Abstract
Height loss starting in middle age is reportedly significantly associated with death due to cardiovascular disease. Impaired blood flow is the main pathology in cardiovascular disease. Hematopoietic stem cells such as CD34-positive cells play an important role in maintaining the microcirculation and preventing impaired blood flow by activating endothelial repair and angiogenesis. Therefore, circulating CD34-positive cell count could be associated with height loss. To clarify the association between circulating CD34-positive cell count and height loss, we conducted a follow-up study of 363 Japanese men aged 60–69 years over 2 years. Height loss was defined as being in the highest quartile of height decrease per year. Independent of known cardiovascular risk factors, circulating CD34-positive cell count was significantly inversely associated with height loss. The fully adjusted odds ratio (OR) and 95% confidence interval (CI) of height loss for circulating CD34-positive cell count (logarithmic values) was 0.49 (0.32, 0.74). This study suggests that a lower capacity to maintain the microcirculation due to a fewer CD34-positive cells might affect height loss.
Similar content being viewed by others
Introduction
A previous Japanese study reported that height loss starting in middle age is significantly associated with death due to coronary heart disease or stroke but not cancer1. However, the mechanism underlying this association has not yet been clarified.
Recent studies have revealed an inverse association between height and cardiovascular disease2,3,4,5,6 and a positive association between height and cancer2,7. Those studies indicated that participants with height loss and participants with comparative short stature have a higher risk of cardiovascular disease but not cancer.
Impaired blood flow is the main pathology in cardiovascular disease. Growth of feeding vessels (angiogenesis) is the main pathological condition in cancer8. Hematopoietic stem cells known as CD34-positive cells contribute to angiogenesis9,10 and play an important role in endothelial repair11,12. Our previous cross-sectional studies of men aged 65–69 years showed a significant positive association between height and circulating CD34-positive cell count among those with hypertension13 and among those with circulating CD34-positive cell count below the median value14. Thus, deficiencies in the development of angiogenesis or endothelial repair (lower capacity to maintain the microcirculation) caused by lower production of CD34-positive cells lead to a higher risk of cardiovascular disease in people with short stature15. However, the association between circulating CD34-positive cell count and height loss has not yet been clarified.
Regarding the risk for cardiovascular disease and cancer, individuals with height loss have characteristics in common with individuals with short stature1,2,3,4,5,6,7. Therefore, height loss could be inversely associated with circulating CD34-positive cell count since circulating CD34-positive cell count indicates the capacity to maintain the microcirculation. To clarify this association, we conducted a 2-year follow-up study of 363 Japanese men aged 60–69 years who participated in a general health check-up at least twice from 2014–2017.
Materials and methods
Study population
The present study was performed in two locations in Nagasaki prefecture, the city of Goto and the town of Saza. Detailed descriptions were provided elsewhere15. The methods related to the present risk survey, including circulating CD34-positive cell measurement, also have been described elsewhere16,17,18.
Written consent forms were made available to ensure that the participants understood the objective of the study. Informed consent was obtained from all participants. All procedures performed in this study were in accordance with the ethical standards of the institutional research committee and the 1964 Declaration of Helsinki and its later amendments. Ethical approval was obtained from the Ethics Committee for Human Use of Nagasaki University. The study was approved by the Ethics Committee of Nagasaki University Graduate School of Biomedical Sciences (Project Registration Number: 14051404-13).
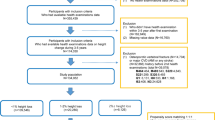
The study population comprised 498 male residents of Goto city and Saza town, rural communities in western Japan, aged 60–69 years who underwent an annual medical check-up from 2014–2015, which was considered the baseline evaluation. Individuals with no data on CD34-positive cells (n = 2) or serum test results (n = 2) were excluded. In order to avoid the influence of chronic inflammatory disease, subjects with a high white blood cell count (WBC count ≥ 10,000 cells/μL) (n = 5) were excluded. We also excluded 126 subjects who did not undergo an annual health check-up during the follow-up period (2016 to 2017). The remaining participants, 363 men aged 65.4 years ± 2.6 years (range, 60–69 years) were enrolled in the study. The mean follow-up period was 2.20 ± 0.53 years.
Data collection and laboratory measurements
Medical history was ascertained by specially trained interviewers. An automatic body composition analyzer (BF-220; Tanita, Tokyo, Japan) calculated body mass index (BMI, kg/m2) after measuring height and weight. A current drinker was defined as an individual with ethanol intake ≥ 23 g/week. After at least 5 min of rest, blood pressure (systolic and diastolic) was measured in the sitting position using a blood pressure measuring device (HEM-907; Omron, Kyoto, Japan). A heparin sodium tube, an EDTA-2 K tube, a siliconized tube, and a sodium fluoride tube were used to collect fasting blood samples for laboratory measurements. The heparin sodium tube was used to measure CD34-positive cell count. The EDTA-2 K tube was used to measure WBC count, hemoglobin (Hb), and platelet count. The siliconized tube was used to measure high-density lipoprotein cholesterol (HDLc), triglyceride, and serum creatinine levels. The sodium fluoride tube was used to measure hemoglobin A1c (HbA1c). To measure circulating CD34-positive cell count, an automated software program on the BD FACSCanto™ II system (BD Biosciences) was used in accordance with International Society of Hematotherapy and Graft Engineering guidelines19.
Using standard laboratory procedures, WBC count, platelet count, and levels of Hb, HDLc, triglycerides, serum creatinine, and HbA1c were measured at SRL, Inc. (Tokyo, Japan).
Using the method proposed by a working group of the Japanese Chronic Kidney Disease Initiative20, the estimated glomerular filtration rate (eGFR) was calculated: eGFR (mL/min/1.73 m2) = 194 × (serum creatinine (enzyme method))-1.094 × (age)-0.287.
Statistical analysis
To clarify the influence of age on circulating CD34-positive cell count and height loss, simple correlation analysis was performed.
Clinical characteristics of the study participants were compared by circulating CD34-positive cell count tertile: T1 (Low), < 0.78 cells/μL; T2, 0.78–1.31 cells/μL, and T3 (High), ≥ 1.32 cells/μL. Data are presented as means ± standard deviation (SD) or n (%), except for triglycerides. Since triglyceride values had a skewed distribution, they were expressed based on the median (interquartile range). Trend tests were also performed.
Height loss was defined as being in the highest quartile of height decrease per year. Logistic regression models were used to calculate odds ratios (ORs) and 95% confidence intervals (95% CIs) to determine the association between circulating CD34-positive cell count and height loss.
Adjustment for confounding factors was made in three ways. In Model 1, we adjusted only for age. In Model 2, we adjusted for age plus known cardiovascular risk factors, namely: BMI, systolic blood pressure (SBP), alcohol consumption status [never, former, or current [weekly alcohol consumption (23–45 g/week, 46–68 g/week, > 68 g/week)], smoking status (never, former, current), HDLc, triglycerides, HbA1c, and eGFR. Model 3 adjusted only for age, WBC count, Hb, platelet count, and height at baseline because they could act as confounding factors in the present study. CD34-positive cells are immature hematological precursors. WBC count could be associated with bone density loss21 while Hb could prevent height loss22. In conjunction with circulating CD34-positive cells, platelets contribute to endothelial repair12 and high platelet count is associated with low bone density23. In addition, height at baseline could influence the production of WBCs24, Hb25,26, platelets27, and CD34-positive cells13,14.
For sensitivity analysis, we re-ran analyses of the association between circulating CD34-positive cell count and height loss when height loss was defined as being in the highest quintile of height decrease per year.
We also performed simple correlation analysis and multiple linear regression analysis to clarify the correlation between circulating CD34-positive cell count and height loss as a continuous variable (mm/year). In multiple linear regression analysis, two models were constructed. The first model (Multivariable model 1) adjusted for age, BMI, SBP, current drinker status, current smoker status, HDLc, triglycerides, HbA1c, and eGFR. Multivariable model 2 adjusted for age, hematological parameters, and height at baseline. Logarithmic transformation was performed because circulating CD34-positive cell count and triglycerides had skewed distributions.
All statistical analyses were performed with SAS for Windows (version 9.4; SAS Inc., Cary, NC). Values of p < 0.05 were regarded as statistically significant.
Results
Characteristics of the study population
Among the study population, age was not significantly correlated with circulating CD34-positive cell count or height loss. The simple correlation coefficient for age and the logarithmic value of circulating CD34-positive cell count was 0.003 (p = 0.951). The correlation coefficient for age and height loss as a continuous variable was 0.02 (p = 0.656).
The characteristics of the study population by circulating CD34-positive cell count are shown in Table 1. Circulating CD34-positive cell count was significantly positively associated with known hematological parameters such as platelet count, Hb, and WBC count. Circulating CD34-positive cell count was also significantly positively associated with BMI and triglycerides and inversely associated with HDLc.
Association between height loss and circulating CD34-positive cell count
Table 2 shows ORs and 95% CIs for the association between height loss and circulating CD34-positive cell count. Circulating CD34-positive cell count was significantly inversely associated with height loss; the age-adjusted OR and 95% CI of height loss for circulating CD34-positive cell count (logarithmic values) was 0.48 (0.32, 0.71). This association remained even after further adjustment for known cardiovascular risk factors (0.49, (0.32, 0.74)). In addition, hematological parameters were significantly positively associated with circulating CD34-positive cell count. The association remained significant in Model 3 that adjusted for known hematological parameters (0.45 (0.28, 0.72)).
Correlation between height loss as a continuous variable and circulating CD34-positive cell count adjusted for cardiovascular risk factors
Table 3 shows the correlation between height loss as a continuous variable and known cardiovascular risk factors associated with circulating CD34-positive cells. Independent of known confounding variables, circulating CD34-positive count was significantly inversely associated with height loss as a continuous variable. No significant correlations between height loss and other variables were found.
Table 4 shows the correlation between height loss as a continuous variable and hematological parameters including circulating CD34-positive cell count. Simple correlation analysis showed that Hb and circulating CD34-positive cell count were significantly inversely associated with height loss. After adjusted for those variables and height at baseline, Hb was no longer significant but circulating CD34-positive cell count remained significant.
Sensitivity analysis
To assess sensitivity, we performed the main analyses with height loss defined as being in the highest quintile of height decrease per year, as in our previous study22. We obtained essentially the same results. The ORs and 95% CIs for height loss with circulating CD34-positive cell count (logarithmic values) were 0.52 (0.34, 0.79) for Model 1, 0.51 (0.32, 0.79) for Model 2, and 0.54 (0.32, 0.89) for Model 3.
Discussion
The major finding of this study with elderly men is that circulating CD34-positive cell count is inversely associated with height loss. A previous study with Japanese workers aged 40–74 years revealed a significant inverse association between Hb and height loss defined as either being in the highest quartile or quintile of height decrease per year among men with BMI < 25 kg/m2 but not among men with BMI ≥ 25 kg/m222. In the present study, simple correlation analysis showed that Hb is slightly but significantly inversely correlated with height loss. However, the correlation was not significant after adjusting for age, other known hematological parameters, and height at baseline.
In the present study, we found further evidence that, independent of BMI and Hb, circulating CD34-positive cell count is inversely associated with height loss. However, the mechanisms explaining this association have not yet been clarified.
The major causes of height loss in elderly men are intervertebral disc degeneration and vertebral fractures associated with osteoporosis. Although the development of angiogenesis has been observed in degenerative intervertebral disc lesions28, angiogenesis is not a major course of intervertebral disc degeneration.
The regulation of angiogenesis by hypoxia is an important component of homeostatic mechanisms that link vascular oxygen supply to oxygen demands29. Since hypoxia accelerates intervertebral disc degeneration30,31, inadequate angiogenesis related to lower adaptability to hypoxia might play an important role in the development of intervertebral disc degeneration. In addition, bone is a highly vascularized tissue, and dysregulation of the microcirculation is associated with many bone diseases, including osteoporosis32. Therefore, less angiogenesis related to low levels of circulating CD34-positive cells could be associated with height loss due to a higher risk of intervertebral disc degeneration and osteoporosis.
In older men, height loss is independently associated with an increased risk of all-cause mortality and coronary heart disease33 while a higher number of circulating CD34-positive cells is inversely associated with all-cause and cardiovascular mortality34,35. Therefore, the present results showing a significant inverse association between circulating CD34-positive cell count and height loss can help clarify the potential mechanism for the association between height loss and cardiovascular disease.
Measurement of CD34-positive cells requires fresh samples, analyzed within 24 h after blood collection. Approximately 30 min are required to process one sample. Since a maximum of 20 samples could be processed for CD34-positive cell measurement each day, measuring CD34-positive cell count is hard to realize in a general population study. Therefore, we limited the measurement of CD34-positive cell count to men aged 60–69 years who participated in a general health check-up in the city of Goto and the town of Saza. Detailed descriptions have been described elsewhere15.
The potential limitations of this study warrant consideration. Intervertebral disc degeneration and vertebral fractures associated with osteoporosis might play an important role in height loss among adults, but those data were not available to us, as in our previous study22. Many intervertebral disc degeneration cases are pain free36. Most of the vertebral fracture cases are asymptomatic and diagnosed incidentally37. To identify those diseases among general population, plain radiographs, computed tomography (CT), or magnetic resonance imaging (MRI) are necessary. An efficient cutoff point to define height loss has not been established. In the present study, we defined height loss as being in the highest quartile of height decrease per year. However, our sensitivity analysis based on quintile of height decrease per year showed essentially the same associations. Furthermore, a significant inverse correlation was also observed between height loss as a continuous variable and circulating CD34-positive cell count. Oxidative stress and hypoxia might play an important role in the present associations. However, we had no data to evaluate oxidative stress and hypoxia. Further epidemiological investigations with data on hypoxia inducing factor, superoxide dismutase, and 8-hydroxydeoxyguanosine are required. Due to financial and technical reasons, we limited the measurement of CD34-positive cell count to men aged 60–69 years. Further investigation with larger sample could be informative. Sex steroid hormone could influence on survival of CD34-positive cell38 and bone size39. However, as like previous studies that reported the association between height loss and cardiovascular disease1,33, we have no data of sex steroid hormone. Furter investigation with data of sex steroid hormone is necessary.
In conclusion, circulating CD34-positive cell count is inversely associated with height loss among elderly men. This study indicates that a lower capacity to maintain the microcirculation due to a fewer of CD34-positive cells might affect height loss.
References
Masunari, N. et al. Height loss starting in middle age predicts increased mortality in the elderly. J. Bone. Miner. Res 27(1), 138–145 (2012).
Sawada, N. et al. The association between adult attained height and sitting height with mortality in the European Prospective Investigation into Cancer and Nutrition (EPIC). PLoS ONE 12(3), e0173117 (2017).
Hozawa, A. et al: NIPPON DATA80 Research Group. Relation of adult height with stroke mortality in Japan: NIPPON DATA80. Stroke 38(1), 22–26 (2007).
Honjo, K. et al. Adult height and the risk of cardiovascular disease among middle aged men and women in Japan. Eur. J. Epidemiol 26(1), 13–21 (2011).
Park, C. S. et al. Association between adult height, myocardial infarction, heart failure, stroke and death: a Korean nationwide population-based study. Int. J. Epidemiol 47(1), 289–298 (2018).
Shimizu, Y. et al: CIRCS Investigators. Adult height and body mass index in relation to risk of total stroke and its subtypes: the Circulatory Risk in Communities Study. J. Stroke. Cerebrovasc. Dis 23(4), 667–674 (2014).
Ong, J. S. et al. Height and overall cancer risk and mortality: evidence from a Mendelian randomisation study on 310,000 UK Biobank participants. Br. J. Cancer 118(9), 1262–1267 (2018).
Viallard, C. et al. Tumor angiogenesis and vascular normalization: alternative therapeutic targets. Angiogenesis 20(4), 409–426 (2017).
Siemerink, M. J. et al. CD34 marks angiogenic tip cells in human vascular endothelial cell cultures. Angiogenesis 15(1), 151–163 (2012).
Takakura, N. et al. A role for hematopoietic stem cells in promoting angiogenesis. Cell 102(2), 199–209 (2000).
Shi, Q. et al. Evidence for circulating bone marrow-derived endothelial cells. Blood 92(2), 362–367 (1998).
Daub, K. et al. Platelets induce differentiation of human CD34+ progenitor cells into foam cells and endothelial cells. FASEB. J 20(14), 2559–2561 (2006).
Shimizu, Y. et al. Height is an indicator of vascular maintenance capacity in older men. Geriatr. Gerontol. Int 17(10), 1729–1736 (2017).
Shimizu, Y. et al. Association between height and circulating CD34-positive cells taken into account for the influence of enhanced production among elderly Japanese men: a cross-sectional study. Aging (Albany NY) 11(2), 663–672 (2019).
Shimizu, Y. et al. Influence of height on endothelial maintenance activity: a narrative review. Environ. Health. Prev. Med 26(1), 19 (2021).
Shimizu, Y. et al. Associations between handgrip strength and hypertension in relation to circulating CD34-positive cell levels among Japanese older men: a cross-sectional study. Environ. Health Prev. Med 26(1), 62 (2021).
Shimizu, Y. et al. Circulating CD34+ cells and active arterial wall thickening among elderly men: A prospective study. Sci. Rep 10(1), 4656 (2020).
Shimizu, Y. et al. Association between chronic kidney disease and carotid intima-media thickness in relation to circulating CD34-positive cell count among community-dwelling elderly Japanese men. Atherosclerosis 283, 85–91 (2019).
Sutherland, D. R. et al. The ISHAGE guidelines for CD34+ cell determination by flow cytometry: International Society of Hematotherapy and Graft Engineering. J. Hematother 5(3), 213–226 (1996).
Matsuo, S. et al. Revised equations for estimated GFR from serum creatinine in Japan. Am. J. Kidney. Dis 53(6), 982–992 (2009).
Valderrábano, R. J. et al: Osteoporotic Fractures in Men (MrOS) Study Research Group. Bone density loss is associated with blood cell counts. J. Bone. Miner. Res 32(2), 212–220 (2017).
Shimizu, Y. et al. Hemoglobin and adult height loss among Japanese workers: A retrospective study. PLoS ONE 16(8), e0256281 (2021).
Kristjansdottir, H. L. et al. High platelet count is associated with low bone mineral density: The MrOS Sweden cohort. Osteoporos. Int 32(5), 865–871 (2021).
Shimizu, Y. et al. Short stature is an inflammatory disadvantage among middle-aged Japanese men. Environ. Health. Prev. Med 21(5), 361–367 (2016).
Shimizu, Y. et al. Height indicates hematopoietic capacity in elderly Japanese men. Aging (Albany NY) 8(10), 2407–2413 (2016).
Shimizu, Y. et al. Height and drinking status in relation to risk of anemia in rural adult healthy Japanese men: the Nagasaki Islands study. Aging. Male 18(2), 100–105 (2015).
Shimizu, Y. et al. Possible mechanism underlying the association between height and vascular remodeling in elderly Japanese men. Oncotarget 9(8), 7749–7757 (2017).
Koike, Y. et al. Angiogenesis and inflammatory cell infiltration in lumbar disc herniation. Spine (Phila Pa 1976) 28(17), 1928–1933 (2003).
Pugh, C. W. et al. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat. Med 9(6), 677–684 (2003).
Huang, Y. et al. Elevated expression of hypoxia-inducible factor-2alpha regulated catabolic factors during intervertebral disc degeneration. Life. Sci 232, 116565 (2019).
Meng, X. et al. Hypoxia-inducible factor (HIF)-1alpha knockout accelerates intervertebral disc degeneration in mice. Int. J. Clin. Exp. Pathol 11(2), 548–557 (2018).
Tong, X. et al. The effect of exercise on the prevention of osteoporosis and bone angiogenesis. Biomed. Res. Int 2019, 8171897 (2019).
Wannamethee, S. G. et al. Height loss in older men: associations with total mortality and incidence of cardiovascular disease. Arch. Intern. Med 166(22), 2546–2552 (2006).
Muggeridge, D. et al. CD34(+) progenitors are predictive of mortality and are associated with physical activity in cardiovascular disease patients. Atherosclerosis 333, 108–115 (2021).
Mandraffino, G. et al. Circulating progenitor cells and the elderly: a seven-year observational study. Exp. Gerontol 47(5), 394–400 (2012).
Peng, Y. et al. Symptomatic versus asymptomatic intervertebral disc degeneration: Is inflammation the key?. Crit. Rev. Eukaryot. Gene. Expr 25(1), 13–21 (2015).
McCarthy, J. et al. Diagnosis and management of vertebral compression fractures. Am. Fam. Physician 94(1), 44–50 (2016).
Kim, S. W. et al. Direct and indirect effects of androgens on survival of hematopoietic progenitor cells in vitro. J. Korean. Med. Sci 20(3), 409–416 (2005).
Banica, T., et al. Modest changes in sex hormones during early and middle adulthood affect bone mass and size in healthy men. A prospective cohort study. J. Bone. Miner. Res (2022). Online ahead of print.
Acknowledgements
We are grateful to the staff of Goto City Hall and Saza city office for their outstanding support. This study was supported by Grants-in-Aid for Scientific Research from Japan Society for the Promotion of Science (No.21H02575, No.22K06421).
Author information
Authors and Affiliations
Contributions
Y.S. designed the study, performed the statistical analyses, interpreted the data, and drafted and revised the manuscript. Y.S., S.Y.K., K.N., F.N., M.T., Y.H., H.Y., S.N., M.K., N.H., Y.N., and T.M. assisted with the study design, were involved in data collection, and checked the manuscript. M.K., H.Y., Y.N., and T.M. participated in the study concept and checked the manuscript. T.M. was the general coordinator and designed the study. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shimizu, Y., Kawashiri, SY., Nobusue, K. et al. Association between circulating CD34-positive cell count and height loss among older men. Sci Rep 12, 7175 (2022). https://doi.org/10.1038/s41598-022-11040-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-11040-y
This article is cited by
-
X-irradiated umbilical cord blood cells retain their regenerative effect in experimental stroke
Scientific Reports (2024)
-
Association between atherosclerosis and height loss among older individuals
Scientific Reports (2024)
-
Association between serum albumin levels and height loss in Japanese workers: a retrospective study
Journal of Physiological Anthropology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.