Abstract
Coronavirus disease 2019 (COVID-19) is a new pandemic the entire world is facing since December of 2019. Several risk factors are identified in developing severe disease and one of which is preexisting type 2 diabetes mellitus. Metformin is known to have host-directed anti-viral and anti-inflammatory properties. However, whether these effects offer lower mortality remains unclear. In this retrospective study, we aim to address whether metformin use prior to admission decreases mortality in patients with COVID-19 and pre-existing type 2 diabetes mellitus. A total of 1356 hospitalized patients with COVID-19 and pre-existing type 2 diabetes mellitus was analyzed by multivariable regression. Covariates that potentially confound the association were further adjusted using propensity score matching or inverse probability of treatment weighting. We found that metformin therapy prior to admission in patients with COVID-19 and type 2 diabetes mellitus was significantly associated with less primary outcome events including in-hospital mortality and hospice care enrollment with an odds ratio (OR) of 0.25 (95% CI 0.06–0.74) and less in-hospital length of stay, compared to the non-metformin group. Our results provide supporting evidence that metformin may confer increased survival in patients with COVID-19 and type 2 diabetes mellitus treated with metformin prior to hospitalization.
Similar content being viewed by others
Introduction
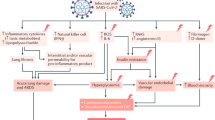
Rapid outbreak and spread of coronavirus disease 2019 (COVID-19), caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), leads to a global health crisis1,2,3,4,5. Risk factors and comorbidities linked to worse outcomes for COVID-19 patients have been identified. These include old age5, chronic pulmonary disease and smoking6, cardiovascular disease6, hypertension7, diabetes mellitus and obesity8. Patients with pre-existing diabetes mellitus, depending on nations and patient cohorts, have been reported to account for patients with COVID-19 from 5 to 20% in China, 17% in Lombardy in Italy to 33% in the US1,2,3,4,5. Diabetic patients hospitalized with COVID-19 are two- to three-fold more likely to be admitted into intensive care units than that of non-diabetics and the mortality rate is at least doubled6,9,10,11, suggesting urgent need for effective treatments in patients with COVID-19 and diabetes mellitus.
Metformin is widely used as the first-line therapy for type 2 diabetes mellitus12. Metformin is effective, safe, and inexpensive and may reduce the risk for cardiovascular events and death in type 2 diabetes mellitus13. Historically, because of its host-directed anti-viral properties, metformin was used during the treatment of influenza outbreak14. Likewise, based on the pathogenesis of SARS-CoV-2, several mechanisms have been speculated about the possible beneficial effects of metformin in COVID-19 patients with pre-existing type 2 diabetes mellitus, such as anti-inflammatory effects15, reduction in neutrophils16, increasing the cellular pH to inhibit viral infection, interfering with the endocytic cycle and reversing established lung fibrosis17. In contrast, metformin use has also been challenged in COVID-19 patients with type 2 diabetes mellitus because of the potential risk for lactic acidosis, particularly in the cases of multi-organ failure and for promoting SARS-CoV-2 infection by possibly increasing in ACE2 availability in the respiratory tract18. Recent accumulating evidence suggests that treating COVID-19 patients with pre-existing type 2 diabetes mellitus with metformin may not be harmful, but whether metformin endows a protective effect and offers a low mortality in patients with COVID-19 and type 2 diabetes mellitus remains inconclusive. Therefore, more clinical data regarding the use of metformin in COVID-19 patients with pre-existing type 2 diabetes mellitus is needed to provide clinical evidence. In the present study, we aimed to address whether metformin use is beneficial in COVID-19 patients with pre-existing type 2 diabetes mellitus. We found that COVID-19 patients with pre-existing type 2 diabetes mellitus treated with metformin prior to hospitalization had favorable outcome compared to metformin non-users. Our findings support the use of metformin as the first-line therapy for type 2 diabetes mellitus in patients with COVID-19 prior to hospitalization.
Methods
Study design and participants
We conducted a retrospective study of COVID-19 patients admitted to the St Luke’s University Health Network (SLUHN) from 3/16/2020 to 2/15/2021. COVID-19 is defined by positive results of RT-PCR for COVID-19 nasal swab specimens. The inclusion criteria included patients (Age ≥ 18) with COVID-19 and pre-existing type 2 diabetes mellitus. The exclusion criteria were incomplete medical records, lack of outcome data and type 1 DM. Patients with in-hospital length of stay less than 1 day were also excluded as in-hospital mortality was not considered in patients with length of stay less than 1 day. Oral hypoglycemic medications including metformin were held during hospitalization according to the SLUHN policy. Patients with pre-existing type 2 diabetes mellitus who took metformin prior to admission were designated as the metformin group and those did not receive metformin prior to admission were assigned as the non-metformin group. Metformin use was determined as documented in the home medication list in EPIC within the 3 months before admission. The primary outcome was a composite of in-hospital death or hospice care enrollment. Death that occurred within 24 h of admission was not counted as in-hospital death. The secondary outcomes were in-hospital length of stay and mechanical ventilation. This study was approved by the SLUHN institutional review board (SLIR 2020-100) with a waiver of informed consent for retrospective analysis of de-identified data. All methods were performed in accordance with the relevant guidelines and regulations.
Multivariable regression
To investigate the potential effect of metformin on the primary outcome, multivariable regression was applied. Clinically relevant confounders including age, height, weight, gender, race, comorbidities [congestive heart failure (CHF), coronary heart disease (CAD), asthma, chronic obstructive pulmonary disease (COPD)], clinical lab tests (BUN, creatinine, ALT, total bilirubin) were entered into a multivariate logistic regression model as covariates. To avoid the introduction of bias, no imputations were performed for missing values. Patients with missing values were excluded from the analysis.
Propensity score matching (PSM) and inverse probability of treatment weighting (IPTW)
To account for the potential confounders associated with the use of metformin and to ensure the robustness of our results, propensity score matching was used based on variables including age, height, weight, gender, race, comorbidities (CHF, CAD, asthma, COPD), clinical lab tests (BUN, creatinine, ALT, total bilirubin). Propensity scores for each patient were estimated by a multivariate logistic regression model. The matched cohort was created at a 1:1 ratio with a caliper size of 0.05. Using the estimated propensity scores as weights, the IPTW method was used to generate additional weighted cohort (IPTW cohort). The balance between covariates was evaluated by estimating standardized mean differences (SMD) in both the propensity score matched cohort and the IPTW cohort. SMD < 0.1 is considered a negligible group imbalance.
Statistical analysis
Data were presented as mean and 95% confidence interval (CI) or median and interquartile range (IQR) for continuous variables. For differences in continuous variables, Student’s t tests (normally distributed) or Mann–Whitney U tests (non-normally distributed) were used in two groups for comparison. Kruskal–Wallis tests with pairwise comparisons using Wilcoxon rank sum test were used for multiple groups, where P values were adjusted with the Benjamini & Hochberg method. Categorical variables were expressed as percentage (%). For the comparison of categorical variables, χ2 tests were used. The risk of primary outcome was calculated as OR using multivariate logistic regression models. P values < 0.05 were considered to be statistically significant. All analyses were conducted with R (version 3.6.1).
Results
Baseline characteristics
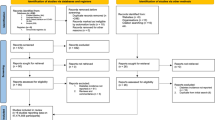
A total of 1872 admissions with confirmed COVID-19 and diabetes from 11 hospitals in the St Luke’s University Health Network were initially included for this study. After excluding multiple admissions (hospital length of stay less than 24 h, type 1 diabetes, or missing value), we included 1356 patients in our study cohort (Fig. 1). In the final cohort, there were 361 patients taking metformin prior to admission and 995 patients treated with anti-diabetic other than metformin. The baseline characteristics of our cohort on admission are summarized in Table 1.
Primary outcomes
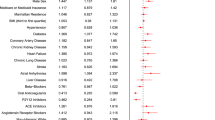
The primary outcome was a composite of in-hospital death or hospice. Of the 361 in the metformin group, primary outcome events occurred in 3 patients (0.83%) including 2 of in-hospital death and 1 for hospice, compared with 40 of 995 (4.02%) in the non-metformin group, specifically 21 of in-hospital death and 19 for hospice. In the multivariable logistic regression analyses, after adjusting age, gender, race, comorbidities, BUN, creatinine, ALT, total bilirubin, outpatient metformin use was significantly associated with lower primary outcome events (OR, 0.25; 95% CI 0.06–0.74; P = 0.027) compared to non-metformin (Fig. 2).
To verify the robustness of the model, we conducted a sensitivity study with randomly excluding patients from two hospitals. The adjust OR from this new cohort (n = 1143) for the primary outcome was 0.25 (95% CI 0.06–0.76; P = 0.031). To account for confounding that could lead to the protective association between metformin and the primary outcome, we further performed propensity score matching (PSM) and propensity score-based inverse probability of treatment weighting (IPTW) analyses. After PSM or IPTW, the covariates between the metformin group and the non-metformin group were well balanced based on SMD as shown in Table 1 and Fig. 3. In the PSM matched cohort, after adjusting the above variables, there was a reduced risk of primary outcome events in the metformin group (adjusted OR, 0.22; 95% CI 0.04–0.81; P = 0.038) (Fig. 2). Similarly, in the IPTW matched cohort, the adjusted OR for the primary outcome was 0.25 (95% CI 0.07–0.89; P = 0.033).
Change in standardized mean difference (SMD) before and after matching. PSM propensity score matching, IPTW inverse probability of treatment weighting, CHF congestive heart failure, CAD coronary artery disease, COPD chronic obstructive pulmonary disease, ALT alanine aminotransferase, BUN blood urea nitrogen, TBILI total bilirubin.
Secondary outcomes
To further investigate the potential effects of metformin use prior to admission, we accessed in-hospital length of stay and the rate of mechanical ventilation in the original cohort and PS matched cohort (Table 2). The in-hospital length of stay was significantly lower with a mean of 6.42 ± 5.51 days in the metformin group compared to 8.04 ± 7.78 days in the non-metformin group (P < 0.001) in the original cohort. A similar result was maintained (P = 0.049) in the propensity score matched cohort with a mean of 6.43 ± 5.51 days in the metformin group and 7.38 ± 7.40 days in the non-metformin group, respectively. For mechanical ventilation, while the rate (3.3%) in the metformin group was markedly smaller than that (6.7%) in the non-metformin group (P = 0.018) in the original cohort, there was no difference in the matched cohort.
Inflammatory responses in the metformin group versus the non-metformin group
Metformin has been shown to have anti-inflammatory effects15. The inflammatory response has been shown to play a pivotal role in the progression of COVID-19, and inflammatory cytokine storm ultimately results in severe COVID-19. To determine whether use of metformin prior to admission affects inflammatory responses in COVID-19 patients, we compared the measured serum levels of ferritin, C-reactive protein (CRP) and interlukin-6 (IL-6) on admission and peak levels during hospitalization between the metformin group and the non-metformin group. In this retrospective cohort, serum ferritin was measured in 92.2% of cases, CRP was measured in 91.5% of cases and IL-6 was measured in 39.0% of cases. There were no statistical differences between the metformin group and non-metformin group for all three markers on admission or peak levels during hospitalization (Fig. 4). Lastly, to further probe whether use of metformin affects inflammatory responses in patients admitted to the intensive care units (ICU) or regular wards, we performed subgroup analyses in four groups based on use of metformin and ICU admission. We found that CRP peak levels, and ferritin on admission and peak levels were higher in ICU patients without metformin use, compared to non-ICU patients without metformin prior to admission (P = 0.008, P < 0.001, P < 0.001, respectively) after adjustment for multiple comparisons, whereas there were no statistical differences among other groups.
Discussion
With the pandemic of COVID-19 and its high mortality especially in patients with pre-existing diabetes mellitus, there is an urgent need to develop treatments that are effective, safe, and available immediately. Metformin is a widely used anti-diabetic agent that is safe, effective, and inexpensive for type 2 diabetes mellitus. In view of host-directed anti-viral properties and anti-inflammatory effects of metformin, we set out to explore whether metformin is beneficial in patients with COVID-19 and preexisting type 2 diabetes mellitus. In this retrospective cohort study, we found that metformin therapy prior to admission in patients with COVID-19 and preexisting type 2 diabetes mellitus was significantly associated with reduced primary outcome events, which was a composite of in-hospital mortality and enrollment in hospice care. Of note, most patients enrolled in hospice/comfort care passed away shortly in the setting of COVID-19 infection in our cohort. In agreement with our findings, in CORONADO study, a large observational multicenter French study, metformin users prior to hospital admission were associated with a significant reduction in mortality at day 7, compared to non-users in an unadjusted analysis (OR 0.59, 95% CI 0.42–0.84). There was a trend but not significant decrease in mortality after the full adjustment19. In a retrospective study of 120 Chinese patients with pre-existing diabetes and confirmed and unconfirmed but clinically diagnosed COVID-19, there was a reduced but not statically significant trend for in-hospital mortality in metformin users (n = 43), compared to metformin non-users (9.3% vs 19.5%)20. Consistent with studies in China and French, a recent retrospective study in USA with 220 COVID-19 patients with pre-existing diabetes including 76 metformin users and 144 non-users showed that metformin treatment was independently and significantly associated with a reduction in mortality in COVID-19 with type 2 diabetes mellitus (OR 0.33; 95% CI 0.13–0.84; p = 0.0210)21. In another large US retrospective study of 6,256 subjects (> 95% diabetes, 52.8% female, mean age 75 years) from UnitedHealth database, a significantly decreased mortality in women (but not in men) was observed in metformin users with an odds ratio of 0.759 by propensity matching and an hazard ratio of 0.785 by Cox proportional-hazards, compared to metformin non-users22. Furthermore, retrospective cohort analysis of US electronic health record (EHR) data from 12 hospitals and 60 primary care clinics in the Midwest have shown that outpatient metformin use was associated with a decrease in mortality from COVID‐19, OR 0.32 (P = 0.002), and a trend towards decreased admission for COVID‐1923. These studies indicate the possible benefit of metformin therapy prior to admission in COVID-19 patients with pre-existing type 2 diabetes mellitus.
While metformin therapy prior to admission is significantly associated with reduced mortality, there is evidence suggesting metformin treatment might be effective in patients with COVID-19 and pre-existing type 2 diabetes mellitus during hospitalization. In a retrospective analysis in China of 283 COVID-19 patients with pre-existing type 2 diabetes mellitus, treating patients with metformin (n = 104) was associated with a significantly lower in-hospital mortality, compared with metformin non-users (n = 179) with similar patients characteristics, laboratory parameters, and treatments (2.9% vs 12.3%, respectively; P = 0.01), despite significantly higher fasting blood glucose levels in metformin users24. Furthermore, another retrospective study of 1213 hospitalized COVID-19 patients with pre-existing type 2 diabetes mellitus on the use of metformin from 16 hospitals in China found that metformin was not only associated with an increased incidence of acidosis (adjusted hazard ratio 2.45, 95% CI 1.08–5.54, P = 0.032), but with a significant reduction in heart failure (adjusted hazard ratio 0.61, 95% CI 0.43–0.87, P = 0.006) as well as inflammatory responses, although there were no differences in 28-day COVID-19-related mortality or the length of hospitalization25. These results further support continuing use of metformin in COVID-19 patients with pre-existing type 2 diabetes mellitus with closely monitoring acidosis and kidney function.
In response to SARS-CoV-2 infection, it seems that there are two distinct but overlapping pathologic phases; the first viral response phase triggered by the virus itself and the second inflammatory phase medicated by the host response26. Accumulating evidence indicates that many patients with severe COVID-19 manifest cytokine storm syndrome. Therefore, agents with evidence of reducing hyper-inflammation in COVID-19 patients could be therapeutic. It has been reported that metformin has anti-inflammatory effects15 and can modulate the immune responses and restore immune homeostasis in immune cells via AMPK-dependent mechanisms27. In a retrospective study of 120 Chinese patients with pre-existing diabetes and confirmed and unconfirmed but clinically diagnosed COVID-19, a significantly lower increase in interleukin-6 was observed in metformin users prior to admission, compared to non-users (n = 77)20. In addition, the dynamic trajectories of serum inflammatory factors, including CRP, IL-6, IL-2, and tumor necrosis factor-alpha (TNF-α), have demonstrated lower degrees of elevation in the hospitalized COVID-19 patients with metformin treatment than the non-metformin group, particularly in patients with severe COVID-1925. Conversely, there were no significant differences in the levels of ferritin, CRP, or IL-6 on admission and peak value during hospitalization between metformin users and non-metformin users in our study. More studies are needed to clarify whether metformin could mitigate inflammatory responses in patients with COVID-19.
In our study, although efforts were made to balance and control for potential confounding factors by multiple variable adjustments and propensity score matching, due to the inherent nature of retrospective observational studies, residual confounders are likely to exist and could not be balanced. Future prospective randomized controlled trials are needed to determine any definitive clinical beneficial outcomes in patients with COVID-19 and pre-existing type 2 diabetes mellitus treated with metformin.
In conclusion, our findings demonstrate that metformin therapy prior to admission in patients with COVID-19 and pre-existing type 2 diabetes mellitus is associated with a significant reduction of in-hospital mortality and provide clinical evidence in support of metformin as first line anti-diabetic agent for patients with COVID-19 and pre-existing type 2 diabetes mellitus.
Data availability
The de-identified dataset for the current study is available from the corresponding author on reasonable request.
References
Guan, W. J. et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 382, 1708–1720. https://doi.org/10.1056/NEJMoa2002032 (2020).
Wu, Z. & McGoogan, J. M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72314 cases from the Chinese center for disease control and prevention. JAMA https://doi.org/10.1001/jama.2020.2648 (2020).
Yang, X. et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 8, 475–481. https://doi.org/10.1016/S2213-2600(20)30079-5 (2020).
Cummings, M. J. et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: A prospective cohort study. Lancet 395, 1763–1770. https://doi.org/10.1016/S0140-6736(20)31189-2 (2020).
Grasselli, G. et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA https://doi.org/10.1001/jama.2020.5394 (2020).
Guan, W. J. et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: A nationwide analysis. Eur. Respir. J. https://doi.org/10.1183/13993003.00547-2020 (2020).
Yang, Q. et al. Effect of hypertension on outcomes of adult inpatients with COVID-19 in Wuhan, China: A propensity score-matching analysis. Respir. Res. 21, 172. https://doi.org/10.1186/s12931-020-01435-8 (2020).
Lighter, J. et al. Obesity in patients younger than 60 years is a risk factor for COVID-19 hospital admission. Clin. Infect. Dis. 71, 896–897. https://doi.org/10.1093/cid/ciaa415 (2020).
Zhou, F. et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 395, 1054–1062. https://doi.org/10.1016/S0140-6736(20)30566-3 (2020).
Roncon, L., Zuin, M., Rigatelli, G. & Zuliani, G. Diabetic patients with COVID-19 infection are at higher risk of ICU admission and poor short-term outcome. J. Clin. Virol. 127, 104354. https://doi.org/10.1016/j.jcv.2020.104354 (2020).
Huang, I., Lim, M. A. & Pranata, R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia: A systematic review, meta-analysis, and meta-regression. Diabetes Metab. Syndr. 14, 395–403. https://doi.org/10.1016/j.dsx.2020.04.018 (2020).
American Diabetes. Promoting health and reducing disparities in populations. Diabetes Care 40, 6–10. https://doi.org/10.2337/dc17-S004 (2017).
Holman, R. R., Paul, S. K., Bethel, M. A., Matthews, D. R. & Neil, H. A. 10-year follow-up of intensive glucose control in type 2 diabetes. N. Engl. J. Med. 359, 1577–1589. https://doi.org/10.1056/NEJMoa0806470 (2008).
Garcia, E. Y. Flumamine, a new synthetic analgesic and anti-flu drug. J. Philipp. Med. Assoc. 26, 287–293 (1950).
Cameron, A. R. et al. Anti-inflammatory effects of metformin irrespective of diabetes status. Circ. Res. 119, 652–665. https://doi.org/10.1161/CIRCRESAHA.116.308445 (2016).
Dalan, R. Metformin, neutrophils and COVID-19 infection. Diabetes Res. Clin. Pract. 164, 108230. https://doi.org/10.1016/j.diabres.2020.108230 (2020).
Esam, Z. A proposed mechanism for the possible therapeutic potential of Metformin in COVID-19. Diabetes Res. Clin. Pract. 108282. doi:https://doi.org/10.1016/j.diabres.2020.108282 (2020).
Ursini, F., Ciaffi, J., Landini, M. P. & Meliconi, R. COVID-19 and diabetes: Is metformin a friend or foe?. Diabetes Res. Clin. Pract. 164, 108167. https://doi.org/10.1016/j.diabres.2020.108167 (2020).
Cariou, B. et al. Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: The CORONADO study. Diabetologia 63, 1500–1515. https://doi.org/10.1007/s00125-020-05180-x (2020).
Chen, Y. et al. Clinical characteristics and outcomes of patients with diabetes and COVID-19 in association with glucose-lowering medication. Diabetes Care 43, 1399–1407. https://doi.org/10.2337/dc20-0660 (2020).
Crouse, A. B. et al. (2020). Metformin use is associated with reduced mortality in a diverse population with COVID-19 and diabetes. Front Endocrinol 11, 600439, https://doi.org/10.3389/fendo.2020.600439
Bramante, C. T. et al. Metformin and risk of mortality in patients hospitalised with COVID-19: A retrospective cohort analysis. Lancet Healthy Longev. 2, e34–e41. https://doi.org/10.1016/S2666-7568(20)30033-7 (2021).
Bramante, C. T. et al. Outpatient metformin use is associated with reduced severity of COVID-19 disease in adults with overweight or obesity. J. Med. Virol. https://doi.org/10.1002/jmv.26873 (2021).
Luo, P. et al. Metformin treatment was associated with decreased mortality in COVID-19 patients with diabetes in a retrospective analysis. Am. J. Trop. Med. Hyg. 103, 69–72. https://doi.org/10.4269/ajtmh.20-0375 (2020).
Cheng, X. et al. Metformin is associated with higher incidence of acidosis, but not mortality, in individuals with COVID-19 and pre-existing type 2 diabetes. Cell. Metab. 32, 537–547. https://doi.org/10.1016/j.cmet.2020.08.013 (2020).
Siddiqi, H. K. & Mehra, M. R. COVID-19 illness in native and immunosuppressed states: A clinical-therapeutic staging proposal. J. Heart Lung. Transplant. 39, 405–407. https://doi.org/10.1016/j.healun.2020.03.012 (2020).
Ursini, F. et al. Metformin and autoimmunity: A “new deal” of an old drug. Front Immunol. 9, 1236. https://doi.org/10.3389/fimmu.2018.01236 (2018).
Acknowledgements
We thank Dr. Douglas S. Corwin for helpful discussion with data collection and interpretation.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Z.M. and M.K. designed the study and wrote the manuscript. Z.M., N.P., and P.V. collected the data. Z.M. performed and analyzed data. M.K. reviewed data. All authors reviewed the results and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ma, Z., Patel, N., Vemparala, P. et al. Metformin is associated with favorable outcomes in patients with COVID-19 and type 2 diabetes mellitus. Sci Rep 12, 5553 (2022). https://doi.org/10.1038/s41598-022-09639-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-09639-2
This article is cited by
-
Risk phenotypes of diabetes and association with COVID-19 severity and death: an update of a living systematic review and meta-analysis
Diabetologia (2023)
-
Beauty and the beast: host microRNA-155 versus SARS-CoV-2
Human Cell (2023)
-
A protective erythropoietin evolutionary landscape, NLRP3 inflammasome regulation, and multisystem inflammatory syndrome in children
Human Cell (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.