Abstract
Capture-mark-recapture/resight (CMR) methods are used for survival-rate studies, yet are often limited by small sample sizes. Advances in passive integrated transponder (PIT) technology have enabled passive detection or ‘resight’ of marked individuals using large antennas with greater read-ranges than previously possible. We used passively-detected resight data and CMR models to study survival rates of the southern bent-winged bat Miniopterus orianae bassanii, a critically endangered, cave-dwelling bat. Over three years, we used PIT-tagging to monitor 2966 individuals at the species’ largest breeding aggregation, using daily detection data (> 1.6 million detections) to estimate seasonal survival probabilities, structured by age, sex and reproductive status, and parameterise population projection matrices. This has hitherto been impossible using traditional CMR methods due to disturbance risk and low recapture rates. Bats exhibited lowest apparent seasonal survival over summer and autumn, particularly for reproductive females in summer (when lactating) and juveniles in autumn (after weaning), and high survival in winter. Lowest survival rates coincided with severe drought in summer–autumn 2016, suggesting that dry conditions affect population viability. Under all likely demographic assumptions, population projection matrices suggested the population is in deterministic decline, requiring urgent action to reduce extinction risk. Passively-collected resight data can now be used in combination with CMR models to provide extensive, robust information for targeted wildlife population management.
Similar content being viewed by others
Introduction
Survival analyses are a key tool for understanding wildlife population variation and can provide valuable information for the effective recovery of threatened species1,2. Survival-rate studies for terrestrial vertebrates have traditionally relied on labour intensive mark-recapture studies; however, technological advances are providing new opportunities. One technique that can be employed for estimating survival rates in animals uses passive integrated transponder (PIT) tag technology to re-detect tagged individuals at key locations without the need for physical recapture3. This method has been demonstrated to significantly increase resight probability and more precisely estimate survival compared to traditional methods4. A limitation of PIT technology for undertaking long-term passive mark-resight studies has been the size and read-range capabilities of PIT antennas, with animals needing to pass close (usually c. 15 cm) to the readers to be detected, thereby restricting the potential application of this approach for many species. However, PIT-tracking systems have recently become available that can be successfully optimised to detect small bats flying in cave passages up to 2 × 5 m in size5.
The use of PIT-tag technology has been recommended for studying insectivorous bats due to challenges such as low recaptures rates6 and adverse impacts associated with traditional banding in many species7. To date, survival estimates using mark-resight approaches using passively collected data have been published only for a few cool-temperate species, including big brown bats Eptesicus fuscus8,9,10 and little brown bats Myotis lucifugus11 roosting in buildings, lesser short-tailed bats Mystacina tuberculata using tree hollows12,13, and Daubenton’s bats Myotis daubentonii and Natterer’s bats Myotis nattereri hibernating in an old well shaft14. Undertaking survival analyses using passively collected data for further species in different environments and seasons could provide critical information to inform conservation and recovery of bat populations, which are declining worldwide15.
Survival studies of bats have been published for almost a century, but most early studies had methodological issues including reliance on potentially injurious banding and repeated sampling at hibernacula (creating disturbance which may lower bat survival due to the bats burning valuable winter fat stores)8. A global synthesis of survival estimates found that bat survival rates were strongly associated with age, sex and the number of young produced per year, as well as additional factors including season, species guild and data collection methods16. Survival was highest for adult females in summer and those that produce fewer young per year16. First-year survival is commonly lower than annual adult survival8,16,17,18. Several studies have reported higher survival rates in females than males16,17,19, while others found no significant difference between the sexes20,21,22. Similarly, a number of studies found no effect of seasonal or climatic effects on bat survival20,23, whilst others identified higher survival in summer16 or winter10,14. CMR studies using traditional mark-recapture methods require intermittent capture periods that often suffer from low recapture rates6, so the power to detect such sex- and season-specific differences in survival can be small. Whilst much is yet to be learned about bat demography, bats typically have significantly higher survival rates and slower life histories than expected for their small size24.
To investigate the capacity of PIT technology to improve estimates of structured survival rates for small, cave-dwelling bats, our case study focused on the critically endangered southern bent-winged bat Miniopterus orianae bassanii, an insectivorous bat with a restricted range in south-eastern Australia. The southern bent-winged bat has undergone serious decline since the 1960s. However, the cause of this historic decline remains uncertain25 and the current population trajectory is also unclear. The maximum longevity of the species is at least 20.5 years26 and current population mortality does not appear to be due to parasitic or pathogenic factors27,28,29,30,31. Survival estimates by age, sex and season are urgently needed to assist managers to identify the most likely population threats, determine the current population trend and understand whether any cohort or time of year is contributing disproportionately to mortality25. Historical literature on other Australian bent-winged bats suggest that the cold season may be associated with highest mortality, particularly if individuals are unable to accumulate sufficient fat stores prior to winter32,33. We therefore predicted that apparent survival rates would be lowest in winter, particularly for juveniles that probably enter this period with lower fat stores34.
Here, we investigate age-, sex- and season-specific survival rates of the southern bent-winged bat over three years, by PIT-tagging and monitoring almost 3,000 individuals at their largest breeding aggregation. We analyse seasonal variation in estimates of apparent survival for the respective age, sex and reproductive classes, consider potential explanations of mortality, calculate population growth rates to assess whether population decline is ongoing, and discuss the benefits and limitations of the PIT methods employed. The results provide essential data for implementing targeted recovery actions for the southern bent-winged bat, and highlights areas that would benefit from further research for analysing rich capture-mark-resight (CMR) datasets in other wildlife species.
Results
Capture demographics
A total of 2966 southern bent-winged bats were PIT-tagged, with approximately 1000 bats tagged per year (Table 1). The juvenile sex ratio approached the expected 1:1 in 2016 and 2018 but had statistically significant (P < 0.01) male bias in 2017 (Table 1). As expected at a maternity site, adult captures had female bias across all study years. The proportion of adult females classed as lactating ranged between 62.5 and 100% of adult females captured in January capture periods (across study years), suggesting that the majority of adult females bred each year (Supplementary Table S1). Just 28.5% of captured adult females were classed as lactating on a trapping trip on 3 February 2017, suggesting that juveniles were being weaned at this time.
A small number of tagged females recaptured in subsequent years provided some observations of reproductive maturity. Two females tagged as juveniles were recaptured one year after tagging and classed as pre-parous (i.e. non-reproductive). A further two females tagged as juveniles were recaptured two years after tagging and classed as lactating. Whilst the sample size was small, these observations suggest that females may not typically breed until two years of age.
Body mass
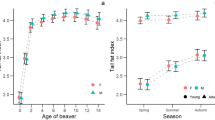
Sexual dimorphism was evident, with males consistently weighing more than females of the same age cohort (Fig. 1, Supplementary Table S2). In January, juveniles were newly volant, yet already approximated adult proportions and juvenile males exceeded the weight of adult females. Highest body mass in adults was observed on 3 February 2017, which appeared to coincide with weaning and some decrease in juvenile body mass. A capture period in mid-February 2016 after weaning saw a marked decline in body mass for juveniles of both sexes compared to during the previous month and all other capture periods. No adults were tagged (and hence weighed) during this later trip so it is unknown whether adults also experienced decline in body mass at this time. The top-ranked body mass model showed that sex, age and capture period were important predictors of body mass at tagging (as per Supplementary Table S3 and plotted in Fig. 1).
Body mass of southern bent-winged bats for each capture period over the study. Error bars show standard error. Only juveniles were tagged and weighed during the 19 February 2016 capture period, so adult body mass during this period is unknown. Full summary data for body mass is available in Supplementary Table S2.
Survival analysis
The top AIC-ranked survival model included the variables age, sex, female reproductive condition and the interaction between season and year (Table 2). Survival probabilities were therefore best presented as apparent seasonal survival (i.e. the probability of surviving the three-month period) for each individual season across the study period (Fig. 2). All population cohorts had lowest apparent survival in summer and autumn, particularly in 2016 (Fig. 2 and Supplementary Table S4), which coincided with record-breaking drought conditions in the study region (a 43-month severe rainfall deficiency with rainfall totals at the lowest on record)35. By contrast, monthly regional rainfall for the remainder of the study period was classed as ‘very much above average’ from mid-late 2016, ‘above average’ in 2017, ranging between ‘below average’ to ‘above average’ in 2018, and ‘average’ in early 201935.
Winter survival was high for all population cohorts (Fig. 2). Apparent survival estimated for juveniles was generally lower than for adult classes, particularly in autumn and spring—the exception being summer estimates which were even lower in reproductive females. Adult females that were assessed as reproductive (lactating) had lower survival estimates than those individuals classed as non-reproductive or for which reproductive condition was unknown (i.e. individuals tagged in previous years).
Estimated population growth rates
The exponential rate of population growth (\(r\)) was calculated for each study year using a variety of different parameter assumptions (Table 3) and annual apparent survival rates from the top-ranked AIC model (Supplementary Table S5). All calculations of \(r\) predicted population decline under the apparent survival rates experienced in 2016 (i.e., all r values were negative), with the baseline value as low as -0.603. Predicted rates of decline were markedly smaller for 2017 and 2018, though all r values were negative (Table 3) unless including a permanent migration rate of at least 0.1. Emigration rates in southern bent-winged bats are unknown, however, historical banding records suggest a rate less than 0.0536. Varying other parameters, including juvenile sex ratio, pre-volant survival and potential tag loss, made comparatively small differences to the population growth rate.
Discussion
Passive monitoring of PIT-tagged wildlife populations can provide rich data on survival rates compared with traditional recapture-based methods4, due to high encounter rates. Using PIT technology we found that all sex and age classes of the critically endangered southern bent-winged bat have high winter survival, with lower survival in summer and autumn. This is contrary to our expectation that survival would be lowest in winter, based on historical research on eastern bent-winged bats Miniopterus orianae oceanensis32,33. Our study adds to an increasing body of literature showing no evidence of increased mortality risk in insectivorous bats during winter20,23,37, except during severe or exceptional winters14,37, or with additional factors, such as human disturbance38 or white-nose syndrome39.
Factors affecting survival and population decline
Apparent survival rates varied across years, with markedly lower survival in the summer and autumn of 2016. This period (January–May 2016) coincided with the last few months of a severe 43-month rainfall deficiency (with rainfall totals the lowest on record) in south-east South Australia35. Our general finding of lower summer survival aligns with the results of other studies using passive PIT-tag monitoring to estimate survival in temperate insectivorous bats9,10,14, including lower survival in drought years in big brown bats9,10. In contrast, a review of global survival studies in insectivorous bats has suggested higher survival rates in summer, particularly for adult females16; however, this finding may be confounded by sampling issues in early survival studies8. In temperate regions, many bats have high survival associated with winter hibernation24,40, whereas summer survival is more likely to be affected by factors such as adequate availability of food resources. For example, high summer rainfall is associated with increased survival estimates in adult females of the little brown myotis Myotis lucifugus18.
Our study suggests summer and autumn are key times of mortality for the southern bent-winged bat population. The probable impact of drought and human activity on prey and water availability may explain the seasonal patterns in the apparent survival estimates. The southern bent-winged bat congregates en masse from spring to autumn25,41, thereby placing concentrated demand for resources around the maternity cave. The impact of dry conditions on resource availability may be exacerbated by diminished foraging availability due to habitat loss. Forested areas and wetlands are thought to be favoured foraging habitats of the southern bent-winged bat25. In the geographic range of the southern bent-winged bat, over 90% of native vegetation has been cleared25. Most wetlands in the region (which previously regularly inundated approximately 40% of south-east South Australia) have been drained for agriculture42 and significant loss of wetlands has also been attributed to groundwater decline, as a result of both groundwater extraction and reduced rainfall43. Southern bent-winged bats are heavier than its congener the eastern bent-winged bat, despite no significant difference in forearm length. This suggests that southern bent-winged bats may have higher energy demands and may be more susceptible to adverse conditions such as drought44. A changing climate is bringing drier conditions to this region in south-eastern Australia, with droughts predicted to become longer and more severe45. As a result, warm/dry season survival rates for this critically endangered bat may worsen in the future.
Adult survival is a major driver of population dynamics in long-lived species19. Here, apparent survival in adults was lowest in lactating females during the summer, particularly during the drought period when reproductive females had 6% lower apparent seasonal survival compared to non-reproductive females. This is one of the first studies to show increased survival costs in lactating females, compared to non-reproductive females—though Culina et al.46 recently reported reduced winter survival in breeding females of Daubenton’s bat but not Natterer’s bat. This included a 33% lower winter survival in successful first-time breeders compared to failed first-time breeders46. Lactation places high energy and water demands on nursing females47,48. Home-ranges and foraging distance can be significantly decreased during lactation49,50, there is increased dependence on water sources48,51, and lactation typically coincides with peaks in insect activity, enabling energetic costs of nursing to be met52. As such, it is expected that resource limitation arising from drought disproportionately affects lactating females compared to other adult classes. Indeed, with juvenile southern bent-winged bats weighing roughly the same or more than adult females when they first start exiting the cave there must be an enormous energetic investment from adult females, presumably to increase survivability of their young. Additionally, observations of lactating females in this study suggest that juveniles continued to be nursed by their mothers for a number of weeks after they commence flying.
Apparent survival rates of juveniles were lowest in summer and autumn 2016, and juveniles generally had lower survival than adults (except for lactating females in summer) (Fig. 2). Whilst apparent survival often underestimates true survival, our summer survival rates do not include pre-volant juvenile survival (as bats were tagged after they began emerging from Bat Cave), so true summer survival of juveniles may be even lower than our results suggest. First-year individuals commonly have lower survival rates than adults9,16,17,20. Juvenile mortality is likely related to inexperience. In summer, juveniles newly emerging from the cave with little flying experience appear to have higher chance of injurious collisions, as observed in newly-flying juveniles at Bat Cave53. Inexperience may also place juveniles at increased risk of predation due to being less able to evade predators. During the study, owls were observed hunting at the entrance of Bat Cave during emergence and, on one occasion, an owl was observed roosting inside the cave (E.vH, pers. obs.). Furthermore, when young lose their mother due to mortality, as indicated by lower apparent survival in lactating females, their chance of subsequent survival is presumably low.
After weaning, juvenile mortality may be influenced by a lack of foraging experience. There was a conspicuous loss of body mass during and following the period of weaning (Fig. 1, Supplementary Table S2). Declining body mass is commonly observed in juvenile bats following the onset of flight54 and experience of juvenile bats appears to influence foraging success55. Body mass is a strong predictor of fat stores in insectivorous bats56 and is associated with higher survival9,52. For example, in big brown bats, female juveniles that did not return to monitored roosts as one-year-olds had lower body condition in late summer of their natal year than those known to survive their first year9. In our study, juveniles had markedly lower spring survival than adults, which could suggest that whilst winter is not a time of high mortality as initially expected, juveniles may enter spring with lower fat stores affecting subsequent spring survival. Juvenile survival estimates can be negatively biased by permanent migration from the natal site16. However, migration between the two major maternity populations of the southern bent-winged bat is thought to be very low, as suggested by historical banding records36, relative pesticide loads57,58 and viral diversity27, and natal philopatry appears to be strong in both sexes41.
The apparent survival estimates and derived rates of population growth in this study indicate further decline of the southern bent-winged bat population, potentially exacerbated by dry years. The highest population growth rates are estimated if permanent juvenile emigration is higher than previously assumed, although all estimates predict population decline under the apparent survival rates identified in 2016 (Table 3). There has been a severe historical decline at this maternity site since the 1960s25 and at least two mortality events have been observed during droughts in 1967 and late 200625. Widespread clearing of natural forest and woodland vegetation did not occur in this region until after World War II. Whilst most wetland drainage in the lower southeast of South Australia occurred prior to 1970s, in the upper southeast (where Bat Cave is located) widespread drainage mostly occurred later—undertaken privately in the 1980s and then through a government program in the 1990s42. Therefore, habitat loss approximately coincides with reported population declines25. Additional pressure on foraging may include decreased prey availability due to the use of pesticides25. Taken together, our results suggest that challenging conditions during the breeding season are affecting population viability of the southern bent-winged bat. Given the timing of mortality, an emphasis of recovery strategies should be on boosting foraging habitat and prey availability within foraging range of maternity caves.
The southern bent-winged bat is just one of more than a third of bat species listed as threatened or data-deficient globally15. Many bats provide important ecosystem services such as agricultural pest control59. Drought, and other climatic extremes, can negatively impact bat survival9,10,14,37, and climate change will likely have a profound impact on population dynamics into the future60,61. Long-term data has linked warmer summers with increasing body mass and mortality risk in the Bechstein’s bat Myotis bechsteinii62. An increasingly drier and hotter climate has also been linked to increasing male bias in juvenile sex ratios of several bat species63. In our study, the male bias of juveniles in 2017 differed significantly from the expected 1:1 sex ratio. Male-biased sex-allocation as a result of drought is a plausible explanation because conception occurs immediately after mating in late autumn64 and drought-breaking rainfall did not occur until the winter of 201635. Further sampling is required to test the relationship between climate and sex ratios in southern bent-winged bats, an aspect of clear importance for predicting long-term population dynamics.
Use of PIT technology and CMR analyses for survival-rate estimation
Low physical recapture rates in bats provides challenges for the use of traditional mark-recapture approaches. Here, the use of passive monitoring at a roost provided an abundance of re-sight data, resulting in very high encounter probabilities with reduced disturbance5. For example, in this study even during periods of relatively lower daily encounter probabilities, such as over winter (Supplementary Fig. S1), \(p\) for a tagged individual was close to 1 when calculated across a 3-month season. However, challenges also occur in using PIT-data for survival analyses. Continuous data violates the assumption of instantaneous sampling in CJS models and can bias resulting survival estimates, particularly if sampling periods are long65. The Barker joint model outperforms CJS models in estimating survival from continuous data when using monthly and ten-day sample bins66. Increasing sample size in these comparisons did not overcome bias in CJS models, because increasing the number of individuals resulted in overly narrow confidence intervals. A drawback is that the Barker joint model is prohibitively complex for use in many real-life applications66. Additionally, the Barker model requires primary capture periods dispersed between the continuous mark-resight data, and this may not always be possible or appropriate for reasons such as disturbance to vulnerable populations, or very low recapture rates for elusive species that are hard to capture, such as bats. During trapping we recaptured just 52 of our 2966 tagged individuals67.
Our approach to the problem of instantaneous sampling was to choose daily time intervals for binning detection data, to ensure the data was as close to instantaneous as possible (whilst still ecologically and practically reasonable). However, this study was based on a dataset of in excess of 1.6 million unique individual detections. Fitting models for this quantity of data is time consuming, requires the use of high-performance computing (HPC) clusters and it becomes increasingly difficult to converge more complex models (for example with higher numbers of interacting variables). An alternative approach by Reusch et al.14 used simpler mixed-effects logistic regression (with an individual-specific random intercept) to examine bat survival from continuous PIT-tag data, but limited explanation was provided on how this approach compares to traditional CMR models. There is a need to develop robust methods for the analysis of increasingly large, continuous mark-resight datasets emerging from the use of new technologies. These solutions should balance the challenges that can arise from larger datasets, such as analysis time, computing power and false confidence, with usability in applied ecology for informing species management.
Another assumption of CJS models is that tags are not lost: therefore, tag loss can negatively bias estimates68. PIT-tag loss predominantly occurs soon after tagging due to tags working their way out of the insertion hole69 and can be minimised by using surgical adhesive on the injection site70, as in this study. Double-tagging (with a second alternative marking method) can allow for estimating tag loss rates for interpreting subsequent survival results, but requires continued physical recapture to confirm tag loss69. We decided that ongoing trapping to confirm tag loss rates posed too much disturbance for this critically endangered bat. Although the ‘first6months’ variable (which fitted separate survival estimates for the first six months after an individual’s tagging and for its remaining resight history) did not provide the best fit for the data (Table 2), survival results from after six months of tagging showed similar seasonal results of lower summer and autumn survival. Thus, the seasonal survival pattern identified could not be explained solely by tag loss or other marking effects (Supplementary Fig. S2).
PIT-tagging appears to be a safe marking method for bats, with no evidence of effects on body condition, reproductive success, infection or other detrimental effects67,71,72. It is unlikely that apparent survival differences are due to PIT-tagging affecting survivability, but we cannot preclude this possibility. Ongoing research and transparent documentation of tag loss rates and marking effects in a range of species is critical to inform data interpretation and to ensure the marking procedures continue to be informed by the best available knowledge to minimise disturbance to wildlife.
Our CMR approach using large-scale passive detection has provided rich data on apparent survival rates of the southern bent-winged bat, including sex, age and reproductive classes across all seasons in consecutive years, as well as population growth estimates. This information will be critical for developing targeted recovery actions to reduce extinction risk in this critically endangered species, and has particularly highlighted the need for increased foraging resources around maternity caves. In addition, we showcase the potential of this approach for providing complex, robust analysis on population dynamics for wildlife species to address knowledge gaps into the future, such as the demographic responses to climate change73, and the large proportion of species with unknown population trends worldwide, particularly for bats74.
Methods
PIT-tagging and data collection
Southern bent-winged bats were trapped and PIT-tagged at Bat Cave within the Naracoorte Caves National Park, South Australia, which serves as a maternity and congregation site from spring to autumn41. Trapping occurred over six nights in 2016, and four nights in each of 2017 and 2018, at the end of the breeding season (January and February) by which time juveniles born in November were emerging from the cave to forage at night. Bats were trapped with Austbat harp traps (Faunatech, Mount Taylor) surrounding the cave entrance. Trapping continued throughout the night, catching bats as they left or re-entered the cave. Both females and males congregate at Bat Cave, so both sexes could be tagged. There was no targeting of one sex over the other, and hence the tagged samples reflect the sex ratio of trapped individuals. A potential male bias (from the expected 1:1) in juvenile sex ratios in 2017 was tested for statistical significance using a Chi-squared test.
Individual covariates were recorded for each PIT-tagged bat, including sex, body mass and age. Age was described as juvenile (first year) or adult based on the respective presence or absence of a cartilaginous core at the metacarpal-phalangeal joints75. The reproductive condition of adult females was classified as pre-parous, lactating or post-lactating through examination of the nipples76. PIT-tags were subcutaneously injected dorsally using a sterilised 12-gauge needle and applicator (Biomark MK10 implanter and N125 needles in 2016, Biomark MK 25 Implant Guns and HPT12 Pre-load Trays in 2017–18). The injection site was sealed with a drop of surgical adhesive (3 M™ VetBond™) to minimise tag loss, and allowed to dry prior to release67. All PIT-tags (Biomark HPT 12) were checked for correct function using a hand-held PIT-tag scanner (Trovan LID560 and Biomark 601) both before and after insertion. During handling and tagging, bats typically remained calm and were able to fly within minutes of the procedure. Recaptured individuals were in good physical condition, with no sign of infection or other detrimental effects67. Linear models were used to explore the relationship between capture periods, demographic variables, and body mass recorded at the time of tagging (Supplementary Table S3).
Tagged bats were monitored by using a large PIT-tracking system (Biomark IS1001) which employed a 2 × 5 m loop antenna as described in van Harten et al.5, installed within Bat Cave, which detected tagged individuals in real time as they flew through the cave passage. When the system was working optimally, there was a large read-range before and after the antenna plane and high detection success5. Data files were recorded directly to USB flash drives plugged into the data logger board of the Biomark IS1001. Data were collected from the cave regularly (approximately monthly) over a 37-month period by manually retrieving the flash drives.
Survival analyses
To prepare the data for analysis, we derived capture-resight histories for each of the 2966 PIT-tagged bats to produce a binary response variable (undetected/detected) for each individual across each day of the 1120-day study period, with a ‘day’ being defined as the 24 h between successive middays. Accumulative age functions were incorporated to allow juvenile bats to age appropriately (i.e., bats tagged as juveniles were classified as adults at 1 year of age). As reproductive status in adult females could be determined only by physical examination during PIT-tagging, these individuals were defined as ‘reproductive’ (i.e. lactating) or ‘non-reproductive’ for the summer and autumn following tagging, and then pooled into an ‘unknown’ reproductive category for all subsequent times. We then modelled daily survival using Cormack-Jolly-Seber (CJS) models which were fitted using the R package ‘RMark’77. CJS models allow estimation of apparent survival probabilities (\(\varphi\)) whilst also accounting for (potentially variable) encounter probabilities (\(p\)).
Given the impact of environmental noise on antenna performance5, and the seasonal behaviour and movement patterns of the southern bent-winged bat41, we modelled encounter probability with flexible spline functions such that
where \(yday\) is the day of year, \(noise\) is the average environmental noise (summation of unwanted signal being received by the PIT tracking system, %) over the course of that day, and \(k\) is the dimension of the spline. The basis functions for the splines were calculated with the R package ‘mgcv’, and a cubic regression spline was assumed for the day-of-year effect to ensure continuity in the response between the first and last day of year.
We then developed a candidate set of models for daily survival probability \((\varphi )\) that included different covariates including age, sex, season, year and reproductive condition (for adult females only), as well as possible interaction terms. When modelling the effect of both season and year, we coded December as a component of summer in the following year (e.g. December 2016 data were coded as summer 2017). We also tested a variable \(first6months\) which fitted separate survival estimates for the first six months after an individual’s tagging and for the remainder of the re-sight history, to explore any possible marking effects. Model selection was undertaken by comparing Akaike information criterion (AIC) for each alternative model. The AIC includes a penalty for increasing complexity (i.e. number of parameters) in the model78. The ‘best’ or top-ranked model is the one with the lowest AIC value.
Estimation of population growth rates
In this study, we use ‘population’ to refer to the colony of southern bent-winged bats that occupy the Bat Cave maternity site. To estimate the exponential rate of population growth (r), we constructed pre-breeding Leslie matrices79. To achieve this, we initially used annual survival-rate estimates for each age, sex and reproductive class (derived from daily survival estimates from the top AIC-ranked model), assumed a female age of reproductive maturity of two years (as described for eastern bent-winged bats80 and observed in this study as reported above) and fertility estimates of one offspring per adult female per year81 as baseline parameterisation. We then calculated the effect on population growth rates under different demographic assumptions, including differing juvenile sex ratios (from observations in this study), potential emigration rates, breeding probabilities and tag loss. Two potential rates of tag loss were tested: 2.7% which was calculated for the Gould’s wattled bat Chalinolobus gouldii in a double-tagging experiment using the same PIT-tagging procedures as this study69; and 5%, the approximate proportion of bats not detected more than 10 days after tagging during this study in 2017 and 20185 which provides an estimate of the maximum tag loss over this period.
Ethics approval
Animal capture, handling and data collection were approved by the La Trobe University Animal Ethics Committee (Project Number AEC15-67) and were carried out in accordance with relevant guidelines and regulations prescribed by the South Australian Department of Environment and Water (Research Permit Number U26453). This study was carried out in compliance with the ARRIVE guidelines on animal research.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Odonnell, C. Population dynamics and survivorship in bats. In Ecology and Behavioral Methods for the Study of Bats (eds Kunz, T. H. & Parsons, S.) 158–176 (The Johns University Press, 2009).
Lebreton, J.-D., Burnham, K. P., Clobert, J. & Anderson, D. R. Modeling survival and testing biological hypotheses using marked animals: A unified approach with case studies. Ecol. Monogr. 62, 67–118 (1992).
Gibbons, J. W. & Andrews, K. M. PIT tagging: Simple technology at its best. Bioscience 54, 447–454 (2004).
Ellison, L. E. et al. A comparison of conventional capture versus PIT reader techniques for estimating survival and capture probabilities of big brown bats (Eptesicus fuscus). Acta Chiropterologica 9, 149–160 (2007).
van Harten, E. et al. High detectability with low impact: Optimizing large PIT tracking systems for cave-dwelling bats. Ecol. Evol. 9, 10916–10928 (2019).
Schorr, R. A., Ellison, L. E. & Lukacs, P. M. Estimating sample size for landscape-scale mark-recapture studies of North American migratory tree bats. Acta Chiropterologica 16, 231–239 (2014).
Baker, G. B. et al. The effect of forearm bands on insectivorous bats (Microchiroptera) in Australia. Wildl. Res. 28, 229–237 (2001).
O’Shea, T. J., Ellison, L. E. & Stanley, T. R. Survival estimation in bats: Historical overview, critical appraisal, and suggestions for new approaches. In Sampling Rare or Elusive Species: Concepts, Designs, and Techniques for Estimating Population Parameters (ed. Thompson, W. L.) 297–336 (Island Press, 2004).
O’Shea, T. J. et al. Recruitment in a Colorado population of big brown bats: Breeding probabilities, litter size, and first-year survival. J. Mammal. 91, 418–428 (2010).
O’Shea, T. J., Ellison, L. E. & Stanley, T. R. Adult survival and population growth rate in Colorado big brown bats (Eptesicus fuscus). J. Mammal. 92, 433–443 (2011).
Schorr, R. A. & Siemers, J. L. Population dynamics of little brown bats (Myotis lucifugus) at summer roosts: Apparent survival, fidelity, abundance, and the influence of winter conditions. Ecol. Evol. 11, 7427–7438 (2021).
O’Donnell, C. F. J., Edmonds, H. & Hoare, J. M. Survival of PIT-tagged lesser short-tailed bats (Mystacina tuberculata) through a pest control operation using the toxin pindone in bait stations. N. Z. J. Ecol. 35, 291–295 (2011).
Edmonds, H., Pryde, M. & O’Donnell, C. Survival of PIT-tagged lesser short-tailed bats (Mystacina tuberculata) through an aerial 1080 pest control operation. N. Z. J. Ecol. 41, 186–192 (2017).
Reusch, C. et al. Differences in seasonal survival suggest species-specific reactions to climate change in two sympatric bat species. Ecol. Evol. 9, 7957–7965 (2019).
IUCN. The IUCN red list of threatened species. Version 2020-2. http://www.iucnredlist.org (2020).
Lentini, P. E., Bird, T. J., Griffiths, S. R., Godinho, L. N. & Wintle, B. A. A global synthesis of survival estimates for microbats. Biol. Lett. 11, 20150371 (2015).
Culina, A., Linton, D. M. & Macdonald, D. W. Age, sex, and climate factors show different effects on survival of three different bat species in a woodland bat community. Glob. Ecol. Conserv. 12, 263–271 (2017).
Frick, W. F., Reynolds, D. S. & Kunz, T. H. Influence of climate and reproductive timing on demography of little brown myotis Myotis lucifugus. J. Anim. Ecol. 79, 128–136 (2010).
Schorcht, W., Bontadina, F. & Schaub, M. Variation of adult survival drives population dynamics in a migrating forest bat. J. Anim. Ecol. 78, 1182–1190 (2009).
Sendor, T. & Simon, M. Population dynamics of the pipistrelle bat: Effects of sex, age and winter weather on seasonal survival. J. Anim. Ecol. 72, 308–320 (2003).
Sripathi, K., Raghuram, H., Rajasekar, R., Karuppudurai, T. & Abraham, S. G. Population size and survival in the indian false vampire bat Megaderma lyra. Acta Chiropterologica 6, 145–154 (2004).
Papadatou, E., Butlin, R. K., Pradel, R. & Altringham, J. D. Sex-specific roost movements and population dynamics of the vulnerable long-fingered bat, Myotis capaccinii. Biol. Conserv. 142, 280–289 (2009).
López-Roig, M. & Serra-Cobo, J. Impact of human disturbance, density, and environmental conditions on the survival probabilities of pipistrelle bat (Pipistrellus pipistrellus). Popul. Ecol. 56, 471–480 (2014).
Wilkinson, G. S. & Adams, D. M. Recurrent evolution of extreme longevity in bats. Biol. Lett. 15, 20180860 (2019).
DELWP. National Recovery Plan for the Southern Bent-wing Bat Miniopterus orianae bassanii (2020).
Lumsden, L. & Gray, P. Longevity record for a southern bent-wing bat Miniopterus schreibersii bassanii. Australas. Bat Soc. Newsl. 16, 43–44 (2001).
Holz, P. H. et al. Virus survey in populations of two subspecies of bent-winged bats (Miniopterus orianae bassanii and oceanensis) in south-eastern Australia reveals a high prevalence of diverse herpesviruses. PLoS ONE 13, e0197625 (2018).
Holz, P. H., Lumsden, L. F., Marenda, M. S., Browning, G. F. & Hufschmid, J. Two subspecies of bent-winged bats (Miniopterus orianae bassanii and oceanensis) in southern Australia have diverse fungal skin flora but not Pseudogymnoascus destructans. PLoS ONE 13, e0204282 (2018).
Holz, P. H., Lumsden, L. F. & Hufschmid, J. Ectoparasites are unlikely to be a primary cause of population declines of bent-winged bats in south-eastern Australia. Int. J. Parasitol. Parasites Wildl. 7, 423–428 (2018).
Holz, P. H., Lumsden, L. F., Legione, A. R. & Hufschmid, J. Polychromophilus melanipherus and haemoplasma infections not associated with clinical signs in southern bent-winged bats (Miniopterus orianae bassanii) and eastern bent-winged bats (Miniopterus orianae oceanensis). Int. J. Parasitol. Parasites Wildl. 8, 10–18 (2019).
Holz, P. H., Clark, P., McLelland, D. J., Lumsden, L. F. & Hufschmid, J. Haematology of southern bent-winged bats (Miniopterus orianae bassanii) from the Naracoorte Caves National Park, South Australia. Comp. Clin. Pathol. 29, 231–237 (2020).
Dwyer, P. D. The population pattern of Miniopterus schreibersii (Chiroptera) in north-eastern New South Wales. Aust. J. Zool. 14, 1073–1137 (1966).
Dwyer, P. D. Mortality factors of the bent-winged bat. Vic. Nat. 83, 31–36 (1966).
Dwyer, P. D. Seasonal changes in activity and weight of Miniopterus schreibersii blepotis (Chiroptera) in north-eastern NSW. Aust. J. Zool. 12, 52–69 (1964).
Bureau of Meteorology. Drought archive. http://www.bom.gov.au/climate/drought/archive.shtml (2019).
Dwyer, P. D. Population ranges of Miniopterus schreibersii (Chiroptera) in south-eastern Australia. Aust. J. Zool. 17, 665–686 (1969).
Fleischer, T., Gampe, J., Scheuerlein, A. & Kerth, G. Rare catastrophic events drive population dynamics in a bat species with negligible senescence. Sci. Rep. 7, 7370 (2017).
Thomas, D. W. Hibernating bats are sensitive to nontactile human disturbance. J. Mammal. 76, 940–946 (1995).
Reeder, D. M. et al. Frequent arousal from hibernation linked to severity of infection and mortality in bats with white-nose syndrome. PLoS ONE 7, e38920 (2012).
Turbill, C., Bieber, C. & Ruf, T. Hibernation is associated with increased survival and the evolution of slow life histories among mammals. Proc. R. Soc. B Biol. Sci. 278, 3355–3363 (2011).
van Harten, E. Population Dynamics of the Critically Endangered, Southern Bent-Winged Bat Miniopterus orianae bassanii (La Trobe University, 2020).
PIRSA. History of the south east drainage system - summary. https://www.pir.sa.gov.au/aghistory/natural_resources/water_resources_ag_dev/history_of_the_south_east_drainage_system_-_summary/history_of_the_south_east_drainage_system_-_summary#_ftnref2 (2017).
Harding, C., Herpich, D. & Cranswick, R. H. Examining temporal and spatial changes in surface water hydrology of groundwater dependent ecosystems using WOfS (Water Observations from Space): Southern Border Groundwaters Agreement area, South East South Australia. (2018).
Holz, P. H., Lumsden, L. F., Reardon, T., Gray, P. & Hufschmid, J. Does size matter? Morphometrics of southern bent-winged bats (Miniopterus orianae bassanii) and eastern bent-winged bats (Miniopterus orianae oceanensis). Aust. Zool. AZ https://doi.org/10.7882/AZ.2019.019 (2020).
Rashid, M. M. & Beecham, S. Characterization of meteorological droughts across South Australia. Meteorol. Appl. 26, 556–568 (2019).
Culina, A., Linton, D. M., Pradel, R., Bouwhuis, S. & Macdonald, D. W. Live fast, don’t die young: Survival–reproduction trade-offs in long-lived income breeders. J. Anim. Ecol. 88, 746–756 (2019).
Kunz, T. H., Whitaker, J. O. & Wadanoli, M. D. Dietary energetics of the insectivorous Mexican free-tailed bat (Tadarida brasiliensis) during pregnancy and lactation. Oecologia 101, 407–415 (1995).
Adams, R. A. & Hayes, M. A. Water availability and successful lactation by bats as related to climate change in arid regions of western North America. J. Anim. Ecol. 77, 1115–1121 (2008).
Henry, M., Thomas, D. W., Vaudry, R. & Carrier, M. Foraging distances and home range of pregnant and lactating little brown bats (Myotis lucifugus). J. Mammal. 83, 767–774 (2002).
Lučan, R. & Radil, J. Variability of foraging and roosting activities in adult females of Daubenton’s bat (Myotis daubentonii) in different seasons. Biologia (Bratisl.) 65 (2010).
Amorim, F., Jorge, I., Beja, P. & Rebelo, H. Following the water? Landscape-scale temporal changes in bat spatial distribution in relation to Mediterranean summer drought. Ecol. Evol. 8, 5801–5814 (2018).
O’Donnell, C. F. J. Timing of breeding, productivity and survival of long-tailed bats Chalinolobus tuberculatus (Chiroptera: Vespertilionidae) in cold-temperate rainforest in New Zealand. J. Zool. 257, 311–323 (2002).
Holz, P. H., Stent, A., Lumsden, L. F. & Hufschmid, J. Trauma found to be a significant cause of death in a pathological investigation of bent-winged bats (Miniopterus orianae). J. Zoo Wildl. Med. 50, 966–971 (2020).
Hughes, P. M., Rayner, J. M. V. & Jonesg, G. Ontogeny of ‘true’ flight and other aspects of growth in the bat Pipistrellus pipistrellus. J. Zool. 236, 291–318 (1995).
Wund, M. A. Learning and the development of habitat-specific bat echolocation. Anim. Behav. 70, 441–450 (2005).
McGuire, L. P. et al. Common condition indices are no more effective than body mass for estimating fat stores in insectivorous bats. J. Mammal. 99, 1065–1071 (2018).
Mispagel, C. et al. DDT and metabolites residues in the southern bent-wing bat (Miniopterus schreibersii bassanii) of south-eastern Australia. Chemosphere 55, 997–1003 (2004).
Allinson, G. et al. Organochlorine and trace metal residues in adult southern bent-wing bat (Miniopterus schreibersii bassanii) in southeastern Australia. Chemosphere 64, 1464–1471 (2006).
Kolkert, H., Andrew, R., Smith, R., Rader, R. & Reid, N. Insectivorous bats selectively source moths and eat mostly pest insects on dryland and irrigated cotton farms. Ecol. Evol. https://doi.org/10.1002/ece3.5901 (2019).
Sherwin, H. A., Montgomery, W. I. & Lundy, M. G. The impact and implications of climate change for bats. Mammal Rev. 43, 171–182 (2013).
O’Shea, T. J., Cryan, P. M., Hayman, D. T. S., Plowright, R. K. & Streicker, D. G. Multiple mortality events in bats: A global review. Mammal Rev. 46, 175–190 (2016).
Mundinger, C., Scheuerlein, A. & Kerth, G. Long-term study shows that increasing body size in response to warmer summers is associated with a higher mortality risk in a long-lived bat species. Proc. R. Soc. B Biol. Sci. 288, 20210508 (2021).
Adams, R. A. & Hayes, M. A. Assemblage-level analysis of sex-ratios in Coloradan bats in relation to climate variables: A model for future expectations. Glob. Ecol. Conserv. 14, e00379 (2018).
Crichton, E. G., Seamark, R. F. & Krutzsch, P. H. The status of the corpus luteum during pregnancy in Miniopterus schreibersii (Chiroptera: Vespertilionidae) with emphasis on its role in developmental delay. Cell Tissue Res. 258, 183–201 (1989).
Olsen, I. C. The analysis of continuous mark-recapture data (Norwegian University of Science and Technology, 2006).
Barbour, A. B., Ponciano, J. M. & Lorenzen, K. Apparent survival estimation from continuous mark-recapture/resighting data. Methods Ecol. Evol. 4, 846–853 (2013).
van Harten, E. et al. Recovery of southern bent-winged bats (Miniopterus orianae bassanii) after PIT-tagging and the use of surgical adhesive. Aust. Mammal. 42, 216–219 (2020).
McDonald, T. L., Amstrup, S. C. & Manly, B. F. Tag loss can bias Jolly-Seber capture-recapture estimates. Wildl. Soc. Bull. 31, 814–822 (2003).
van Harten, E. et al. Low rates of PIT-tag loss in an insectivorous bat species. J. Wildl. Manag. 85, 1739–1743 (2021).
Lebl, K. & Ruf, T. An easy way to reduce PIT-tag loss in rodents. Ecol. Res. 25, 251–253 (2010).
Rigby, E. L., Aegerter, J., Brash, M. & Altringham, J. D. Impact of PIT tagging on recapture rates, body condition and reproductive success of wild Daubenton’s bats (Myotis daubentonii). Vet. Rec. 170, 101 (2012).
Locatelli, A. G., Ciuti, S., Presetnik, P., Toffoli, R. & Teeling, E. Long-term monitoring of the effects of weather and marking techniques on body condition in the Kuhl’s pipistrelle bat, Pipistrellus kuhlii. Acta Chiropterologica 21, 87–102 (2019).
Paniw, M. et al. The myriad of complex demographic responses of terrestrial mammals to climate change and gaps of knowledge: A global analysis. J. Anim. Ecol. 90, 1398–1407 (2021).
Frick, W. F., Kingston, T. & Flanders, J. A review of the major threats and challenges to global bat conservation. Ann. N. Y. Acad. Sci. 1469, 5–25 (2020).
Brunet-Rossinni, A. K. & Wilkinson, G. S. Methods for age estimation and the study of senescence in bats. In Ecological and Behavioral Methods for the Study of Bats (eds Kunz, T. H. & Parsons, S.) 315–325 (Johns Hopkins University Press, 2009).
Churchill, S. Australian Bats (Allen and Unwin, 2008).
Laake, J. L. RMark: An R interface for analysis of capture-recapture data with MARK. 25 (2013).
Burnham, K. P. & Anderson, D. R. Model Selection and Multimodel Inference (Springer, 2002). https://doi.org/10.1007/b97636.
Caswell, H. Matrix population models. In Encyclopedia of Environmetrics (eds El-Shaarawi, A. H. & Piegorsch, W. W.) (Wiley, Berlin, 2006). https://doi.org/10.1002/9780470057339.vam006m.
Dwyer, P. D. The breeding biology of Miniopterus schreibersii blepotis (Termminck) (Chiroptera) in north-eastern NSW. Aust. J. Zool. 11, 219–240 (1963).
Richardson, E. G. The biology and evolution of the reproductive cycle of Miniopterus schreibersii and M. australis (Chiroptera: Vespertilionidae). J. Zool. 183, 353–375 (1977).
Acknowledgements
We sincerely thank the 70+ volunteers that worked throughout the nights during trapping and tagging. A special thanks to Rose Thompson and Dennis Matthews who helped throughout the study. We are grateful for the continued time and in-kind support from the staff at Naracoorte Caves National Park, in particular Andrew Hansford and Tom Shortt. Andrew Bennett provided helpful comments which improved the manuscript. This work was financially supported by an Australian Government Research Training Scholarship and funded by the Holsworth Wildlife Research Endowment, Australian Speleological Federation Karst Conservation Fund, Department of Environment and Water (South Australia) and Lirabenda Endowment Fund.
Author information
Authors and Affiliations
Contributions
E.v.H., R.L., L.F.L., and T.R. designed the study; E.v.H. collected the data with assistance from T.R., L.F.L. and R.L.; E.v.H. and T.A.A.P analysed the data. E.v.H. interpreted the data and wrote the manuscript; all authors contributed critically to the manuscript and assisted with revision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
van Harten, E., Lawrence, R., Lumsden, L.F. et al. Novel passive detection approach reveals low breeding season survival and apparent lactation cost in a critically endangered cave bat. Sci Rep 12, 7390 (2022). https://doi.org/10.1038/s41598-022-11404-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-11404-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.