Abstract
Aim of this study was to validate the prognostic impact of clinical parameters and baseline 18F-FDG-PET/CT derived textural features to predict histopathologic response and survival in patients with esophageal squamous cell carcinoma undergoing neoadjuvant chemoradiation (nCRT) and surgery. Between 2005 and 2014, 38 ESCC were treated with nCRT and surgery. For all patients, the 18F-FDG-PET-derived parameters metabolic tumor volume (MTV), SUVmax, contrast and busyness were calculated for the primary tumor using a SUV-threshold of 3. The parameter uniformity was calculated using contrast-enhanced computed tomography. Based on histopathological response to nCRT, patients were classified as good responders (< 10% residual tumor) (R) or non-responders (≥ 10% residual tumor) (NR). Regression analyses were used to analyse the association of clinical parameters and imaging parameters with treatment response and overall survival (OS). Good response to nCRT was seen in 27 patients (71.1%) and non-response was seen in 11 patients (28.9%). Grading was the only parameter predicting response to nCRT (Odds Ratio (OR) = 0.188, 95% CI: 0.040–0.883; p = 0.034). No association with histopathologic treatment response was seen for any of the evaluated imaging parameters including SUVmax, MTV, busyness, contrast and uniformity. Using multivariate Cox-regression analysis, the heterogeneity parameters busyness (Hazard Ratio (HR) = 1.424, 95% CI: 1.044–1.943; p = 0.026) and contrast (HR = 6.678, 95% CI: 1.969–22.643; p = 0.002) were independently associated with OS, while no independent association with OS was seen for SUVmax and MTV. In patients with ESCC undergoing nCRT and surgery, baseline 18F-FDG-PET/CT derived parameters could not predict histopathologic response to nCRT. However, the PET/CT derived features busyness and contrast were independently associated with OS and should be further investigated.
Similar content being viewed by others
Introduction
Esophageal cancer ranks sixth in cancer-related mortality and has caused more than 5,00,000 cancer deaths in 2018 worldwide1. Mortality rate of esophageal cancer is particularly high because most patients are diagnosed with locally advanced or metastatic disease2. Despite improvements in diagnostic and therapeutic techniques over the last decades the 5-year OS remains poor.
The standard treatment regimen for patients with locally advanced tumors suitable for surgery is neoadjuvant chemoradiation followed by surgery (nCRT + S)3,4. Thereby, nCRT can improve survival and R0 resection rate compared to surgery alone5,6. Patients who are unsuitable for surgery or decline surgery are generally treated with definitive chemoradiation (dCRT). Despite direct comparisons between dCRT and nCRT + S are often impaired by heterogeneous patient cohorts, it is known there is no significant difference for overall survival in patients with good response to (neoadjuvant) chemoradiation7. Therefore, pretherapeutic prediction of treatment response could guide the treating radiation oncologist to select personalized therapeutic strategies for each patient.
In patients with curative treatment intent, 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG-PET/CT) is typically used for staging and can help to detect distant metastases8,9. SUV-based parameters like maximum standardized uptake value (SUVmax) or metabolic tumor volume (MTV) have been analyzed for their ability to predict OS or histopathological response to chemoradiation in esophageal cancer patients10,11,12,13,14,15,16,17,18,19,20. These studies demonstrate contradictory results and interpretation of the data is also impaired by the fact, that most studies included different histologic subtypes. In recent years, studies focusing on heterogeneity parameters like contrast or busyness demonstrated that intratumor heterogeneity might be more suitable than SUV-based parameters in predicting treatment response or survival11,21,22. In addition, the contrast-enhanced CT-based parameter uniformity was associated with treatment response and survival in different types of cancer including esophageal carcinoma23,24,25,26.
In this study, the potential of the most promising 18F-FDG-PET/CT-based parameters to predict survival and histopathological response shall be further analyzed in a cohort of ESCC patients undergoing neoadjuvant chemoradiation and surgery.
Patients and methods
Baseline characteristics
Clinical data of 38 ESCC patients who underwent nCRT and esophagectomy with regional lymphadenectomy between 2005 and 2014, were retrospectively evaluated. 53% of the patients were male and median age was 62 years. The median craniocaudal tumor length was 5 cm (range 2–12 cm). 55% of patients had moderately differentiated tumors (G2) and 45% had poorly differentiated tumors (G3). Most patients (92%) had locally advanced tumor lesions (T3) and 87% of the patients had clinically suspected lymph node metastases (cN +). In addition, one patient had a supraclavicular lymph node metastasis that was classified as M1 (LYM) (Table1).
Neoadjuvant chemoradiation
All patients received nCRT up with a total dose of 45 Gy (daily dose 1.8 Gy, 5 times/week) and concomitant platinum-based chemotherapy. 29 patients (76%) received chemotherapy with cisplatin and 5-fluorouracil (5-FU). In 8 patients the combination of oxaliplatin, 5-FU and cetuximab was used. One patient received combination chemotherapy with cisplatin and irinotecan. The median time interval between completion of neoadjuvant therapy and surgery was 39 days (range 9–84 days).
18F-FDG-PET
Prior to treatment a 18F-FDG-PET/CT was done either using the ´Biograph 16´ (Siemens Medical Solutions, Germany) or the ´SOMATOM Definition AS´ (Siemens Medical Solutions, Germany). In all patients the 3D-Ordered Subsets Expectation Maximization (OSEM3D) algorithm27, was used resulting in a comparable slice thickness of 5.3 mm and 4.1 mm.
Patients were instructed to fast for at least 6 h before injection of 18F-FDG (4.6 MBq/kg). Median time interval between injection of 18F-FDG and imaging was 69 min and imaging was done with 5–14 bed positions and an acquisition time of 1.5–4 min per bed position.
For calculation of PET-derived parameters (SUVmax, MTV, busyness and contrast) the region of interest (ROI) was semi-automatically generated in two consecutive steps: The ROI was automatically contoured using a SUV-threshold of 3. To assure, that only voxels containing tumor were included within the ROI all adjacent physiological 18F-FDG-avid structures beside the primary tumor area were then excluded manually. The resulting ROI included voxels with a SUV-uptake of ≥ 3 and assigned to the primary tumor. In the following the heterogeneity parameters were calculated automatically using Matlab software version 2018. The parameter contrast, which indicates differences between neighboring voxels, was calculated on 3D matrices as described by Tixier and colleagues21. In addition, the parameter busyness, which corresponds to the spatial frequency of intensity changes, was calculated as reported by Amadasun and King 28.
Diagnostic contrast-enhanced computed tomography
Standardized pre-therapeutic contrast-enhanced (portal venous phase) CT-imaging with a maximum slice thickness of 5 mm was available in 30 patients (79%). The primary tumor in the esophagus was manually delineated using all available diagnostic information (endoscopy, endoscopic ultrasound (EUS) and 18F-FDG-PET scans). Voxels containing air or fluid within the esophagus were manually excluded. The image parameter uniformity quantifies the distribution of grey levels. Thus, uniformity is an indicator of image heterogeneity. Calculation of uniformity was done as described by Ganeshan and colleagues29 using IBEX (Imaging Biomarker Explorer) software30.
Follow-up
All patients underwent regular follow-up examinations following international guidelines including physical examination, esophago-gastro-duodenoscopy and computed tomography.
Histopathologic assessment
The basis for our analysis was the differentiation between patients with histopathological good response (R) and non-responders (NR). Therefore, resected specimen of all 38 patients were evaluated by extensive and standardized histomorphological workup as described by Becker et al.31. Complete tumor regression with 0% residual tumor was classified as grade 1a, subtotal tumor regression with < 10% residual tumor per tumor bed was classified as grade 1b, partial tumor regression with 10–50% residual tumor per tumor bed was classified as grade 2 and minimal or no tumor regression with > 50% residual tumor per tumor bed was classified as grade 3. For this analysis, good response (R) was defined as < 10% residual tumor (Becker grade 1a + 1b), while non-response (NR) was defined as ≥ 10% residual tumor (Becker grade 2 + 3).
Statistics
Statistical tests were performed using the SPSS Statistics software version 18.0.0 (IBM SPPS Statistics, Armonk, U.S.) and R package version 2019-07-2532. P-values < 0.05 were considered statistically significant. Univariate binary logistic regression analyses were conducted to evaluate the correlation of each parameter and response to therapy. OS was determined as the time between esophagectomy and last follow-up or death. Cox regression analyses were used to identify parameters predicting OS. The optimal cut-off value of significant parameters was determined by the maximally selected LogRank test33 and a test on association using the R package maxstat.test. Survival curves were estimated using the Kaplan Meier method.
Ethics approval and consent to participate
The ethical committee of the Technical University of Munich has approved the retrospective study protocol (ethical vote N° 490/19 S). All patients gave their written informed consent for radiotherapy. All methods were performed in accordance with the relevant guidelines and regulations.
Results
Tumor response and imaging parameters
27 (71%) patients were classified as good responders (R) and eleven patients were classified as non-responders (NR) (29%). Initial median MTV was 14.6cm3 in responders, and 19.1cm3 in non-responders. Median SUVmax (18F-FDG) was 16.8 (R) and 17.6 (NR). Median busyness, contrast and uniformity were 1.53, 0.38, and 0.18 (R) and 2.82, 0.53 and 0.19 (NR), respectively. Using binary logistic regression analysis, neither MTV nor any other pre-therapeutic imaging parameter could significantly predict treatment response (Table 2).
Tumor response and clinical parameters
Table 3 shows the association of clinical parameters and histopathological response to nCRT. No significant difference between responders and non-responders was seen for T-stage, N-stage, tumor length, age and sex. In contrast, tumor grading was significantly associated with treatment response. In summary, the odds of good tumor response was 5.3 times higher in patients with moderate tumor differentiation (G2) than in patients with poor tumor differentiation (G3) (OR = 0.188, p = 0.034).
Survival prediction
With a median follow-up of 68 months for surviving patients, median OS for all patients was 40 months. The 3-year cumulative survival was 56% and 5-year survival was 46%. Results concerning the prognostic value of all analyzed parameters are shown in Table 4.
Using a univariate Cox-regression model, none of the clinical parameters (age, sex, grading, T-stage, N-stage and tumor length) showed good predictive performance for OS, whereas the textural features busyness (Hazard Ratio (HR) = 1.564, p = 0.001), contrast (HR = 9.340, p < 0.001) and SUVmax (HR = 0.934, p = 0.019) were significantly associated with OS. While each increasement for the parameters busyness and contrast by “1” (busyness) and “0.1” (contrast) was associated with a 1.564 times (busyness) and a 1.250 times (contrast) higher risk of death, each increasement by “1” for the SUVmax was associated with a 1.07 times lower risk of death. Because of a significant high correlation (Pearson´s index = 0.899, p < 0.001) between busyness and contrast, both parameters were not tested within the same multivariate cox-regression analysis. When using multivariate cox-regression models including the parameters SUVmax and busyness or SUVmax and contrast, the impact of SUVmax was not statistically significant anymore (p = 0.301/p = 0.224) while the parameters busyness (HR = 1.424, 95% CI: 1.044–1.943; p = 0.026) and contrast (HR = 6.678, 95% CI: 1.969–22.643; p = 0.002) were independently associated with OS. Thereby, an increasement by “1” (busyness) or “0.1” (contrast) was still associated with a 1.424 times (busyness) and a 1.209 times (contrast) higher risk of death.
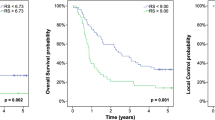
Using the maximally selected LogRank test optimal cut-off values of 0.407 (contrast) and 1.76 (busyness) were calculated and used for further analysis. Median OS was 1.8 years in the subgroup of patients with tumor contrast > 0.407 and 9.7 years for patients with tumor contrast < 0.407 (p = 0.021) (Fig. 1a). Similarly, busyness of < 1.76 was associated with a better outcome and a median survival of 9.7 years than busyness of > 1.76 (1.8 years; p = 0.017) (Fig. 1b). Finally, there was no statistically significant correlation between OS and the 18F-FDG-PET/CT based image parameters MTV and uniformity.
Discussion
Assessment of prognosis and response to therapy prior to treatment plays an increasing role in patient management as well as treatment planning. Being able to predict prognosis and response to nCRT based on a standard pre-treatment imaging would be practicable, cost- and timesaving in clinical routine. In this context, the value of baseline PET-based parameters for prediction of treatment response and prognosis has been promisingly described in the literature for different tumor entities34,35,36,37,38. However, regarding ESCC, only few data showing conflicting results are available. Therefore, we validated the impact of 18F-FDG-PET/CT derived textural features for prediction of treatment response to neoadjuvant chemoradiation in a cohort of only ESCC patients treated at our institution. Neoadjuvant treatment was very homogenous with all patients receiving neoadjuvant radiotherapy with a total dose of 45 Gy and simultaneous platin-based chemotherapy. This is an important aspect because the efficiency of chemoradiation differs between the two histological subtypes6 and radiation doses can also affect patients´ response to chemoradiation.
None of the analyzed pretreatment 18F-FDG-PET/CT parameters including SUVmax, MTV, contrast, busyness and uniformity were associated with histopathological response to nCRT. Regarding histopathological parameters, patients with moderate differentiated tumors showed higher response rates than patients with poorly differentiated tumors. However, in terms of OS, a lower SUVmax, higher contrast and higher busyness were associated with inferior OS.
The PET derived SUV quantifies the FDG accumulation rate and is particularly high in tissues with pathological processes such as inflammation or tumor tissue. However, the typically used image parameter SUVmax does not represent the glucose metabolism of the entire tumor but indicates the highest value within a defined region of interest. While pretreatment SUVmax was associated with response to chemoradiation in two studies primarily including patients with adenocarcinoma17,18, results for patients with squamous cell carcinoma are heterogenous10,16,20,21. In a study by Zhang and colleagues10 treatment response was assessed 4 weeks after chemoradiation in 48 ESCC patients. While the reduction of SUVmax between baseline PET and an interim-PET after 40 Gy was predictive for treatment response, no significant association was seen for baseline SUVmax alone. In addition, another study mainly including ESCC patients (> 70%) also found no association between pretreatment PET and response to chemoradiation16. While this is in line with our results, in two other studies with mainly ESCC patients, lower baseline SUVmax was associated with higher rates of clinical complete response20,21. In contrast to the presented data, in all these studies, treatment response was assessed by computed tomography using RECIST (Response Evaluation Criteria in Solid Tumours)39 and not by histopathological workup.
There was no independent association between SUVmax and OS. This is in line with several other studies analyzing the predictive value of baseline SUVmax in patients with predominantly ESCC12,13,40,41. When looking to other survival endpoints like relapse-free survival or disease-free survival, contradictory results are presented in the literature12,42. While patients with a SUVmax > 7 of the primary tumor tended to have a lower RFS after R0-esophagectomy than patients with a SUVmax < 7 in a study by Shimizu and colleagues42, in another study by Lemarignier et al.12 a higher baseline SUVmax of the primary tumor predicted longer disease-free survival in ESCC patients after chemoradiotherapy. Beside the mix-up of different histologic subtypes, contradictory results seen in the literature, might be partly explained by the fact that SUVmax generally depends on the assessment method and the technical standard of the PET equipment 43,44.
As shown MTV was not found to predict treatment response or OS in ESCC patients undergoing neoadjuvant chemoradiation. In contrast to our results large MTV was associated with shorter OS in previous trials12,13,41. However, while we used an absolute SUV threshold of 3 to determine MTV, all these studies used just relative or even individual SUV thresholds. Conforming with our data, in a study by Nakajo and colleagues11, who used an absolute SUV threshold of 2.5, MTV was also not associated with OS in a multivariate analysis. Thereby, different MTV definitions may impair the comparability of results and individual SUV threshold seem to be more suitable than absolute thresholds. In addition, most patients included in the mentioned studies were treated with either definite chemoradiation or esophagectomy, while all patients in our study underwent neoadjuvant chemoradiation and subsequent surgery. It remains unclear, to what extent the prognostic significance of MTV might be affected by treatment regimen. Regarding response to chemoradiation, our study confirms the result of an earlier study, in which also no association was seen between MTV and treatment response10.
Although SUVmax and MTV are still the most commonly used PET derived image parameters, recent studies increasingly focus on characterization of local tumor heterogeneity. Generally, heterogeneity within the tumour microenvironment is caused by hypoxia, necrosis and cellular proliferation. These factors are associated with adverse tumour biology and are considered as hallmarks of malignancy. Thus, textural parameters that correspond to increased heterogeneity (e.g. decreased uniformity) have been associated with poorer overall survival. Therefore, the prognostic impact of the three heterogeneity parameters busyness, contrast and uniformity was further evaluated in this study. None of the heterogeneity parameters was predictive for treatment response, which is in line with the results described by Tixier and colleagues21. They analyzed the impact of different textural features to predict response to chemoradiation in patients with mostly ESCC and no significant association was seen between treatment response and the parameters contrast and busyness.
In contrast to a study by Ganeshan and colleagues24, no association was seen between OS and uniformity in our study. However, comparison of the results is strongly impaired by the fact, that the authors included patients with mostly adenocarcinoma and used non-contrast-enhanced CT data for imaging analysis. While there are very limited data regarding the prognostic impact of heterogeneity parameters in patients with ESCC, association of baseline busyness or contrast and survival has already been described in several other tumor entities including rectal cancer, non-small cell lung cancer and hypopharyngeal carcinoma35,37,45.
Because parts of the analysis were done retrospectively, this study has some limitations. First, the study was conducted at a single institution and the total number of patients is limited. This clearly compromises any generalization of results and conclusions. However, the limited number of patients may be more than outweighed by the fact, that only patients with ESCC were included. Secondly, PET acquisition and reconstruction have been developed since 2005, which may further limit the comparability of imaging parameters. But even if the repeatability and reproducibility of the heterogeneity measurement depends on the image processing method and the reconstruction algorithm, local heterogeneity parameters are generally regarded as particularly robust46. Third, the imaging parameters in our study were defined using a SUV threshold of 3. This limits the comparability to other studies, where different SUV thresholds were used. However, the optimum threshold depends amongst others on the clinical setting, tumor characteristics and on the type of PET machine and therefore no standard threshold has been established for universal clinical use so far.
Although the predictive potential of different 18F-FDG-PET/CT derived textural features has successfully been shown for several types of malignancies, this study confirms the current limitation of baseline PET-CT guided response prediction in ESCC patients. This even applies to a particularly homogeneous patient population with only one histological subtype and homogeneous radiotherapy regime.
In conclusion, our data do not support the use of PET/CT derived imaging parameters to predict response to nCRT in patients with ESCC. However, the study supports the thesis that textural analysis of local heterogeneity in the tumor´s tracer uptake might also be suitable to predict survival and should be further evaluated.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Bray, F. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424 (2018).
Pennathur, A. et al. Oesophageal carcinoma. Lancet 381, 400–412 (2013).
Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft DK, AWMF): S3-Leitlinie Diagnostik und Therapie der Plattenepithelkarzinome und Adeno-karzinome des Ösophagus, Langversion 2.0, 2018, AWMF Registernummer: 021/023OLhttps://www.leitlinienprogramm-onkologie.de/leitlinien/oesophaguskarzi-nom/(Accessed December 14, 2019).
National Comprehensive Cancer Network. Esophageal and Esophagogastric Junction Cancers (Version 3.2019). http://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf. Accessed December 14, 2019.
Tepper, J. et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J. Clin. Oncol. 26, 1086–1092 (2008).
van Hagen, P. et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N. Engl. J. Med. 366, 2074–2084 (2012).
Bedenne, L. et al. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J. Clin. Oncol. 25, 1160–1168 (2007).
Flamen, P. et al. Utility of positron emission tomography for the staging of patients with potentially operable esophageal carcinoma. J. Clin. Oncol. 18, 3202–3210 (2000).
Heeren, P. A. et al. Detection of distant metastases in esophageal cancer with (18)F-FDG PET. J. Nucl. Med. 45, 980–987 (2004).
Zhang, P. et al. (18)F-fluorodeoxyglucose positron emission computed tomography for monitoring tumor response in esophageal carcinoma treated with concurrent chemoradiotherapy. Oncol. Lett. 15, 1845–1852 (2018).
Nakajo, M. et al. Texture analysis of (18)F-FDG PET/CT to predict tumour response and prognosis of patients with esophageal cancer treated by chemoradiotherapy. Eur. J. Nucl. Med. Mol. Imaging. 44, 206–214 (2017).
Lemarignier, C. et al. Pretreatment metabolic tumour volume is predictive of disease-free survival and overall survival in patients with oesophageal squamous cell carcinoma. Eur. J. Nucl. Med. Mol. Imaging 41, 2008–2016 (2014).
Hofheinz, F. et al. Confirmation of the prognostic value of pretherapeutic tumor SUR and MTV in patients with esophageal squamous cell carcinoma. Eur. J. Nucl. Med. Mol. Imaging 46, 1485–1494 (2019).
Domachevsky, L. et al. Baseline 18F-FDG PET/CT as predictor of the pathological response to neoadjuvant therapy in esophageal cancer: a retrospective study. Medicine (Baltimore) 97, e13412 (2018).
Hong, D. et al. Value of baseline positron emission tomography for predicting overall survival in patient with nonmetastatic esophageal or gastroesophageal junction carcinoma. Cancer 104, 1620–1626 (2005).
Hatt, M. et al. Baseline (1)(8)F-FDG PET image-derived parameters for therapy response prediction in oesophageal cancer. Eur. J. Nucl. Med. Mol. Imaging 38, 1595–1606 (2011).
Rizk, N. P. et al. Predictive value of initial PET-SUVmax in patients with locally advanced esophageal and gastroesophageal junction adenocarcinoma. J. Thorac. Oncol. 4, 875–879 (2009).
Levine, E. A. et al. Predictive value of 18-fluoro-deoxy-glucose-positron emission tomography (18F-FDG-PET) in the identification of responders to chemoradiation therapy for the treatment of locally advanced esophageal cancer. Ann. Surg. 243, 472–478 (2006).
Elimova, E. et al. 18-fluorodeoxy-glucose positron emission computed tomography as predictive of response after chemoradiation in oesophageal cancer patients. Eur. J. Cancer 51, 2545–2552 (2015).
Kato, H. et al. Prediction of response to definitive chemoradiotherapy in esophageal cancer using positron emission tomography. Anticancer Res. 27, 2627–2633 (2007).
Tixier, F. et al. Intratumor heterogeneity characterized by textural features on baseline 18F-FDG PET images predicts response to concomitant radiochemotherapy in esophageal cancer. J. Nucl. Med. 52, 369–378 (2011).
Desbordes, P. et al. Predictive value of initial FDG-PET features for treatment response and survival in esophageal cancer patients treated with chemo-radiation therapy using a random forest classifier. PLoS One 12, e0173208 (2017).
Yip, C. et al. Primary esophageal cancer: heterogeneity as potential prognostic biomarker in patients treated with definitive chemotherapy and radiation therapy. Radiology 270, 141–148 (2014).
Ganeshan, B. et al. Tumour heterogeneity in oesophageal cancer assessed by CT texture analysis: preliminary evidence of an association with tumour metabolism, stage, and survival. Clin. Radiol. 67, 157–164 (2012).
Giganti, F. et al. Pre-treatment MDCT-based texture analysis for therapy response prediction in gastric cancer: comparison with tumour regression grade at final histology. Eur. J. Radiol. 90, 129–137 (2017).
Goh, V. et al. Assessment of response to tyrosine kinase inhibitors in metastatic renal cell cancer: CT texture as a predictive biomarker. Radiology 261, 165–171 (2011).
Vija, A. H., Hawman, E. G., Engdahl, J. Analysis of a SPECT OSEM reconstruction method with 3D beam modeling and optional attenuation correction: phantom studies (2003).
Amadasun, M. & King, R. Textural features corresponding to textural properties. IEEE Trans. Syst. Man Cybern. 19(5), 1264–1274 (1989).
Ganeshan, B. et al. Tumour heterogeneity in non-small cell lung carcinoma assessed by CT texture analysis: a potential marker of survival. Eur. Radiol. 22, 796–802 (2012).
Zhang, L. et al. IBEX: an open infrastructure software platform to facilitate collaborative work in radiomics. Med. Phys. 42, 1341–1353 (2015).
Becker, K. et al. Histomorphology and grading of regression in gastric carcinoma treated with neoadjuvant chemotherapy. Cancer 98, 1521–1530 (2003).
R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing V, Austria. URL https://www.R-project.org (2021).
Hothorn, T. & Lausen, B. On the exact distribution of maximally selected rank statistics. Comput. Stat. Data Anal. 43, 121–137 (2003).
Gao, S. et al. Multiparametric PET/MR (PET and MR-IVIM) for the evaluation of early treatment response and prediction of tumor recurrence in patients with locally advanced cervical cancer. Eur. Radiol. https://doi.org/10.1007/s00330-019-06428-w (2019).
Lovinfosse, P. et al. FDG PET/CT radiomics for predicting the outcome of locally advanced rectal cancer. Eur. J. Nucl. Med. Mol. Imaging 45, 365–375 (2018).
Hatt, M. et al. Early prediction of pathological response in locally advanced rectal cancer based on sequential 18F-FDG PET. Acta Oncol. 52, 619–626 (2013).
Cook, G. J. et al. Are pretreatment 18F-FDG PET tumor textural features in non-small cell lung cancer associated with response and survival after chemoradiotherapy?. J. Nucl. Med. 54, 19–26 (2013).
Cheng, N. M. et al. Textural features of pretreatment 18F-FDG PET/CT images: prognostic significance in patients with advanced T-stage oropharyngeal squamous cell carcinoma. J. Nucl. Med. 54, 1703–1709 (2013).
Therasse, P. et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J. Natl. Cancer Inst. 92, 205–16 (2000).
Choi, J. Y. et al. 18F-FDG PET in patients with esophageal squamous cell carcinoma undergoing curative surgery: prognostic implications. J. Nucl. Med. 45, 1843–1850 (2004).
Hyun, S. H. et al. Prognostic value of metabolic tumor volume measured by 18F-fluorodeoxyglucose positron emission tomography in patients with esophageal carcinoma. Ann. Surg. Oncol. 17, 115–122 (2010).
Shimizu, D. et al. Clinical significance of SUVmax on preoperative 18F- fl uorodeoxyglucose positron emission tomography in patients who underwent R0-esophagectomy for esophageal cancer. Nagoya J. Med. Sci. 80, 401–409 (2018).
Feuardent, J., Soret, M., de Dreuille, O., Foehrenbach, H. & Buvat, I. Reliability of uptake estimates in FDG PET as a function of acquisition and processing protocols using the CPET. IEEE Trans. Nucl. Sci. 52(5), 1447–1452. https://doi.org/10.1109/TNS.2005.858222 (2005).
Boellaard, R. et al. Effects of noise, image resolution, and ROI definition on the accuracy of standard uptake values: a simulation study. J. Nucl. Med. 45, 1519–1527 (2004).
Oh, J. S. et al. Intratumor textural heterogeneity on pretreatment (18)F-FDG PET images predicts response and survival after chemoradiotherapy for hypopharyngeal cancer. Ann. Surg. Oncol. 22, 2746–2754 (2015).
Hatt, M. et al. Robustness of intratumour (1)(8)F-FDG PET uptake heterogeneity quantification for therapy response prediction in oesophageal carcinoma. Eur. J. Nucl. Med. Mol. Imaging 40, 1662–1671 (2013).
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
L.M. and S.M. initiated the project. L.M., T.P., J.C.P., K.S., S.M. generated and analyzed the data. L.M. and B.H. were responsible for statistical analyzes. L.M. and S.M. wrote the manuscript. M.J., H.F., S.E.C., advised and corrected the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Marr, L., Haller, B., Pyka, T. et al. Predictive value of clinical and 18F-FDG-PET/CT derived imaging parameters in patients undergoing neoadjuvant chemoradiation for esophageal squamous cell carcinoma. Sci Rep 12, 7148 (2022). https://doi.org/10.1038/s41598-022-11076-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-11076-0
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.