ 12.2, 14.3, 18.02, 18.05, 18.9, 22.8, 28.6, 29.0, 29.3, 29.4, 29.6, 29.7, 32.0, 46.8, 57.8, 68.8, 87.7, 145.4, 168.6, 204.9 ppm; HR–MS (ESI–TOF) : m/z C27H49O3Si ([M + H]+) calcd. for 449.3445, found 449.3441.
epi-29; TLC (Hexane:EtOAc, 3:1 v/v): Rf = 0.45; [α]D20 = + 25.6 (c = 1.0 in CHCl3); 1H NMR (400 MHz, CDCl3) δ 0.87 (t, J = 7.0 Hz, 3H), 1.05–1.18 (m, 21H), 1.26–1.39 (m, 14H), 1.48–1.54 (m, 2H), 2.25 (dt, J = 2.0, 7.1 Hz, 2H), 2.43 (dd, J = 2.1, 18.3 Hz, 1H), 2.80 (dd, J = 5.8, 18.3 Hz, 1H), 3.05 (d, J = 4.3 Hz, 1H), 5.02–5.05 (m, 1H), 5.26 (brs, 1H), 7.43 (q, J = 3.5 Hz, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ 12.1, 14.3, 18.02, 18.04, 18.9, 22.8, 28.6, 29.0, 29.3, 29.5, 29.7, 29.8, 32.0, 46.8, 58.1, 68.9, 87.7, 145.1, 168.9, 205.4 ppm; HR–MS (ESI–TOF) : m/z C27H48O3SiNa ([M + Na]+) calcd. for 471.3265, found 471.3251.
Synthesis of 30. K2OsO2(OH)4 (2.3 mg, 6.13 µmol) and NMO (42.8 mg, 0.306 mmol) were added to a stirred solution of 29 (55 mg, 0.122 mmol) in THF–H2O (2.5 mL, 10:1) at room temperature, and the mixture was stirred at 40 °C. After 3.5 h, the reaction mixture was quenched with aqueous Na2S2O3 (10%). The resulting mixture was extracted with EtOAc (2 × 15 mL). The combined extracts were washed with brine (20 mL) then dried with MgSO4. Concentration under vacuum afforded a residue, which was purified by column chromatography (hexane/EtOAc 4:1) to give 30 (24.3 mg, 41%) as a pale-yellow oil. TLC (Hexane:EtOAc, 2:1 v/v): Rf = 0.40; [α]D20 = − 39.0 (c = 0.10 in CHCl3); 1H NMR (400 MHz, CDCl3) δ 0.88 (t, J = 7.1 Hz, 3H), 1.04–1.20 (m, 21H), 1.22–1.37 (m, 14H), 1.46–1.52 (m, 2H), 2.21 (dt, J = 2.0, 7.1 Hz, 2H), 2.44 (ddd, J = 1.0, 3.7, 20.6 Hz, 1H), 2.58 (brs, 1H), 2.85 (dd, J = 7.4, 19.6 Hz, 1H), 3.34 (brs, 1H), 3.72 (d, J = 10.6 Hz, 1H), 4.35 (d, J = 1.9 Hz), 1H), 4.45–4.48 (m, 1H), 4.55 (d, J = 9.8 Hz, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ 12.0, 14.3, 179, 18.9, 22.8, 28.6, 29.0, 29.3, 29.5, 29.6, 29.7, 32.0, 44.5, 65.0, 71.5, 81.5, 89.3, 214.2 ppm; HR–MS (ESI–TOF) : m/z C27H49O5Si− ([M-H]−) calcd. for 481.3355, found 481.3346.
Synthesis of (+)-Hygrophorone B12 (4). 10% Pd/C (1.7 mg, 10 w/w%) were added to a solution of 30 (16.8 mg, 0.0348 mmol) in MeOH (1.7 mL), and the reaction mixture was stirred under hydrogen atmosphere (balloon). After 5.5 h, the reaction mixture was filtered through a pad of celite and concentrated under reduced pressure. An obtained crude product (16.7 mg) which was used without any purification was dissolved in EtOH (1.2 mL). 1 M HCl (0.6 mL) was added to the stirred solution at room temperature, and the mixture was stirred at 90 °C. After 30 min, the reaction mixture was quenched with saturated aqueous NaHCO3. The resulting mixture was extracted with EtOAc (2 × 15 mL). The combined extracts were washed with brine (20 mL) then dried with MgSO4. Concentration under vacuum afforded a residue, which was purified by column chromatography (hexane/EtOAc 1:1) to give (+)-hygrophorone B12 (4) (6.7 mg, 63%, 2 steps) as a white solid. TLC (Hexane:EtOAc, 1:1 v/v): Rf = 0.30; [α]D25 = + 23.0 (c = 0.10 in MeOH), {ref.17 [α]D27 = + 20.7 (c = 0.135, MeOH)}, 1H and 13C NMR, and MS spectra were identical to those of natural ( +)-hygrophorone B12; 1H NMR (400 MHz, CDCl3) δ 0.88 (t, J = 6.9 Hz, 3H), 1.25–1.36 (m, 20H), 1.52–1.62 (m, 2H), 2.07 (d, J = 8.3, 1H), 2.94 (d, J = 7.3, 1H), 3.58 (s, 1H), 3.77 (t, J = 9.6 Hz, 1H), 4.72 (ddd, J = 1.4, 2.2, 7.3 Hz, 1H), 6.30 (dd, J = 1.3, 6.1 Hz, 1H), 7.64 (dd, J = 2.3, 6.1 Hz, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ 14.3, 22.8, 26.3, 29.5, 29.6, 29.67, 29.7, 29.77, 29.8, 31.4, 32.1, 71.6, 73.5, 76.0, 133.7, 163.7, 207.5 ppm; HR–MS (ESI–TOF) : m/z C18H31O4− ([M-H]−) calcd. for 311.2228, found 311.2219.
Synthesis of 31. 1 M aqueous HCl (0.75 mL) was added to a stirred solution of 30 (11.4 mg, 0.0236 mmol) in ethanol (1.5 mL) at room temperature, and the mixture was stirred at 90 °C. After 30 min, the reaction mixture was quenched with saturated aqueous NaHCO3. The resulting mixture was extracted with EtOAc (2 × 15 mL). The combined extracts were washed with brine (20 mL) then dried with MgSO4. Concentration under vacuum afforded a residue, which was purified by column chromatography (hexane/EtOAc 4:3) to give 31 (6.1 mg, 84%) as a colorless amorphous. TLC (Hexane:EtOAc, 1:1 v/v): Rf = 0.2; [α]D25 = − 13.0 (c = 0.10 in CHCl3); 1H NMR (400 MHz, CDCl3) δ 0.88 (t, J = 7.0 Hz, 3H), 1.25–1.31 (m, 15H), 1.41–1.46 (m, 2H), 2.16 (dt, J = 2.0, 7.1 Hz, 1H), 2.88 (d, J = 7.9, 1H), 2.91 (d, J = 8.8, 1H), 3.83 (s, 1H), 4.55 (dt, J = 1.9, 8.8 Hz, 1H), 4.85 (dt, J = 1.7, 7.6 Hz, 1H), 6.31 (dd, J = 1.5, 6.1 Hz, 1H), 7.64 (dd, J = 2.2, 6.1 Hz, 1H) ppm; 13C NMR (100 MHz, CDCl3) δ 14.3, 18.7, 22.8, 28.5, 28.9, 29.2, 29.5, 29.6, 29.7, 32.0, 64.9, 71.9, 75.0, 76.0, 89.5, 133.4, 163.9, 206.1 ppm; HR–MS (ESI–TOF) : m/z C18H27O4− ([M-H]−) calcd. for 307.1914, found 307.1915.
Synthesis of 32. 10% Pd/C (0.27 mg, 10 w/w%) were added to a solution of (+)-4 (2.7 mg, 8.64 µmol) in MeOH (1.0 mL), and the reaction mixture was stirred under hydrogen atmosphere (balloon). After an hour, the reaction mixture was filtered through a pad of celite and concentrated under reduced pressure. The residue was purified by column chromatography (hexane/EtOAc 1:1) to give to 32 (2.7 mg, 99%) as a white solid. TLC (Hexane:EtOAc, 1:3 v/v): Rf. = 0.6; [α]D25 = − 26.0 (c = 0.10 in CHCl3); 1H NMR (400 MHz, CDCl3) δ 0.88 (t, J = 6.8 Hz, 3H), 1.25–1.42 (m, 21H), 1.51–1.53 (m, 1H), 1.87 (d, J = 9.7 Hz, 1H), 2.04–2.12 (m, 1H), 2.23–2.54 (m, 4H), 3.13 (s, 1H), 3.60–3.65 (m, 1H), 4.40–4.41 (m, 1H) ppm; 13C NMR (150 MHz, CDCl3) δ 14.3, 22.8, 25.4, 26.0, 29.5, 29.56, 29.66, 29.71, 29.77 (2C), 29.8, 31.8, 32.1, 32.7, 71.3, 72.0, 83.4, 217.3 ppm; HR–MS (ESI–TOF) : m/z C18H33O4− ([M-H]−) calcd. for 313.2384, found 313.2354.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Fischbach, M. A. & Walsh, C. T. Antibiotics for emerging pathogens. Science 325, 1089–1093 (2009).
Zhu, Y.-G. et al. Continental-scale pollution of estuaries with antibiotic resistance genes. Nat. Microbiol. 2, 16270 (2017).
O’Neill, J. Tackling drug-resistant infections globally: Final report and recommendations. Rev. Antimicrob Resist. 2016, 1–84 (2016).
Bickley, J. F. et al. Reactions of some cyclopentenones with selected cysteine derivatives and biological activities of the product thioethers. Bioorg. Med. Chem. 12, 3221–3227 (2004).
Arjona, O., Gómez, A. M., López, J. C. & Plumet, J. Synthesis and conformational and biological aspects of carbasugars. Chem. Rev. 107, 1919–2036 (2007).
Roche, S. P. & Aitken, D. J. Chemistry of 4-Hydroxy-2-cyclopentenone Derivatives. Eur. J. Org. Chem. 2010, 5339–5358 (2010).
Simeonov, S. P., Nunes, J. P. M., Guerra, K., Kurteva, V. B. & Afonso, C. A. M. Synthesis of chiral cyclopentenones. Chem. Rev. 116, 5744–5893 (2016).
Umino, K. et al. Studies on pentenomycins. I. J. Antibiot. 26, 506–512 (1973).
Kamishima, T. et al. A facile synthesis of (+)/(−)-pentenomycin I and analogs, and their antimicrobial evaluation. Tetrahedron Lett. 60, 1375–1378 (2019).
Lübken, T., Schmidt, J., Porzel, A., Arnold, N. & Wessjohann, L. Hygrophorones A-G: Fungicidal cyclopentenones from Hygrophorus species (Basidiomycetes). Phytochemistry 65, 1061–1071 (2004).
Otto, A. et al. Structural and stereochemical elucidation of new hygrophorones from Hygrophorus abieticola (Basidiomycetes). Tetrahedron 73, 1682–1690 (2017).
Lübken, T., Arnold, N., Wessjohann, L., Böttcher, C. & Schmidt, J. Analysis of fungal cyclopentenone derivatives from Hygrophorus spp. by liquid chromatography/electrospray-tandem mass spectrometry. J. Mass Spectrom. 41, 361–371 (2006).
Teichert, A. et al. Unusual bioactive 4-oxo-2-alkenoic fatty acids from Hygrophorus eburneus. Z. Naturforsch. 60b, 25–32 (2005).
Otto, A. et al. Structure and absolute configuration of pseudohygrophorones A12 and B12, alkyl cyclohexenone derivatives from Hygrophorus abieticola (Basidiomycetes). J. Nat. Prod. 79, 74–80 (2016).
PhD thesis, Lübken, T. Martin-Luther-Universität Halle-Wittenberg, Hygrophorone Neue antifungische Cyclopentenonderivate aus Hygrophorus-Arten (Basidiomycetes). (2006).
Ditfe, T., Bette, E., Sultani, H. N., Otto, A., Wessjohann, L. A., Arnold, N. & Westermann, B. Synthesis and biological evaluation of highly potent fungicidal deoxy-hygrophorones. Eur. J. Org. Chem. 2021, 3827–3836 (2021).
Bette, E., Otto, A., Dräger, T., Merzweiler, K., Arnold, N., Wessjohann, L. & Westermann, B. Isolation and asymmetric total synthesis of fungal secondary metabolite hygrophorone B12. Eur. J. Org. Chem. 2015, 2357–2365 (2015).
Surender, B., Vinaykumar, A., Kumar, B. S., Vemulapalli, S. P. B. & Rao, B. V. Stereoselective total synthesis of (–)-6-epi-hygrophorone B14 and (–)-4-epi-hygrophorone D14. ChemistrySelect 3, 9596–9599 (2018).
Das, S., Dalal, A. & Gholap, S. L. Stereoselective total syntheses of (−)-hygrophorone A12, 4-O-acetyl-hygrophorone A12 and (+)-hygrophorone B12. Org. Biomol. Chem. 19, 1100–1108 (2021).
Kamishima, T., Nonaka, T., Watanabe, T., Koseki, Y. & Kasai, H. One-step conversion to a disubstituted cyclopentenone from 2-deoxy-D-glucose and application to synthesis of prostaglandin E1 methyl ester. Bull. Chem. Soc. Jpn. 91, 1691–1696 (2018).
Koseki, Y., Watanabe, T., Kamishima, T., Kwon, E. & Kasai, H. Formation of five-membered carbocycles from D-glucose: A concise synthesis of 4-hydroxy-2-(hydroxymethyl)cyclopentenone. Bull. Chem. Soc. Jpn. 92, 1324–1328 (2019).
Corey, E. J., Niimura, K., Konishi, Y., Hashimoto, S. & Hamada, Y. A new synthetic route to prostaglandins. Tetrahedron Lett. 27, 2199–2202 (1986).
Timms, G. H. & Wildsmith, E. The reduction of oximes with tervalent titanium, a mild deoximation procedure and the partial synthesis of erythromycylamine. Tetrahedron Lett. 12, 195–198 (1971).
Shaabani, A., Hezarkhania, Z. & Badali, E. Wool supported manganese dioxide nano-scale dispersion: A biopolymer based catalyst for the aerobic oxidation of organic compounds. RSC Adv. 5, 61759–61767 (2015).
Jie, Z., Rammoorty, V. & Fischer, B. Diethyl chlorophosphite: A versatile reagent. J. Org. Chem. 67, 711–719 (2002).
DePuy, C. H. & Ponder, B. W. Levulinic acid as a reagent for the hydrolysis of oximes and 2,4-dinitrophenylhydrazones. J. Am. Chem. Soc. 81, 4629–4631 (1959).
Yamaguchi, J. et al. Concise enantio- and diastereoselective total syntheses of fumagillol, RK-805, FR65814, ovalicin, and 5-demethylovalicin. Angew. Chem. Int. Ed. 45, 789–793 (2006).
Hughes, J. M. E. & Gleason, J. L. A concise enantioselective total synthesis of (−)-Virosaine|A. Angew. Chem. Int. Ed. 56, 10830–10834 (2017).
Shibata, N. et al. Thiyl radical-mediated cyclization of ω-alkynyl O-tert-butyldiphenylsilyloximes. Org. Biomol. Chem. 15, 3025–3034 (2017).
Wang, Z.-M., Qian, X.-H. & Zhou, W.-S. Stereoselective synthesis of (-)-(5R,6S)-6-acetoxy-5-hexadecanolide, the mosquito oviposition attractant pheromone. Tetrahedron 46, 1191–1198 (1990).
Miller, J., Gregoriou, G. & Mosher, H. S. Relative rates of Grignard addition and reduction reactions1-3. J. Am. Chem. Soc. 83, 3966–3971 (1961).
Rachwal, S., Kawalek, B., Gorecki, T. & Milart, P. Spiroferrocenophanes II. Grignard addition and reduction reactions of spiro[5]ferrocenophane-3,1’-cyclohexane-1,5-dione. J. Organomet. Chem. 384, 165–179 (1990).
Meerwein, H. & Schmidt, R. Ein neues Verfahren zur Reduktion von Aldehyden und Ketonen. Justus Liebigs Ann. Chem. 444, 221–238 (1925).
Ponndorf, W. D. reversible austausch der oxydationsstufen zwischen aldehyden oder ketonen einerseits und primären oder sekundären alkoholen anderseits. Angew. Chem. 39, 138–143 (1926).
Verley, A. Exchange of functional groups between two molecules. Bull. Soc. Chim. Fr. 37, 537–542 (1925).
Baraldi, P. G. et al. Stereospecific nitromethane conjugate addition to 4-oxygenated-2-substituted-cyclo pent-2-enones: A simple approach to prostaglandins. Tetrahedron 43, 4669–4678 (1987).
Cunico, R. F. & Bedell, L. The triisopropylsilyl group as a hydroxyl-protecting function. J. Org. Chem. 45, 4797–4798 (1980).
CLSI. Performance Standards for Antimicrobial Susceptibility Testing. 30th ed. CLSI guideline M100 (2020).
Acknowledgements
We would like to thank Emeritus Profs. Kunio Arai and Hachiro Nakanishi (Tohoku University) as well as Dr. Jun Hasegawa and Mr. Toshiaki Tanaka (Genesis Research Institute Inc.), who supported our research. This work was performed under the Cooperative Research Program “Network Joint Research Center for Materials and Devices, Grants for Research Program on Emerging, the Research Program of “Dynamic Alliance for Open Innovation Bridging Human, Environment and Materials” in “Network Joint Research Center for Materials and Devices” and “Re-emerging Infectious Diseases” (JP21fk0108093, JP21fk0108139, JP21fk0108133, JP21wm0325003, JP21wm0325022, JP21wm0225004, JP21wm0225008, and JP21gm1610003 to M.S.) from the Japan Agency for Medical Research and Development (AMED).
Author information
Authors and Affiliations
Contributions
T. K. and H. K. directed the research. T. K., T. N., H. N. and H. H. conceived the idea and designed the experiments. T. K. and K. N. synthesized compounds. T. K. and Y. K. analyzed NMR and MASS data. M. S. tested antimicrobial evaluation. T. K. wrote the main manuscript and supporting information with comments from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kamishima, T., Suzuki, M., Narita, K. et al. Total synthesis and antimicrobial evaluation of (+)-hygrophorone B12 and its analogues. Sci Rep 12, 7471 (2022). https://doi.org/10.1038/s41598-022-11608-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-11608-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.