Abstract
Interstitial lung disease associated with primary Sjögren’s syndrome (SJS-ILD) has a variable clinical course. We aimed to investigate the role of blood biomarkers in predicting prognosis for SJS-ILD. Clinical data of 46 SJS-ILD patients were retrospectively reviewed. Plasma biomarker levels, including Krebs von den Lungen-6 (KL-6), CC chemokine ligand 18 (CCL18), chitinase-3-like-1 (YKL-40), interleukin-4 receptor alpha (IL-4Ra), and matrix metalloproteinase-7 (MMP-7) were measured using the multiplex Luminex assays (R&D Systems, Minneapolis, USA). The median follow-up period was 69.0 months. The mean age of the patients was 59.4 years; 17.4% were men. The KL-6 level was significantly higher in non-survivors (n = 12; 119.6 vs. 59.5 pg/mL, P = 0.037) than survivors (n = 34), while the levels of the other biomarkers did not differ. Receiver operating characteristic analysis indicated that KL-6 shows the best performance for predicting survival (area under the curve = 0.705, P = 0.037; best cut-off value = 53.5 pg/mL). Multivariable Cox analysis that was adjusted by age and diffusing capacity for carbon monoxide suggested a high KL-6 level (> 53.5 pg/mL) as an independent prognostic factor for survival (hazard ratio = 5.939, 95% confidence interval 1.312–26.881, P = 0.021). Our results suggest that blood KL-6 might be a useful in predicting the prognosis for patients with SJS-ILD.
Similar content being viewed by others
Introduction
Primary Sjögren syndrome (SJS) is a chronic systemic inflammatory disorder that is characterized by diminished function of the lacrimal and salivary glands1,2,3. Lung involvement is a common extraglandular complication; the most frequent lung complication, interstitial lung disease (ILD), has a reported prevalence of 10–20%4,5,6. ILD has been reported to be associated with increased mortality in patients with SJS7,8. Although SJS-ILD generally follows a mild and self-limited course, it can exhibit a more severe and progressive course in some patients9. Therefore, it is important to find predicting factors that could differentiate those patients who will have the progressive disease from those that are expected to experience slow or stable disease, in order to provide appropriate intervention. Prognostic factors for patients with SJS-ILD, such as the baseline partial pressure of carbon dioxide and oxygen, forced vital capacity (FVC), the extent of reticular abnormality on high resolution computed tomography (HRCT), and the severity of fibroblastic foci, or the presence of microscopic honeycomb in a surgical lung biopsy have been reported previously as predicting factors for mortality in SJS-ILD10,11,12. However, the utility of these variables can be limited by insufficient effort on the part of the patient, reader variability in the interpretation of images, or invasiveness.
On the contrary, blood biomarkers are relatively easy to test, independent of patient effort or reader ability. Biomarkers for idiopathic pulmonary fibrosis (IPF) or connective tissue disease (CTD)-ILD have mainly been investigated using proteins that are associated with epithelial damage, matrix turnover, or inflammation13. The most-studied epithelial-specific biomarker in ILD is Krebs von den Lungen-6 (KL-6)10,14,15. KL-6 is a mucin-like glycoprotein that is of high molecular weight and is classified as a human MUC1 mucin protein. It is secreted by type II alveolar pneumocytes and bronchial epithelial cells in response to cellular damage16 and is therefore frequently considered an indicator for pulmonary damage and has been studied as a biomarker for disease activity in ILD17,18. The cytokines and enzymes that have been reported to be associated with the clinical course of IPF or CTD-ILDs include CC chemokine ligand 18 (CCL18), chitinase-3-like protein 1 (YKL 40), and interleukin-4 receptor alpha (IL-4Ra)12,14,15,19,20,21,22. CCL18 is a chemokine that is secreted mainly by alveolar macrophages (M2 phenotype)23 and is known to play an important role in the immune-mediated lung fibrosis processes in IPF24, and YKL 40 is a glycoprotein that is primarily secreted by macrophages, neutrophils, and certain types of local epithelial cells and is known to have a role in inflammation and tissue remolding25. A recent meta-analysis study revealed that serum YKL-40 is correlated with lung function and can therefore be used as a predictive biomarker for survival with IPF and CTD-ILD26. IL-4R mainly promotes the proliferation of T cells and B cells22, and can also stimulate the proliferation, differentiation, and activation of several other cell types including fibroblasts while also increasing the recruitment of inflammatory cells22. The extracellular matrix markers in ILD include matrix metalloproteinase-7 (MMP-7)27,28,29. The MMPs are a group of proteins that contribute to the activation of pro-fibrotic molecules, crucially contributing to tissue remodeling and fibrosis30,31. Of these, MMP-7 is reported to be associated with the severity and prognosis of IPF, suggesting its role in the fibrotic process27,28. However, the role of blood biomarkers in predicting prognosis in SJS-ILD is not well defined, and no study has yet compared the performance of various biomarkers in predicting SJS-ILD prognosis. Our study therefore aimed to compare the prognostic value of the different blood biomarkers to find the best biomarker for use in patients with SJS-ILD.
Methods
Study population
A total of 62 patients diagnosed with primary SJS-ILD (biopsy-proven cases, n = 16) between January 2000 and December 2016 at Asan Medical Center, Seoul, Republic of Korea, were screened in this study. Of these, only subjects with available blood samples were finally included in this study (n = 46). The patients excluded from the cohort showed lower C-reactive protein level than those included (see Supplementary Table S1). All patients met the diagnostic criteria of the American College of Rheumatology and the European League Against Rheumatism (EULAR), and the presence of ILD was confirmed by HRCT images32. The study was approved by the Institutional Review Board of Asan Medical Center (2018-1115), and written informed consent for the use of the blood samples for clinical research was obtained from all patients. All methods were performed in accordance with the relevant guidelines and regulations.
Clinical data
The clinical and survival data of all patients were retrospectively collected from medical records, and/or the records of the National Health Insurance Service of Korea. To measure disease activity in patients with primary SJS, EULAR Sjögren’s Syndrome Disease Activity Index (ESSDAI) scores at the time of ILD diagnosis was calculated33. Spirometry, total lung capacity (TLC) and the diffusing capacity of the lung for carbon monoxide (DLCO) were measured according to the recommendations from the American Thoracic Society (ATS)/European Respiratory Society (ERS). The results were presented as percentages of the normal predicted values34,35,36. A six-minute walk test (6MWT) was performed according to ERS/ATS recommendations37, and bronchoalveolar lavage (BAL) was performed according to the ATS guildelines38. Data from follow-up assessments at 3–6-month intervals or from hospitalization events were reviewed to determine the development of acute exacerbation (AE). AE was defined according to the criteria suggested by Collard et al.39, which is worsening dyspnea within 30 days with new bilateral lung infiltration with no evidence of infection or other alternative causes for the dyspnea (e.g., pulmonary embolism or heart failure).
Measurement of blood biomarkers
Blood samples were obtained at the time of diagnosis by venipuncture and immediately centrifuged. The separated plasma samples were then stored at − 80 °C until biomarker measurement. The plasma levels of KL-6, CCL18, YKL-40, IL-4Ra, and MMP-7 were measured using the multiplex Luminex assays (R&D Systems, Minneapolis, USA) in accordance with the manufacturer’s instructions.
HRCT evaluation
HRCT scans were obtained in accordance with standard protocols and reviewed by a radiologist (J.C.) who was blinded to the clinical information. The HRCT patterns were categorized into usual interstitial pneumonia (UIP), probable UIP, indeterminate for UIP, or an alternative diagnosis based on the 2018 Fleischner Society guidelines3. A UIP pattern was defined by a subpleural and basal predominance of reticular abnormalities, honeycombing with or without traction bronchiectasis, and the absence of inconsistent findings with a UIP pattern such as extensive ground-glass opacities (GGO), micro-nodules, discrete cysts, or segmental/lobar consolidations40.
Statistical analysis
All values were expressed as mean ± standard deviation for continuous variables or percentages for categorical variables. The Student’s t-test or Mann–Whitney U test were used for the continuous data, while the Pearson’s chi-squared or Fisher’s exact test were utilized to analyze the categorical data. ROC curve analysis was performed to evaluate the optimal cut-off value of blood biomarkers for predicting survival. The risk factors for mortality were evaluated using a Cox proportional hazard model. Due to the limited number of death events, among variables with P < 0.1, age, and DLCO, which were previously prognostic factors in SJS-ILD41,42. were used as adjustment variables for the multivariable Cox analysis. Survival was evaluated using Kaplan–Meier survival analysis and the log-rank test. Survival time was calculated from the date of ILD diagnosis to death or censoring, which took place on December 31, 2016 and included all patients who were still alive on this date. Spearman’s rank correlation coefficients were performed to evaluate the correlation between blood KL-6 levels and lung function or exercise capacity. All P-values were two-tailed with statistical significance set at a P value of < 0.05, and all statistical analyses were performed using SPSS Statistics, Version 24.0. (IBM Corp., Armonk, NY, USA).
Results
Baseline characteristics
The mean age of the 46 patients with primary SJS-ILD was 59.4 years and 17.4% were males (Table 1). The median follow-up period was 69.0 months (interquartile range [IQR], 23.0–101.8 months), with 12 patients (26.1%) dying during follow-up. The major cause of death was underlying ILD progression (66.7%), followed by pneumonia, tuberculosis, heart failure, and unknown cause (8.3% each) (Supplementary Table S2).
The non-survivors had lower TLC and lymphocyte levels in their BAL fluid, and showed positive anti SS-A/Ro and a UIP pattern on the HRCT more frequently than the survivors (Table 1). Total of 39 patients (84.8%) received steroid and/or immunosuppressants (median treatment duration: 15 months [interquartile range: 6–33 months]. However, there was no difference between non-survivors and survivors in terms of the number of patients treated, the initial steroid dose, and the duration of the treatment given.
Comparison of blood biomarkers
The level of KL-6 was significantly elevated in the non-survivors (119.6 vs 59.5 pg/mL, P = 0.037) as compared to the survivors (Table 2). However, no significant differences were observed between the two groups for the other biomarker levels. KL-6 was the most significant predictor of mortality (area under a curve [AUC] = 0.705, 95% confidence interval [CI] 0.509–0.901, P = 0.037) using ROC analysis for 10-year survival, and the optimal cut-off value was 53.5 pg/mL (sensitivity = 66.7%, specificity = 79.4%) (Fig. 1). The rest of the biomarkers were lesser predictive of survival than KL-6 (CCL18 [AUC = 0.569, 95% CI 0.391–0.746, P = 0.484], YKL-40 [AUC = 0.642, 95% CI 0.431–0.854, P = 0.147], IL-4Ra [AUC = 0.690, 95% CI 0.518–0.862, P = 0.053], and MMP-7 [AUC = 0.676, 95% CI 0.513–0.840, P = 0.072]).
Comparison of the ROC curves of biomarkers for 10-year mortality in patients with SJS-ILD. KL-6 (AUC = 0.750, 95% CI 0.509–0.901; P = 0.037), CCL18 (AUC = 0.569, 95% CI 0.391–0.746, P = 0.484), YKL-40 (AUC = 0.642, 95% CI 0.431–0.854, P = 0.147), IL-4Ra (AUC = 0.692, 95% CI 0.539–0.820, P = 0.053), MMP-7 (AUC = 0.676, 95% CI 0.513–0.840, P = 0.072). AUC area under the curve, CCL18 CC chemokine ligand 18, CI confidence interval, ILD interstitial lung disease, IL-4Ra interleukin-4 receptor alpha, KL-6 Krebs von den Lungen-6, MMP-7 matrix metalloproteinase-7, SJS Sjögren syndrome, YKL-40 chitinase-3-like-1.
The unadjusted Cox proportional hazards model showed that age, smoking status, higher C-reactive protein levels, lower DLCO and TLC, a shorter six-minute walk test distance (6MWD), a UIP pattern on the HRCT, and a higher level of KL-6 (> 53.5 pg/mL) were significantly associated with 10-year mortality (Table 3). In the multivariable analysis adjusted by age and DLCO, a high KL-6 level (> 53.5 pg/mL) was independently associated with a poor prognosis (hazard ratio [HR] = 5.939, 95% CI 1.312–26.881, P = 0.021) (Table 4). However, no association was observed between any of the other biomarkers and mortality in patients with SJS-ILD.
Survival according to KL-6 levels
Classification of the patients in accordance with the baseline level for KL-6 demonstrated lower lung functions (FVC, DLCO, TLC) and poorer exercise capacities (shorter walking distance and lower the minimum oxygen saturation on 6MWT) in the high KL-6 group (> 53.5 pg/mL, n = 15) than the low KL-6 group (≤ 53.5 pg/mL, n = 31) (Table 5). Patients with high KL-6 levels (with a mean follow-up period of 61.9 months) also showed more frequent AE (4 patients [27%] vs 1 patient [3%], P = 0.017) than the low KL-6 group (81.5 months; P = 0.288) and were less likely to survive (5-year survival: 64% vs 96%; 10-year survival: 30.0% and 75.0%; P = 0.001) than those with low KL-6 levels. (Fig. 2).
Comparison of the Kaplan–Meier survival curves according to KL-6 levels in patients with SJS-ILD. Vertical axis represents survival probability (%); horizontal axis represents time (months) after diagnosis. Black line indicates high KL-6 group (> 53.5 pg/mL) and dotted line indicates low KL-6 group (≤ 53.5 pg/mL). Vertical bar indicates a censored case. ILD interstitial lung disease, KL-6 Krebs von den Lungen-6, SJS Sjögren syndrome.
Correlation between KL-6 and physiological parameters
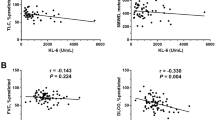
Significant negative correlations were observed between the KL-6 levels and FVC (r = − 0.499, P = 0.001), DLCO (r = − 0.498, P = 0.001), and 6MWD (r = − 0.575, P = 0.001) (Fig. 3). However, no significant correlation was observed between the other biomarkers and lung function or exercise capacity.
Correlation between KL-6 levels and physiological parameters in patients with SJS-ILD. Serum KL-6 showed negative correlation with (A) FVC (r, − 0.499; P = 0.001), (B) DLCO (r, − 0.498, P = 0.001), (C) 6MWD (r, − 0.575; P = 0.001). Spearman’s correlation coefficients were used to analyze the linear relationship between the variables. 6MWD six-minute walk test distance, DLCO diffusing capacity of the lung for carbon monoxide, FVC forced vital capacity, ILD interstitial lung disease, KL-6 Krebs von den Lungen-6, SJS Sjögren syndrome.
Discussion
In this study, the prediction of survival using various biomarkers was evaluated for patients with primary SJS-ILD. We focused on evaluating whether biomarkers that were previously studied in other CTD-ILDs are useful in patients with SJS-ILD in terms of the severity and prognosis for ILD. KL-6 was found to be superior to CCL18, YKL-40, IL-4Ra, and MMP-7 in predicting the prognosis of patients with SJS-ILD. High KL-6 levels were independently associated with an increased risk of 10-year mortality in patients with SJS-ILD when adjusted by age and DLCO. Patients with high KL-6 levels showed poorer lung function, with more frequent AE and death than those with low KL-6 levels. Moreover, KL-6 levels were found to correlate with the severity of the disease in patients with SJS-ILD.
KL-6 showed the best performance in predicting 10-year mortality as compared to the other biomarkers. A high KL-6 level (> 53.5 pg/mL) was found to be an independent risk factor for mortality after adjustment for age and DLCO when using multivariable Cox analysis. These findings are consistent with those in previous reports12,19. Kamiya et al. studied 99 patients with SJS-ILD (with a median follow-up period of 5.97 years) and reported that a higher level of KL-6 (> 800 U/mL) was associated with poor survival (HR = 2.91, 95% CI 1.04–8.10, P = 0.04) using multivariable Cox analysis adjusted by age and gender12. Kim et al. also showed that a high KL-6 level (≥ 640 U/mL) was an independent prognostic factor for survival (HR = 3.235, 95% CI 1.394–7.510, P = 0.006) in 158 patients with rheumatoid arthritis (RA)-ILD1919. These findings suggest that KL-6 might be useful for predicting the clinical outcomes of CTD-ILD, including SJS-ILD. In addition to identifying the usefulness of KL-6 for predicting the prognosis of patients with SJS-ILD, our study revealed that KL-6 showed better performance for predicting survival compared to other biomarkers.
In this study, patients with high KL-6 levels experienced AE more frequently, and KL-6 levels were negatively correlated with lung function and exercise capacity, suggesting the value of KL-6 for predicting AE and disease severity. Previous reports support our findings17,43. Ohshimo et al. studied 77 patients with IPF and reported that a high KL-6 level (≥ 1300 U/mL) was an independent risk factor for the development of AE (HR = 11.8, 95% CI 1.43–97.8, P = 0.022) when adjusted for age, sex, smoking history, and treatment43. They also showed that baseline KL-6 levels were significantly higher in patients who experienced AE than those who did not (2528 ± 1645 U/mL vs. 1584 ± 1000 U/mL, P < 0.0001)43. Lee et al. studied 165 patients with CTD-ILD (41 RA, 53 systemic sclerosis, 56 inflammatory myopathy, 15 systemic lupus erythematosus or SJS), and also showed that the semiquantitative grades of ILD on the HRCT (grade 1, 0–25%; grade 2, 26–50%; grade 3, 51–75%; grade 4, 76–100%) were significantly proportional to serum KL-6 levels, from which grades could be successfully differentiated (grades 1 vs. 2, P = 0.022; grades 2 vs. 3, P < 0.001; grades 3 vs. 4, P = 0.002)17. They also showed that serum KL-6 level had a moderate negative correlation with both FVC% (r = − 0.399, P < 0.001) and DLCO% (r = − 0.578, P < 0.001)17.
In our study, CCL18, YKL-40, IL-4Ra, and MMP-7 were not found to be associated with the prognosis or severity of ILD. However, previous studies have reported contradictory findings20,21,22,29, with several reporting these biomarkers useful in the prognosis of CTD-ILD. Tiev et al. studied 83 patients with systemic sclerosis-ILD, and reported that a high baseline CCL18 level (> 187 mg/mL) was a predictive factor (HR = 5.36, 95% CI 2.44–11.75, P = 0.001) for worsening of the subsequent disease (with a decrease of > 10% predicted in FVC or TLC) within 2 years using multivariable Cox analysis20. Hozumi et al. also demonstrated a correlation between serum YKL-40 levels and a lower arterial oxygen pressure (r = − 0.40, P < 0.001) in 72 patients with polymyositis/dermatomyositis-ILD, and independent association with poor prognosis under multivariable Cox analysis when adjusted by the anti-aminoacyl tRNA synthetase status (per 10 ng/ml increase, HR = 1.15, 95% CI 1.04–1.28, P < 0.01) or the anti-CADM-140/melanoma differentiation-associated gene 5 antibody status (per 10 ng/mL increase, HR = 1.15, 95% CI 1.04–1.29, P < 0.01)21. Moreover, Nakatsuka et al. studied 52 patients with polymyositis/dermatomyositis-ILD, and showed that higher levels of serum MMP-7 were associated with 6-month mortality (odds ratio [OR] = 1.57, 95% CI 1.01–2.45, P = 0.046) using univariate logistic regression analysis, and that high serum MMP-7 (> 5.08 ng/mL) was associated with a worse prognosis (OR = 14.60, 95% CI 1.11–192.00, P = 0.027) when using multivariable logistic regression analysis adjusted by hypoxia and serum ferritin levels29. Due to these inconsistent results, the prognostic value of the above markers is not clear for SJS-ILD. Further verification on a larger scale is required for clinical use in patients with SJS-ILD.
In this study, positive anti-SSA was more frequent in survivors but was not associated with prognosis in the unadjusted Cox analysis. Moreover, there was no difference in the frequency of positive autoantibodies such as ANA and anti-SSB between the non-survivors and survivors. The previous study support our findings; Gao et al., in 178 patients with SJS-ILD, reported that the frequency of positive ANA (65.2 vs 75.8%, P = 0.329), anti-SSA (56.0 vs 57.3%, P = 0.907), and anti-SSB (40.0 vs 37.3%, P = 0.812) was not different between the non-survivors and survivors, suggesting that autoantibodies were not associated with prognosis of ILD44. However, there is also a contradictory report; Boitiaux et al., in 45 newly diagnosed patients with idiopathic interstitial pneumonia, showed that the anti-SSA (+) group (n = 15) had lower vital capacity (63 ± 22 vs. 87 ± 23% predicted, P = 0.006) and more frequent GGO (87 vs 67%, P = 0.001) and reticulation (33 vs 21%, P = 0.030) on HRCT than the anti-SSA(−) group (n = 30)45. Due to these inconsistent findings, it is still insufficient to draw a conclusion about the association between autoantibodies and prognosis of SJS-ILD.
Our study has some limitations. First, this was a retrospective study conducted at a single center, which might lead to selection biases or a lack in generalizability. However, the baseline characteristics of our patients were comparable to those in other studies12,17. Second, we did not include a validation cohort to confirm our findings. Our sample size was relatively small; however, the rarity of SJS-ILD means that the number of patients studied is not considered low, and the long-term observation in our study can provide important insights into this rare condition. Finally, detailed treatment information such as type, dose, timing, and the duration over which medication was given were not considered in the analysis of the prognostic factors. However, the treatment given to survivors and non-survivors did not differ.
In conclusion, our results suggest that blood KL-6 might be a useful predictor of the prognosis for patients with SJS-ILD.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- 6MWD:
-
Six-minute walk test distance
- 6MWT:
-
Six-minute walk test
- AE:
-
Acute exacerbation
- ATS:
-
American Thoracic Society
- AUC:
-
Area under a curve
- BAL:
-
Bronchoalveolar lavage
- CCL18:
-
CC chemokine ligand 18
- CI:
-
Confidence interval
- CTD:
-
Connective tissue disease
- DLCO :
-
Diffusing capacity of the lung for carbon monoxide
- ERS:
-
European Respiratory Society
- ESSDAI:
-
European League Against Rheumatism Sjögren’s Syndrome Disease Activity Index
- FVC:
-
Forced vital capacity
- GGO:
-
Ground glass opacities
- HR:
-
Hazard ratio
- HRCT:
-
High resolution computed tomography
- IL-4Ra:
-
Interleukin-4 receptor alpha
- ILD:
-
Interstitial lung disease
- IPF:
-
Idiopathic pulmonary fibrosis
- IQR:
-
Interquartile range
- KL-6:
-
Krebs von den Lungen-6
- MMP-7:
-
Matrix metalloproteinase-7
- OR:
-
Odds ratio
- RA:
-
Rheumatoid arthritis
- ROC:
-
Receiver operating characteristic
- SJS:
-
Sjögren syndrome
- TLC:
-
Total lung capacity
- UIP:
-
Usual interstitial pneumonia
- YKL 40:
-
Chitinase-3-like
References
Pertovaara, M. et al. Clinical follow up study of 87 patients with sicca symptoms (dryness of eyes or mouth, or both). Ann. Rheum. Dis. 58, 423–427. https://doi.org/10.1136/ard.58.7.423 (1999).
Ramos-Casals, M., Tzioufas, A. G. & Font, J. Primary Sjogren’s syndrome: New clinical and therapeutic concepts. Ann. Rheum. Dis. 64, 347–354. https://doi.org/10.1136/ard.2004.025676 (2005).
Lynch, D. A. et al. Diagnostic criteria for idiopathic pulmonary fibrosis: A Fleischner Society White Paper. Lancet Respir. Med. 6, 138–153. https://doi.org/10.1016/s2213-2600(17)30433-2 (2018).
Kokosi, M., Riemer, E. C. & Highland, K. B. Pulmonary involvement in Sjogren syndrome. Clin. Chest Med. 31, 489–500. https://doi.org/10.1016/j.ccm.2010.05.007 (2010).
Stojan, G., Baer, A. N. & Danoff, S. K. Pulmonary manifestations of Sjögren’s syndrome. Curr. Allergy Asthma Rep. 13, 354–360. https://doi.org/10.1007/s11882-013-0357-9 (2013).
Koo, S. M., Kim, S. Y., Choi, S. M. & Lee, H. K. Korean Guidelines for Diagnosis and Management of Interstitial Lung Diseases: Part 5. Connective tissue disease associated interstitial lung disease. Tuberc. Respir. Dis. (Seoul). 82, 285–297. https://doi.org/10.4046/trd.2019.0009 (2019).
Palm, O. et al. Clinical pulmonary involvement in primary Sjogren’s syndrome: Prevalence, quality of life and mortality—A retrospective study based on registry data. Rheumatology (Oxford) 52, 173–179. https://doi.org/10.1093/rheumatology/kes311 (2013).
Ramos-Casals, M. et al. Characterization of systemic disease in primary Sjögren’s syndrome: EULAR-SS Task Force recommendations for articular, cutaneous, pulmonary and renal involvements. Rheumatology (Oxford) 54, 2230–2238. https://doi.org/10.1093/rheumatology/kev200 (2015).
Ramos-Casals, M. et al. Primary Sjögren syndrome in Spain: Clinical and immunologic expression in 1010 patients. Medicine (Baltimore) 87, 210–219. https://doi.org/10.1097/MD.0b013e318181e6af (2008).
Enomoto, Y. et al. Prognostic factors in interstitial lung disease associated with primary Sjögren’s syndrome: A retrospective analysis of 33 pathologically-proven cases. PLoS ONE 8, e73774. https://doi.org/10.1371/journal.pone.0073774 (2013).
Ito, I. et al. Pulmonary manifestations of primary Sjogren’s syndrome: A clinical, radiologic, and pathologic study. Am. J. Respir. Crit. Care Med. 171, 632–638. https://doi.org/10.1164/rccm.200403-417OC (2005).
Kamiya, Y. et al. Prognostic factors for primary Sjögren’s syndrome-associated interstitial lung diseases. Respir. Med. 159, 105811. https://doi.org/10.1016/j.rmed.2019.105811 (2019).
Tzouvelekis, A., Kouliatsis, G., Anevlavis, S. & Bouros, D. Serum biomarkers in interstitial lung diseases. Respir. Res. 6, 78. https://doi.org/10.1186/1465-9921-6-78 (2005).
Fukaya, S. et al. KL-6 as a novel marker for activities of interstitial pneumonia in connective tissue diseases. Rheumatol. Int. 19, 223–225. https://doi.org/10.1007/s002960000064 (2000).
Yanaba, K., Hasegawa, M., Takehara, K. & Sato, S. Comparative study of serum surfactant protein-D and KL-6 concentrations in patients with systemic sclerosis as markers for monitoring the activity of pulmonary fibrosis. J. Rheumatol. 31, 1112–1120 (2004).
Hu, Y. et al. Serum Krebs von den Lungen-6 level as a diagnostic biomarker for interstitial lung disease in Chinese patients. Clin. Respir. J. 11, 337–345. https://doi.org/10.1111/crj.12341 (2017).
Lee, J. S. et al. Serum KL-6 levels reflect the severity of interstitial lung disease associated with connective tissue disease. Arthritis Res. Ther. 21, 58. https://doi.org/10.1186/s13075-019-1835-9 (2019).
Qin, H. et al. Krebs von den Lungen-6 associated with chest high-resolution CT score in evaluation severity of patients with interstitial lung disease. Pulmonology 25, 143–148. https://doi.org/10.1016/j.pulmoe.2018.05.008 (2019).
Kim, H. C., Choi, K. H., Jacob, J. & Song, J. W. Prognostic role of blood KL-6 in rheumatoid arthritis-associated interstitial lung disease. PLoS ONE 15, e0229997. https://doi.org/10.1371/journal.pone.0229997 (2020).
Tiev, K. P. et al. Serum CC chemokine ligand-18 predicts lung disease worsening in systemic sclerosis. Eur. Respir. J. 38, 1355–1360. https://doi.org/10.1183/09031936.00004711 (2011).
Hozumi, H. et al. Clinical utility of YKL-40 in polymyositis/dermatomyositis-associated interstitial lung disease. J. Rheumatol. 44, 1394–1401. https://doi.org/10.3899/jrheum.170373 (2017).
Shen, H., Xia, L. & Lu, J. Interleukin-4 in rheumatoid arthritis patients with interstitial lung disease: A pilot study. Indian J. Med. Res. 138, 919–921 (2013).
Schutyser, E., Richmond, A. & Van Damme, J. Involvement of CC chemokine ligand 18 (CCL18) in normal and pathological processes. J. Leukoc. Biol. 78, 14–26. https://doi.org/10.1189/jlb.1204712 (2005).
Prasse, A. et al. CCL18 as an indicator of pulmonary fibrotic activity in idiopathic interstitial pneumonias and systemic sclerosis. Arthritis Rheum. 56, 1685–1693. https://doi.org/10.1002/art.22559 (2007).
Lee, C. G. et al. Role of chitin and chitinase/chitinase-like proteins in inflammation, tissue remodeling, and injury. Annu. Rev. Physiol. 73, 479–501. https://doi.org/10.1146/annurev-physiol-012110-142250 (2011).
Tong, X. et al. Can YKL-40 be used as a biomarker for interstitial lung disease? A systematic review and meta-analysis. Medicine (Baltimore) 100, e25631. https://doi.org/10.1097/md.0000000000025631 (2021).
Richards, T. J. et al. Peripheral blood proteins predict mortality in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 185, 67–76. https://doi.org/10.1164/rccm.201101-0058OC (2012).
Sokai, A. et al. Matrix metalloproteinase-10: A novel biomarker for idiopathic pulmonary fibrosis. Respir. Res. 16, 120. https://doi.org/10.1186/s12931-015-0280-9 (2015).
Nakatsuka, Y. et al. Serum matrix metalloproteinase levels in polymyositis/dermatomyositis patients with interstitial lung disease. Rheumatology (Oxford) https://doi.org/10.1093/rheumatology/kez065 (2019).
Amălinei, C., Căruntu, I. D. & Bălan, R. A. Biology of metalloproteinases. Roman. J. Morphol. Embryol Revue roumaine de morphologie et embryologie. 48, 323–334 (2007).
Pardo, A., Cabrera, S., Maldonado, M. & Selman, M. Role of matrix metalloproteinases in the pathogenesis of idiopathic pulmonary fibrosis. Respir. Res. 17, 23. https://doi.org/10.1186/s12931-016-0343-6 (2016).
Shiboski, C. H. et al. 2016 American College of Rheumatology/European League Against Rheumatism Classification Criteria for Primary Sjögren’s Syndrome: A consensus and data-driven methodology involving three international patient cohorts. Arthritis Rheumatol. Hoboken NJ. 69, 35–45. https://doi.org/10.1002/art.39859 (2017).
Seror, R. et al. EULAR Sjogren’s syndrome disease activity index: Development of a consensus systemic disease activity index for primary Sjogren’s syndrome. Ann. Rheum. Dis. 69, 1103–1109. https://doi.org/10.1136/ard.2009.110619 (2010).
Graham, B. L. et al. Standardization of spirometry 2019 update. An official American Thoracic Society and European Respiratory Society Technical Statement. Am. J. Respir. Crit. Care Med. 200, e70–e88. https://doi.org/10.1164/rccm.201908-1590ST (2019).
Graham, B. L. et al. 2017 ERS/ATS standards for single-breath carbon monoxide uptake in the lung. Eur. Respir. J. https://doi.org/10.1183/13993003.00016-2016 (2017).
Wanger, J. et al. Standardisation of the measurement of lung volumes. Eur. Respir. J. 26, 511–522. https://doi.org/10.1183/09031936.05.00035005 (2005).
Holland, A. E. et al. An official European Respiratory Society/American Thoracic Society technical standard: Field walking tests in chronic respiratory disease. Eur. Respir. J. 44, 1428–1446. https://doi.org/10.1183/09031936.00150314 (2014).
Meyer, K. C. et al. An official American Thoracic Society clinical practice guideline: The clinical utility of bronchoalveolar lavage cellular analysis in interstitial lung disease. Am. J. Respir. Crit. Care Med. 185, 1004–1014. https://doi.org/10.1164/rccm.201202-0320ST (2012).
Collard, H. R. et al. Acute exacerbations of idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 176, 636–643. https://doi.org/10.1164/rccm.200703-463PP (2007).
Assayag, D. et al. Rheumatoid arthritis-associated interstitial lung disease: Radiologic identification of usual interstitial pneumonia pattern. Radiology 270, 583–588. https://doi.org/10.1148/radiol.13130187 (2014).
Ley, B. et al. A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann. Intern. Med. 156, 684–691. https://doi.org/10.7326/0003-4819-156-10-201205150-00004 (2012).
Ryerson, C. J. et al. Predicting survival across chronic interstitial lung disease: The ILD-GAP model. Chest 145, 723–728. https://doi.org/10.1378/chest.13-1474 (2014).
Ohshimo, S. et al. Baseline KL-6 predicts increased risk for acute exacerbation of idiopathic pulmonary fibrosis. Respir. Med. 108, 1031–1039. https://doi.org/10.1016/j.rmed.2014.04.009 (2014).
Gao, H. et al. Characteristics and mortality in primary Sjögren syndrome-related interstitial lung disease. Medicine (Baltimore) 100, e26777. https://doi.org/10.1097/md.0000000000026777 (2021).
Boitiaux, J. F. et al. Idiopathic interstitial lung disease with anti-SSA antibody. Rheumatology (Oxford) 50, 2245–2250. https://doi.org/10.1093/rheumatology/ker267 (2011).
Acknowledgements
This study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and Technology (NRF-2019R1A2C2008541, NRF-2022R1A2B5B02001602), Seoul, Republic of Korea. The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
J.W.S. is the guarantor of the paper and takes responsibility for the integrity of the work as a whole. J.W.S. contributed to study design. J.C. contributed to the radiologic evaluation of study subjects. Y.J.K., S.M. and J.W.S. contributed to the data analysis and interpretation of results. Y.J.K. and J.W.S. drafted the initial manuscript. All authors discussed the results and reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, Y.J., Choe, J., Moon, SJ. et al. Blood KL-6 predicts prognosis in primary Sjögren’s syndrome-associated interstitial lung disease. Sci Rep 12, 5343 (2022). https://doi.org/10.1038/s41598-022-09283-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-09283-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.