Abstract
Toxoplasma gondii is a protozoan parasite, causing one of the most prevalent parasitic infections in the world. In the present study water sources of the Qinghai-Tibet Plateau (QTP), China, where the hygienic infrastructure is still developing, were investigated. A total of 214 water samples of 10 L volume, were collected from wastewater treatment plants (WWTPs), a slaughterhouse and rivers. The samples were filtered and then analysed using real-time PCR and multilocus genotyping. T. gondii DNA was found in four (1.9%) samples representing T. gondii type I; in one of them T. gondii-like oocysts were also confirmed microscopically. The approximate level of contamination of positive samples ranged between 30 and 2300 T. gondii sporozoites. The results of this study confirmed that T. gondii is present in wastewater in the greater metropolitan area of Xining and a neighbouring county. Contamination of wastewater at this level constitutes rather a moderate source of Toxoplasma infections in humans and animals. It suggests, however, a link between environmental exposure of animals, meat processing facilities and WWTPs. To our knowledge, this is the first investigation describing T. gondii detection in wastewater and environmental water samples collected from the territory of P.R. China using sensitive molecular tools.
Similar content being viewed by others
Introduction
Toxoplasma gondii is a worldwide distributed protozoan parasite that can infect humans as well as all warm-blooded animals. In the majority of cases, toxoplasmosis is asymptomatic in immunocompetent individuals; it may have, however, significant repercussions in immunodeficient patients and immature foetuses or infants whose mother suffered from primary infection during pregnancy1. Toxoplasmosis is one of the most prevalent parasitic infections in humans. Various authors suggest that one-tenth up to half the human population has been chronically infected with T. gondii in a global scale2,3. Humans acquire the disease mainly via the oral route through consumption of raw or undercooked meat of infected animals containing cysts filled with parasites4 and through the ingestion of oocysts as a result of drinking contaminated water, eating contaminated food or contact with contaminated soil3,5. Thus, the contaminated environment may be an important source of human exposure. Oocysts are excreted to the environment only by infected felids, being the definitive hosts of T. gondii, therefore, cats play a significant role in the epidemiology of toxoplasmosis6,7.
Waterborne toxoplasmosis presents a considerable threat to public health throughout the world8,9. T. gondii oocysts has been detected in water samples, in different parts of the world, including France10, Poland11, Germany12, Russia, Bulgaria13, Scotland14, Turkey15, Brazil16, Ecuador17. There is limited number of studies, however, reporting the presence of T. gondii in wastewater. DNA of the parasite has been detected in Bulgaria in raw sewage13 and in Germany in both the influent and effluent of a WWTP12; in other studies performed in Greece, Bulgaria, France and Germany18,19,20 Toxoplasma has been examined but not detected. Regarding quantitative analysis, even fever studies have been published for Toxoplasma in water, including the study conducted in Brazil21.
In China, scientific interest in T. gondii is visible, regarding, however, epidemiological studies of T. gondii research has primarily revolved around high risk populations and animals. The most recent status of toxoplasmosis in China was revealed with the nationwide survey for parasites in human population conducted between 2001 and 2004. The survey resulted in an average national prevalence rate of T. gondii infection of 7.9% out of a 47,444 population sample22 indicated that in China the national infection rate is well below the world average. Also many studies have been conducted regarding the prevalence of T. gondii in commercially raised livestock23,24,25,26,27,28 and in meat tissue sampled from meat available in retail stores, particularly pork29 and poultry30; as well as cats and dogs both companion and stray31,32,33. Even though T. gondii is being investigated in China, only a few studies have examined the presence of this parasite in the environment, mostly in soil34,35,36,37,38. Regarding studies performed in the Qinghai Tibetan Plateau (QTP), 12.7% of 268 soil samples taken from urban agglomerations in Qinghai and Gansu provinces, including the two respective capitals, Xining and Lanzhou were found positive for T. gondii39. In addition, a recent study carried out in QTP revealed that fresh vegetables offered at open markets in Qinghai province may be contaminated with T. gondii40. Thus, above studies demonstrate that T. gondii is present in the environment in different parts of China. However, to our knowledge, there are no environmental studies related to the presence of T. gondii in waters in China and therefore possible contribution of this medium in transmission of the parasite is unknown.
Globally, the clonal population structure of T. gondii encompass three major types I, II and III frequently observed in North America and Europe41. Atypical types were observed in wildlife in North America42 as well as in humans and animals in South America43,44. In China, type China 1 (ToxoDB#9) is widespread and likely the major lineage mainly in Northern, Southern and Central parts of the country45,46,47,48. The second most commonly found genotype in China is the type I (ToxoDB#10) that predominates in the eastern and southern provinces of China. It has also been found in the north-western China, including Qinghai Province45,49. Toxoplasma genotypes belonging to type II are less commonly detected in China, mostly in the northern, north-western and central provinces45.
The present study aimed to determine the possible occurrence of T. gondii in water and wastewater samples collected from Qinghai Province, P.R. China. Sensitive molecular tools (real-time PCR, qPCR, multilocus PCR/RFPL analysis) were used to determine the genotype of obtained T. gondii isolates and the equivalent of a number of parasites in investigated samples.
Results
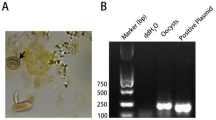
In total, 214 water and wastewater samples were collected and examined with real-time PCR assays based on the T. gondii B1 gene. The presence of T. gondii DNA was recorded in four samples investigated (4/214; 1.9%), including three samples collected from WWTPs and one sample collected from the slaughterhouse (Table 1, Fig. 1). In case of one positive sample collected from WWTPs, presence of T. gondii-like oocysts was also confirmed microscopically; oocysts appear as cystic structures, approximately 10 μm in diameter (Table 1, Supplementary Fig. S1). Sequencing of positive water samples showed that the PCR products were fragments of the T. gondii B1 gene. T. gondii DNA was not found in water samples collected from surface waters (Table 1, Fig. 1). Positive samples were collected in April and August (Table 2); samples collected during the months of January, March, May, September and October were negative.
Results of the Toxoplasma gondii DNA detection in water samples collected from the area of Qinghai Province, P.R. China using real-time PCR. A) Results of real-time PCR performed for samples 1-94; C+-T. gondii positive control, 67-positive wastewater sample (template No. 67), 83-positive wastewater sample (template No. 83), C--negative control. B) Results of real-time PCR performed for samples 95-188; C+-T. gondii positive control, 120-positive wastewater sample (template No. 120), 125-positive wastewater sample (template No. 125), C--negative control. Figure was prepared using MATLAB R2015a.
Among the wastewater samples tested, four were positive for T. gondii (4/188; 2.1%) (Table 1). Parasite DNA was detected in one sample collected from the Xining WWTP (1/38; 2.6%), and in two other samples collected from the Huangyuan WWTP (2/101; 2%). Among the samples collected from the slaughterhouse, one was positive for T. gondii (1/49; 2%). None of the 26 samples collected from rivers located in the Qinghai Province, were positive for T. gondii (Table 1).
It was possible to perform full multilocus genotyping of three out of four positive wastewater samples. PCR/RFLP analysis based on a set of 10 genetic markers showed that all detected isolates represent T. gondii genotype I (Table 2, Fig. 2). The pattern obtained presented in Fig. 2 is clearly different from other strains found in China50,51,52.
Results of multiplex multilocus nested PCR-RFLP (Mn-PCR-RFLP) analysis of Toxoplasma gondii samples using 10 different genetic markers. M (pUC19 DNA/MspI (HpaII) Marker, Thermo Fisher Scientific) – molecular weight marker, I+ – T. gondii type I positive control, II+ – T. gondii type II positive control, 67 – positive wastewater sample (template No. 67), 83 – positive wastewater sample (template No. 83), 120 – positive wastewater sample (template No. 120). The samples derive from the same experiment and gels were processed in parallel. The gels were properly cropped to improve the clarity and conciseness of the presentation; the full-length gels are presented in Supplementary Fig. S2–S5. Figure was prepared using MATLAB R2015a.
Results of quantitative real-time PCR enabled the calculation of equivalent of T. gondii cells and oocysts present in positive wastewater samples, in the final suspension obtained after concentration procedure. The approximate level of contamination of positive samples ranged between 32 and 2360 Toxoplasma cells which correspond with respectively 4 and 295 T. gondii oocysts per sample (Table 2).
Discussion
In the present study, we successfully detected Toxoplasma DNA in wastewater samples from QTP in China. To the best of our knowledge, this is the first investigation describing T. gondii detection in environmental water samples collected from the territory of P.R. China using sensitive molecular tools, supported by microscopy. T. gondii DNA was detected in four wastewater samples (4/188; 2.1%), originating from WWTPs inlets, before treatment, and a slaughterhouse located in the vicinity of Xining, Qinghai Province, P.R. China. In one of these positive samples we confirmed presence of T. gondii-like oocysts by microscopic investigation. The results of the study confirmed that T. gondii was present in the wastewater of two cities in QTP. This is an indicator of possible contamination of water sources in QTP since Toxoplasma12 and other protozoan parasites53,54,55,56,57,58 have been found in the effluent of WWTPs. Previous studies performed specifically in the area of QTP have revealed the presence of different protozoan parasites including Acanthamoeba, Giardia, Cryptosporidium in both surface waters and wastewaters57,59,60. In Xining City since 2000, a major effort has been put in the modernization of the wastewater treatment facilities, including the construction of new plants and the modernization of older ones61; this is indicating lack of wastewater treatment facilities the previous decades as well as lack of such facilities in smaller cities and communities in the countryside. Simultaneously, almost half the water supply for the city of Xining comes from surface water sources62, therefore, the presence of T. gondii in wastewater constitutes a potential risk for toxoplasmosis in humans and animals in the investigated area. The level of positive wastewater samples obtained in this study, however, indicates that this risk may be relatively low. It can also be suggested that water contaminated with oocysts may serve as indirect source of infection in humans when used for irrigation, washing and processing of agricultural products. Many studies performed in different parts of the world have already confirmed presence of Toxoplasma on agricultural products indicating irrigation as one of possible sources of contamination63,64,65. A concurrent with this study investigation, conducted in Xining open markets particularly, presented a contamination level of 3.6% of fresh vegetables samples originating from different parts of China40. The irrigational water is considered as a plausible contamination pathway for such vegetables and in the case of surface water used as a source, loadings from WWTPs further exacerbate the contamination.
The overall rate of positive for T. gondii samples collected from WWTPs in QTP, China was 2.2% (3/139). Presence of Toxoplasma in wastewaters was previously confirmed in Bulgaria13 and in Germany12. In other studies performed in Paris, Hamburg, Bulgaria and Greece, wastewater was tested but T. gondii was not detected18,19,20. All of these studies, however, have differences in the sampling, concentration and identification methods affecting their detection sensitivity and their results are subject to the specific epidemiological conditions. Therefore level of contamination established in our study cannot be simply compared to results obtained in cited articles. Until now, there is no established one sensitive protocol for detection of T. gondii oocysts in water. We can presume that oocysts, if present, are dispersed in waters in low numbers. Therefore, to fulfil basic purpose of our study, which was to check possibility of presence of T. gondii in water and wastewater, it was legitimated to select more sensitive and specific method, which is PCR. Microscopy, characterized by lower detection limit, has been added only to complement molecular techniques.
Both WWTPs in this study perform secondary treatment but differ on the connected sewer system; the Huangyuan WWTP treats wastewater from a combined sewer system, while the Xining WWTP is connected to a separate sewer system. This specification renders the Huangyuan WWTP more vulnerable to T. gondii contamination since in an event of overload of the capacity of the plant, e.g. during extreme rainfall events, the WWTP is bypassed and wastewater is disposed of in the river untreated. Furthermore, presence of T. gondii in this WWTP may be a result of environmental pollution of the streets and open spaces of the city of Huangyuan, which the WWTP is accommodating, owning to the combined sewer system. Surface runoff may be transporting T. gondii oocysts in the sewer system. In the study performed in Xining City and other cities of QTP, T. gondii presence was reported in soil samples taken from green spaces39. There were no positive samples in Huangyuan County, possibly due to the examination of only 10 samples from the particular district, however, whenever an adequate number of samples was taken (> 30), prevalence rate was between 11 and 16%, including the city of Lanzhou that is situated in the borders of QTP39. In a study from 2012 it has been suggested that felids such as Pallas’s cat (Otocolobus manul) and Chinese mountain cat (Felis bieti) may be sources of T. gondii oocysts in the environment of the QTP area66. Although there is no study for cats in Qinghai Province, in Lanzhou City, Gansu Province, which is a major city in the same region and very close to Xining, several investigations reported the presence of T. gondii in cats31,67,68,69. Taking into account the presence of cats in an urban environment and their role as definitive hosts of T. gondii, contamination of the urban environment by cats may be considered as a putative source of Toxoplasma in the sewer system of Huangyuan.
Similarly, contamination of wastewater with Toxoplasma in Xining City may be a result of contaminated wastewater coming from slaughterhouses like the one examined in this study, as it has been recently confirmed for Cryptosporidium57. Infected animals, meat and meat processing by-products are the primary sources; during the washing process, water transports T. gondii into the sewer system, the WWTP and possibly the receiving waterbody, Huangshui River. Some studies have reported T. gondii prevalence in the two most commonly bred animals in QTP, intended for human consumption, sheep27 and yak66,70. These animals free-graze in the high pasturelands, living closely together with other domesticated and wild animals, including felids and have therefore chances to ingest T. gondii oocysts through the faecal-oral route. Therefore, infected meat could be an important source of infection for humans in QTP and other parts of China supplied with meat from QTP. Apart from infected meet, another source of Toxoplasma in the wastewater samples of the slaughterhouse could have been contamination of processing area with oocysts of the parasite. We can speculate with great caution that free-grazing animals may transport contaminated soil and/or cats faeces from the pasture area on their body surface, their hooves or legs for example. However, taking into account very limited data regarding presence of T. gondii oocysts on the body of animals in general71, to verify this hypothesis additional wider studies should be carried out on this type of transportation in livestock. Moreover, we cannot exclude presence of stray cats eating unwanted tissues from positive animals and subsequently contaminating the area of slaughterhouse with oocysts. Contamination of the wastewater sample originating from the slaughterhouse is not surprising and suggests further dispersion of contamination into the wastewater treatment system and possibly water supplies.
All water samples taken from rivers were negative. According to literature, the range of positive T. gondii surface water samples ranges from 0 to 9% in studies conducted in developed countries, and up to 77% in low and middle income countries72. This fact has to be addressed with caution because the use of different methods for sampling, concentration, detection of oocysts together with epidemiological conditions makes direct comparison difficult. Most likely T. gondii oocysts, if present, are widely dispersed in running water and in such low numbers that it was not possible to obtain any positive result due to the sampling method limitations in conjunction with detection limits of the applied concentration method. Sampling was done in 10 L batches; this corresponds to low sampling volume and a very narrow timeframe of detection. Recovery losses during concentration method enhance the limitations of the methodology and detection strategy. In retrospect, a continuous sampling method may have produced different results. This method can provide an adequate number of samples to conclude on the prevalence of Toxoplasma oocysts in rivers, although such a method presents technical challenges that are difficult to overcome, especially in remote areas. It should be also highlighted that in this particular study we investigated a relatively low number of surface water samples which may not fully reflect reality. Therefore, additional studies screening larger volume surface water are needed in the future to draw unequivocal conclusions about the presence of T. gondii in water supplies in this area.
An approximation of the Toxoplasma charge was performed using qPCR. The order of magnitude of the number of oocysts that might be present in the positive samples ranged from one to 102. Actual contamination, however, could be even higher than demonstrated due to possible loss of material during the purification and recovery process. The number of oocysts responsible for the development of infection in humans is unknown and the examination of the viability of oocysts was out of the scope of this study, therefore we cannot comment on the infectiousness of the oocysts in water samples. Quantitative analysis, that helps to estimate approximate oocysts load of the samples, however, can be combined with infectiousness in the future and should not be neglected, even though currently only a few studies21 have performed such analyses regarding Toxoplasma in water.
Multilocus genotyping revealed that the detected isolates in this study represent the T. gondii genotype I (Fig. 2). According to Chaichan et al. (2017) in China, thirteen ToxoDB genotypes and 5 atypical genotypes were identified so far45. Among them, genotype Chinese 1 predominates45,47,73, displaying a decreasing gradient from east to west45. When comparing gel electrophoresis images, type I74,75 is distinctively different from the type Chinese 151,52 which is more similar to type II52. It is the second most common genotype found in China characterising around 15% of the isolates45,73. Interestingly, type I of T. gondii has been reported in the area of QTP (Northwest of China) including Gansu and Qinghai Province in wildlife, Plateau pika (Ochotona curzoniae) and Qinghai vole (Microtus fuscus)45,49. The pattern of the genotype distribution in QTP differs from the rest of China and is most probably connected with the subject of the studies conducted so far. These include domestic birds that show a predominance of type II genotype50 while they don’t include domestic animals and cats where Chinese 1 is expected to be more prevalent in China25,45,46,47,51. One such study in Gansu regarding free ranging white yaks (Bos grunniens) with limited results, has resulted in two cases of Chinese 176. The aforementioned argument is further substantiated by the detection of type Chinese 1 in soil samples from Gansu77. On the contrary, Zhang et al. (2013) have suggested that presence of Toxoplasma type I in animals living in QTP area may depend on the unique environment in this high altitude plateau49. In any case, type I from the wildlife reservoir, somehow has to be transported to or transmitted within the urban environment since it is now reported in wastewater. Therefore more research needs to be done towards the genetic characterization of T. gondii in this high-altitude environment including both different hosts as well as environmental matrices; this study further adds some evidence to support the prevalence of Toxoplasma type I in QTP.
The waterborne transmission route of T. gondii to humans via the dissemination of oocysts through surface water and its epidemiological impact has been shown to be more significant than previously believed8. Several waterborne outbreaks have been reported worldwide since 1979, especially in Brazil, where contaminated drinking water was vehicle of infection in humans63,64. Oocysts are very resistant to unfavourable environmental conditions and to chemical deactivation, which makes treatment process difficult7. In the present paper, we describe the detection of T. gondii DNA in wastewater samples in QTP, China, and identified the Toxoplasma genotype, that is known to be pathogenic for humans. Therefore, we provide evidence for the presence of Toxoplasma in wastewater indicating not only the presence of this parasite in the environment in QTP but also the potential risk for humans and animals. In order to estimate, however, the infectivity of putative oocysts in the samples and their ability to infect humans and/or animals, detection of DNA itself is not enough and must be complemented by additional infectivity tests. The amount of viable and infective Toxoplasma oocysts in this environment remains unknown. It is also noted that in the Xining metropolitan area a wastewater reuse program has been programmed, including irrigation and various other purposes, like street cleaning61,62. In order to ascertain the extent of the risk for humans, future monitoring should include widespread and regular sampling from both the inlet and the outlet of WWTPs, surface waters, particularly lakes and pools where the parasite is more likely to be detected, water treatment facilities, the water supply network and could be expanded to other environmental matrices, such as soil. Moreover, finding of T. gondii in the washing water from a slaughterhouse calls for stricter control and monitoring of the meat-producing industry regarding protozoan parasites. It is also advised that food crops already susceptible to T. gondii contamination like fruits and vegetables78, have to be regularly and well inspected, especially in the cases of reclaimed wastewater used as irrigational water. With the new global goals announcement by the Chinese government for sustainable development in the country, it is high time for a concerted effort to tackle the prevalence of toxoplasmosis and other neglected diseases caused by food- and waterborne pathogens.
Conclusions
Toxoplasmosis remains a disconcerting problem for public health in China as well as in other parts of the world. Current methodologies for detection of Toxoplasma in environmental waters, regardless of their limitations, are readily available for application. In the present study, we reported wastewater contamination with T. gondii in Qinghai-Tibet Plateau using sensitive molecular tools. These findings, together with previous studies showing contamination of the soil and vegetables are an indicator of possible contamination of water sources in QTP with Toxoplasma. On the other hand, the level of contamination of investigated wastewater samples in our study is relatively low in comparison with other countries. This corresponds with the generally low level of Toxoplasma infection within Chinese society as compared with the world average. More intense and wide screening of environmental matrices for the presence of Toxoplasma in different provinces of China is needed to enhance knowledge of Toxoplasma contamination of water resources and food matrices in this country.
Methods
Sampling and study area
A total of 214 water and wastewater samples were collected between January and October 2016 at various sites located in the Qinghai part of QTP, Western China (Fig. 3). Water and wastewater samples, 10 L in volume, were taken from two WWTPs, a slaughterhouse, and surface waters (Table 1). The samples originated from: Huangyuan WWTP (n = 101), Xining WWTP (n = 38), Baide slaughterhouse (n = 49), as well as Guoluo River (n = 10), Chahan River (n = 9), and Baoku River (n = 7) (Fig. 3, Table 1). The material was collected in sterile polypropylene vessels and transported to a laboratory in Xining, where it was immediately processed for analysis.
Sampling sites in Qinghai Province, P.R. China. S1-Baide slaughterhouse, S2-Xining WWTP, S3-Huangyuan WWTP, S4-Baoku River, S5-Chahan River, S6-Guoluo River (Modified by ArcGIS 10.2.2.3552 from CHGIS, Version 4” Cambridge: Harvard Yenching Institute, January 2007. Available at http://www.fas.harvard.edu/~chgis/; Contains modified Copernicus Service information [2015]).
Description of the sampling sites
Xining, Qinghai Province, P.R. China is the province capital with a population of 2.2 million people. It forms an extended administrative area including the counties of Datong, Huangyuan and Huangzhong and it is characterised by a densely populated metropolitan area of 1.2 million people, dominated by high-rise buildings, including rural and semi-rural districts with separate smaller cities. Xining WWTP is located in the city centre of Xining. It is one of the many secondary treatment facilities operating in the city, with a separate sewer system, processing domestic and industrial wastewater.
Huangyuan WWTP serves the nearby Huangyuan County. This is also a secondary treatment facility operating with a combined sewer system. It processes domestic wastewater, as well as storm water runoff. All of the wastewater samples from both WWTPs were collected before treatment, from WWTP influents.
The Baide slaughterhouse is a major facility, with 118 slaughtering production lines operating on a daily routine. It is situated in the outskirts of the Xining city centre. Water used for cleaning the processing area was sampled from the slaughterhouse floor, this water in a later processed by a municipal WWTP.
Chahan River, a tributary of Baoku, flows into Heiquan Reservoir across Baoku River, 80 km from Xining city centre. Baoku River is a major river which flows through Datong County into Huangshui He. Heiquan Reservoir is a possible source of drinking water in Qinghai Province. All the aforementioned WWTPs and rivers are connected with Huangshui He, a major Yellow River tributary flowing through the city of Xining. Finally, Guoluo River, the only site which is not connected with Huangshui He, is located in the pastoral area of Guoluo Tibetan Autonomous Prefecture, in the hinterland of QTP. This river serves directly as drinking water source, without treatment, for humans and animals, including domesticated animals (cattle, sheep, and dogs), as well as wild animals. Water samples were taken at the banks of rivers 1–2 m from the shore and from surface layer of water up to approximately 30 cm in depth.
Isolation of Toxoplasma gondii from environmental material
In order to isolate and concentrate T. gondii from collected water and wastewater samples, two different protocols were applied depending on the type of investigated sample as given below.
A filtration method was employed to concentrate T. gondii oocysts from the environmental water samples79. Briefly, 10 L of surface water was filtered through membrane filters with a pore size of 1.2 µm using a 293-mm sanitary disc filter holder (Millipore) and CC-45 vacuum device (JAVAC, Australia). After filtration, the filters were washed at least three times (up to 5 times depending on the turbidity of water sample) with 15 mL 0.1% Tween 80 solution. The eluate and wash solutions were collected in sterile 50 mL conical tubes and centrifuged at 1,500 × g for 15 min. The supernatant was removed and the obtained pellet (1–2 mL, depending on water turbidity) was stored for further analysis.
For the wastewater samples collected from the WWTPs and slaughterhouse flocculation with Al2(SO4)3 according to80 was applied. Water pellets representing the end products of the processed samples were then used for DNA extraction.
Microscopic investigation
Microscopic investigations of collected water samples (final pellet) were performed using fresh preparations observed under light microscope (Opta–Tech, Mn–358) using 40× magnification. Elements similar in size and structure to T. gondii oocysts7,81,82 were detected and recorded.
Molecular detection of Toxoplasma gondii
DNA extraction
Prior to DNA extraction, the material (pellet) obtained from water samples was ten times frozen in liquid nitrogen and thawed at 85 °C in a water bath to improve the efficiency of DNA yield. Then, DNA extraction was performed using a commercial TIANamp Micro DNA Kit (DP 316) (Tiangen Biotech, Beijing, P.R. China) according to the manufacturer’s instructions. The extracted DNA was then stored at − 20 °C.
Specific detection of Toxoplasma gondii DNA
For the specific detection of Toxoplasma gondii DNA, real-time PCR was performed with the use of a pair of primers targeting a 129-bp fragment of the 35-fold repetitive B1 gene and the fluorescent-labelled TaqMan probe83. The amplification reaction mixture consisted of 12.5 μL of Real-Time 2× HS-PCR Master Mix Probe (A&A Biotechnology, Poland), 400 nM of each primer (Metabion, Germany), 80 nM of TaqMan probe (Genewiz SZ, Suzhou, P.R. China), and 5 μL of template DNA in a 25-μL reaction volume. Amplification was performed with an initial polymerase activation step (10 min at 95 °C), followed by 40 cycles of denaturation (15 s at 95 °C) and hybridisation/extension (1 min at 60 °C) in a Mx3005P thermocycler (Stratagene, USA). The obtained PCR products were analysed using MxPro QPCR Software. The cycle threshold (CT) value, determining the cycle number at which the reporter’s fluorescence exceeds the threshold value, was recorded. A sample was considered positive, if the CT value was <40. Next, to confirm the results of real-time PCR, both orientation cycle sequencing was performed using the primers Tox1 and Tox2 targeting fragment of B1 gene84 (Genewiz BJ, Beijing). The sequences obtained were analysed and compared with sequences from GenBank using GeneStudio Pro Software (GeneStudio, Suwanee, Georgia, USA). The results were analysed using GeneStudioTM Professional (GeneStudio, Inc., USA).
All PCR experiments were performed including the T. gondii positive control to ensure the correct functionality of the reaction, as well as negative controls to ensure that no PCR component had been contaminated. For the positive control in experiments, DNA isolated from tachyzoites of the parasite (the T. gondii RH strain), obtained from the National Institute of Hygiene, Poland has been used. Additionally, samples were retested for the presence of PCR inhibitors by mixing 4 μL of DNA template and 1 μL of internal positive control (IPC). Comparison of results of amplification obtained for samples containing a combination of IPC and template DNA with IPC alone allowed estimation of potential interference.
Quantitative analysis, qPCR
Quantitative analysis was performed using the same primers and real-time PCR conditions as described above.
Standard curve
The initial copy number of the detected T. gondii DNA in positive samples was calculated using qPCR based on a standard curve. First, to generate the standard template the insert gene (a fragment of the B1 gene amplified by PCR using the set of primers described above) was cloned into a plasmid. Next, three series of nine dilutions of standard DNA in the range from one to 108 DNA copies per one μL were prepared and amplified. The standard curve was obtained by plotting the log of the initial template copy number against the CT value generated from each dilution (Fig. 4). Amplification of both the standard dilution series and the DNA isolated from positive samples was run on the same plate. Comparing the CT values of the unknown samples with the standard curve thus enabled the quantification of initial copy numbers40.
Standard curve generated from the amplification of nine dilutions of standard DNA in the range from one to 108 DNA copies per one μL; each dilution was run in triplicates and standard deviation for each dilution was calculated and displayed. The standard curve served for the quantification of initial copy numbers of T. gondii B1 gene in investigated wastewater and water samples collected from the area of Qinghai Province, P.R. China. Figure was prepared using MATLAB R2015a.
Determination of the number of T. gondii dispersive forms
The equivalent of T. gondii dispersive forms was estimated from the established initial number of copies of T. gondii DNA in each sample. Thus, the number of T. gondii cells (charge of the sample) was calculated using the following formula: CCS = N/35 × B, where N is the initial copy number determined using qPCR (per amount of DNA template taken for PCR); 35 is the number of copies of the B1 gene per one T. gondii cell; B is the multiplication factor referring to a total amount of DNA extracted from an investigated sample, in this study it is 10 because 5 µL of DNA template was taken for PCR from a total of 50 extracted per sample. Having CCS value, the number of T. gondii oocysts (oocysts charge of the sample) can be calculated as follows: OCS = CCS/A, where A=8 is the number of T. gondii sporozoites per one oocyst (the formula assumes that one sporulated oocyst contains eight fully developed sporozoites). Calculations were performed assuming that it is possible to detect a single T. gondii oocyst in a water suspension with real-time PCR, which has been validated in previous experiments85.
Genotyping
T. gondii genotypes were determined using multilocus PCR–RFLP assay with selected genetic markers: SAG1, SAG2, SAG3, BTUB, GRA6, c22-8, c29-2, L358, PK1 and Apico74,75,86. The set of reaction included nested PCR and restriction analysis of amplified products.
Amplification of genetic markers using nested PCR
The first step of nested PCR was performed using a set of external primers (Table 3) in a 25-μL reaction volume and the amplification reaction mixture consisted of 12.5 μL of the standard and ready-to-use PCR mixture 2xPCR Mix Plus High GC (A&A Biotechnology, Poland), 200 nM of each forward and reverse primer (Genewiz SZ, Suzhou, P.R. China), and 2 μL of template DNA. Amplifications were performed with an initial polymerase activation step (5 min at 95 °C), followed by 35 cycles of denaturation (30 s at 94 °C), primers annealing (1 min at 55 °C), strand extension (2 min s at 72 °C), and final extension (7 min at 72 °C). The second step of nested PCR reactions were performed using an internal set of primers (Table 1) under the following amplification reaction mixture conditions, 12.5 μL of the PCR mixture 2xPCR Mix Plus High GC (A&A Biotechnology, Poland), 400 nM of each primer (Genewiz SZ, Suzhou, P.R. China), and 2 μL of template DNA in a 25-μL reaction volume. Amplifications were performed according to the same protocol as in the first reaction, with the exception that annealing temperature was 60 °C.
Restriction analysis of nested PCR products
In order to perform restriction analysis, 10 µL of PCR product was mixed with 2 µL of 10× digestive buffer and 1 U of restriction enzyme (each of the two restriction enzymes in case of double digestion) (Thermo Scientific, USA). The reaction was carried out according to the manufacturer’s instruction. RFLP products were analysed using a WD-9413B gel imaging analysis system (Beijing Liuyi Biotechnology, P.R. China) following electrophoresis on a 3% gel agarose (Biowest Regular Agarose G-10, Gene Company) stained with ExRed nucleic acid electrophoresis dye (Beijing Zoman Biotechnology, P.R. China).
Statistical analysis
Statistical analysis of results including the confidence intervals for obtained percentages was performed using the Wilson Score without continuity correction. Calculations were performed using the Wilson Score Interval Calculator by Wolfram Alpha.
Data availability
All data generated or analysed during this study are included in this published article.
References
Robert-Gangneux, F. & Dardé, M.-L. Epidemiology of and diagnostic strategies for toxoplasmosis. Clin. Microbiol. Rev. 25, 264–296 (2012).
Flegr, J., Prandota, J., Sovičková, M. & Israili, Z. H. Toxoplasmosis-A global threat. Correlation of latent toxoplasmosis with specific disease burden in a set of 88 countries. PLoS ONE 9, e90203 (2014).
Tenter, A. M., Heckeroth, A. R. & Weiss, L. M. Toxoplasma gondii: from animals to humans. Int. J. Parasitol. 30, 1217–1258 (2000).
Weinman, D. & Chandler, A. H. Toxoplasmosis in man and swine-An investigation of the possible relationship. J. Am. Med. Assoc. 161, 229–232 (1956).
Hall, S., Ryan, M. & Buxton, D. The Epidemiology of Toxoplasma Infection. In Toxoplasmosis: A Comprehensive Clinical Guide (eds Joynson, D. H. M. & Wreghitt, T. G.) 58–124 (Cambridge University Press, 2001).
Miller, N. L., Frenkel, J. K. & Dubey, J. P. Oral infections with Toxoplasma cysts and oocysts in felines, other mammals, and in birds. J. Parasitol. 58, 928–937 (1972).
Dubey, J. P. Toxoplasmosis of Animals and Humans Vol. 319 (CRC Press, 2009).
Karanis, P., Aldeyarbi, H. M., Kirhashemi, M. E. & Khalil, K. M. The impact of the waterborne transmission of Toxoplasma gondii and analysis efforts for water detection: an overview and update. Environ. Sci. Pollut. Res. 20, 86–99 (2013).
Karanis, P., Kourenti, C. & Smith, H. Waterborne transmission of protozoan parasites: A worldwide review of outbreaks and lessons learnt. J. Water Health 5, 1–38 (2007).
Villena, I. et al. Evaluation of a strategy for Toxoplasma gondii oocyst detection in water. Appl. Environ. Microbiol. 70, 4035–4039 (2004).
Sroka, J., Wójcik-Fatla, A. & Dutkiewicz, J. Occurrence of Toxoplasma gondii in water from wells located on farms. Ann. Agric. Environ. Med. 13, 169–175 (2006).
Gallas-Lindemann, C., Sotiriadou, I., Mahmoodi, M. R. & Karanis, P. Detection of Toxoplasma gondii oocysts in different water resources by Loop Mediated Isothermal Amplification (LAMP). Acta Trop. 125, 231–236 (2013).
Sotiriadou, I. & Karanis, P. Evaluation of loop-mediated isothermal amplification for detection of Toxoplasma gondii in water samples and comparative findings by polymerase chain reaction and immunofluorescence test (IFT). Diagn. Mircrobiol. Infect. Dis. 62, 357–365 (2008).
Wells, B. et al. Molecular detection of Toxoplasma gondii in water samples from Scotland and a comparison between the 529bp real-time PCR and ITS1 nested PCR. Water Res. 87, 175–181 (2015).
Koloren, Z. Sensitive and cost-effective detection of Toxoplasma gondii in water supplies of the Black Sea in Turkey by loop-mediated isothermal amplification (LAMP). Biotechnol. Biotechnol. Equip. 27, 3543–3546 (2013).
de Moura, L. et al. Waterborne toxoplasmosis, Brazil, from field to gene. Emerg. Infect. Dis. 12, 326–329 (2006).
Verant, M. L. et al. Attempted detection of Toxoplasma gondii oocysts in environmental waters using a simple approach to evaluate the potential for waterborne transmission in the Galápagos Islands, Ecuador. EcoHealth 11, 207–214 (2014).
Ajonina, C., Buzie, C., Möller, J. & Otterpohl, R. The detection of Entamoeba histolytica and Toxoplasma gondii in wastewater. J. Toxicol. Environ. Health A 81, 1–5 (2017).
Kourenti, C. & Karanis, P. Evaluation and applicability of a purification method coupled with nested PCR for the detection of Toxoplasma oocysts in water. Lett. Appl. Microbiol. 43, 475–481 (2006).
Moulin, L. et al. Contribution of treated wastewater to the microbiological quality of Seine River in Paris. Water Res. 44, 5222–5231 (2010).
Galvani, A. T. et al. Real-time PCR detection of Toxoplasma gondii in surface water samples in São Paulo, Brazil. Parasitol. Res. 118, 631–640 (2019).
CONSIHPD. A national survey on current status of the important parasitic diseases in human population. Chin. J. Parasitol. Parasitic Dis. 23, 332–340 (2005).
Bai, M. et al. Toxoplasma gondii infection in farmed wild boars (Sus scrofa) in three cities of Northeast China. Foodborne Pathog. Dis. 14, 379–385 (2017).
Li, K. et al. Socio-economic burden of parasitic infections in yaks from 1984 to 2017 on Qinghai Tibetan Plateau of China—A review. Acta Trop. 183, 103–109 (2018).
Wang, D. et al. Seroprevalence and genotypes of Toxoplasma gondii isolated from pigs intended for human consumption in Liaoning province, northeastern China. Parasit. Vectors 9, 248 (2016).
Yang, N., Mu, M., Li, H., Long, M. & He, J. Seroprevalence of Toxoplasma gondii infection in slaughtered chickens, ducks, and geese in Shenyang, northeastern China. Parasit. Vectors 5, 237 (2012).
Yin, M. et al. Seroprevalence and risk factors of Toxoplasma gondii in tibetan sheep in Gansu province, Northwestern China. BMC Vet. Res. 11, 41 (2015).
Zhu, Z., Sung, Y., Li, K., Zhou, Z. & Wang, Z. Progress on prevalence and risk factors of toxoplasmosis in cattle, sheep and goats in China. Progress Vet. Med. 38, 107–110 (2017).
Wang, H. et al. Prevalence and genotypes of Toxoplasma gondii in pork from retail meat stores in Eastern China. Int. J. Food Microbiol. 157, 393–397 (2012).
Zou, Y. et al. First genetic characterization of Toxoplasma gondii infection in poultry meat intended for human consumption in eastern China. Infect. Genet. Evol. 55, 172–174 (2017).
Ding, H., Gao, Y., Deng, Y., Lamberton, P. H. L. & Lu, D. A systematic review and meta-analysis of the seroprevalence of Toxoplasma gondii in cats in mainland China. Parasit. Vectors 10, 27 (2017).
Gao, Y., Ding, H., Lamberton, P. H. L. & Lu, D. Prevalence of Toxoplasma gondii in pet dogs in mainland China: A meta-analysis. Vet. Parasitol. 229, 126–130 (2016).
Zhang, H. et al. Antibodies to Toxoplasma gondii in stray and household dogs in Guangzhou, China. J. Parasitol. 96, 671–672 (2010).
Du, F. et al. Survey on the contamination of Toxoplasma gondii oocysts in the soil of public parks of Wuhan, China. Vet. Parasitol. 184, 141–146 (2012).
Du, F. et al. Soil contamination of Toxoplasma gondii oocysts in pig farms in central China. Vet. Parasitol. 187, 53–56 (2012).
Gao, X., Wang, H., Wang, H., Qin, H. & Xiao, J. Land use and soil contamination with Toxoplasma gondii oocysts in urban areas. Sci. Total Environ. 568, 1086–1091 (2016).
Liu, X. et al. Detection of Toxoplasma gondii in chicken and soil of chicken farms in Nanjing region, China. Infect. Dis. Poverty 6, 62 (2017).
Xu, H. et al. Investigation of Toxoplasma gondii contamination in soil samples of parks in urban areas of Tianjin city. J. Tianjin Agric. Univ. 25, 48–51 (2018).
Wang, M. et al. Detection of Toxoplasma gondii oocysts in soils in Northwestern China using a new semi-nested PCR assay. BMC Vet. Res. 10, 238 (2014).
Lass, A. et al. First molecular detection of Toxoplasma gondii in vegetable samples in China using qualitative, quantitative real-time PCR and multilocus genotyping. Sci. Rep. 9, 17581 (2019).
Howe, D. K. & Sibley, L. D. Toxoplasma gondii comprises three clonal lineages: Correlation of parasite genotype with human disease. J. Infect. Dis. 172, 1561–1566 (1995).
Khan, A. et al. Genetic analyses of atypical Toxoplasma gondii strains reveal a fourth clonal lineage in North America. Int. J. Parasitol. 41, 645–655 (2011).
Minot, S. et al. Admixture and recombination among Toxoplasma gondii lineages explain global genome diversity. Proc. Natl. Acad. Sci. U.S.A. 109, 13458–13463 (2012).
Rajendran, C., Su, C. & Dubey, J. P. Molecular genotyping of Toxoplasma gondii from Central and South America revealed high diversity within and between populations. Infect. Genet. Evol. 12, 359–368 (2012).
Chaichan, P. et al. Geographical distribution of Toxoplasma gondii genotypes in Asia: A link with neighboring continents. Infect. Genet. Evol. 53, 227–238 (2017).
Chen, Z. et al. Genotyping of Toxoplasma gondii isolates from cats in different geographic regions of China. Vet. Parasitol. 183, 166–170 (2011).
Wang, L. et al. Genotypes and mouse virulence of Toxoplasma gondii isolates from animals and humans in China. PLoS ONE 8, e53483 (2013).
Zhou, P. et al. Genetic characterization of Toxoplasma gondii isolates from China. Parasitol. Int. 58, 193–195 (2009).
Zhang, X. et al. Genetic characterization of Toxoplasma gondii from Qinghai vole, Plateau pika and Tibetan ground-tit on the Qinghai-Tibet Plateau, China. Parasit. Vectors 6, 291 (2013).
Cong, W. et al. Prevalence and genetic characterization of Toxoplasma gondii in house sparrows (Passer domesticus) in Lanzhou, China. Korean J. Parasitol. 51, 363–367 (2013).
Qian, W. F. et al. Genetic characterization of Toxoplasma gondii from domestic animals in Central China. Trop. Biomed. 32, 540–544 (2015).
Wang, L. et al. Toxoplasma gondii prevalence in food animals and rodents in different regions of China: Isolation, genotyping and mouse pathogenicity. Parasit. Vectors 6, 273 (2013).
dos Santos, P. R. & Daniel, L. A. Occurrence and removal of Giardia spp. cysts and Cryptosporidium spp. oocysts from a municipal wastewater treatment plant in Brazil. Environ. Technol. 38, 1245–1254 (2017).
Berglund, B. et al. Occurrence and removal efficiency of parasitic protozoa in Swedish wastewater treatment plants. Sci. Total Environ. 598, 821–827 (2017).
Hatam-Nahavandi, K. et al. Detection of parasitic particles in domestic and urban wastewaters and assessment of removal efficiency of treatment plants in Tehran, Iran. J. Environ. Health Sci. Eng. 13, 4 (2015).
Javanmard, E. et al. Small-scale risk assessment of transmission of parasites from wastewater treatment plant to downstream vegetable farms. Gastroenterol. Hepatol. Bed Bench 11, 352–358 (2018).
Ma, L. et al. Detection of Cryptosporidium and Giardia in the slaughterhouse, sewage and river waters of the Qinghai Tibetan plateau area (QTPA), China. Parasitol. Res. 118, 2041–2051 (2019).
Ramo, A., Del Cacho, E., Sánchez-Acedo, C. & Quílez, J. Occurrence and genetic diversity of Cryptosporidium and Giardia in urban wastewater treatment plants in north-eastern Spain. Sci. Total Environ. 598, 628–638 (2017).
Lass, A. et al. Detection of Acanthamoeba spp. in water samples collected from natural water reservoirs, sewages, and pharmaceutical factory drains using LAMP and PCR in China. Sci. Total Environ. 584–585, 489–494 (2017).
Ma, L. et al. Detection of Cryptosporidium and Giardia in agricultural and water environments in the Qinghai area of China by IFT and PCR. Parasitol. Res. 113, 3177–3184 (2014).
World Bank. China-Qinghai Xining Water Environment Management Project (English). (International Bank for Reconstruction and Development, 2014).
World Bank. China-Qinghai Xining Water Environment Management Project : environmental assessment (Vol. 2): Environmental and Social Assessment: Executive Summary (English). (Lanzhou University, 2013).
Ferreira, F. P. et al. The effect of water source and soil supplementation on parasite contamination in organic vegetable gardens. Revista Brasileira e Parasitologia Veterinária 27, 327–337 (2018).
Shapiro, K. et al. Environmental transmission of Toxoplasma gondii: Oocysts in water, soil and food. Food Waterborne Parasitol. 15, e00049 (2019).
Slany, M. et al. Toxoplasma gondii in vegetables from fields and farm storage facilities in the Czech Republic. FEMS Microbiol. Lett. 366, fnz170 (2019).
Wang, M. et al. Serological survey of Toxoplasma gondii in Tibetan mastiffs (Canis lupus familiaris) and yaks (Bos grunniens) in Qinghai, China. Parasit. Vectors 5, 35 (2012).
Cong, W. et al. Toxoplasma gondii, Dirofilaria immitis, feline immunodeficiency virus (FIV), and feline leukemia virus (FeLV) infections in stray and pet cats (Felis catus) in northwest China: Co-infections and risk factors. Parasitol. Res. 115, 217–223 (2016).
Lu, W., Ha, L., Cao, L., Yang, L. & Xue, Y. Lanzhou deng 5 shi (zhou) quan mao gongxin chong bing liuxing xianzhuang diaocha [The current prevalence of toxoplasmosis in dogs and cats in Lanzhou and other five cities (states)]. Anim. Husb. Vet. Med. 42, 109 (2010).
Zheng, H., Ye, D. & Qi, S. Serological detection and analysis of Toxoplasma gondii infection in dogs in Lanzhou. China Anim. Husb. Vet. Med. 37, 172–174 (2010).
Liu, Q. et al. Seroprevalence of Toxoplasma gondii infection in yaks (Bos grunniens) in northwestern China. Trop. Anim. Health Prod. 43, 741–743 (2011).
Lindsay, D. S., Dubey, J. P., Butler, J. M. & Blagburn, B. L. Mechanical transmission of Toxoplasma gondii oocysts by dogs. Vet. Parasitol. 15, 27–33 (1997).
Bahia-Oliveira, L., Gomez-Marin, J. & Shapiro, K. Toxoplasma gondii. In Global Water Pathogen Project (eds Rose, J. B. & Jiménez-Cisneros, B.) 1–39 (Michigan State University, 2017). https://doi.org/10.14321/waterpathogens.37.
Shwab, E. K. et al. Geographical patterns of Toxoplasma gondii genetic diversity revealed by multilocus PCR-RFLP genotyping. Parasitology 141, 453–461 (2014).
Su, C., Shwab, E. K., Zhou, P. & Zhu, X. Moving towards an integrated approach to molecular detection and identification of Toxoplasma gondii. Parasitology 137, 1–11 (2010).
Su, C., Zhang, X. & Dubey, J. P. Genotyping of Toxoplasma gondii by multilocus PCR-RFLP markers: A high resolution and simple method for identification of parasites. Int. J. Parasitol. 36, 841–848 (2006).
Qin, S. et al. Seroprevalence, risk factors and genetic characterization of Toxoplasma gondii in free-range white yaks (Bos grunniens) in China. Vet. Parasitol. 211, 300–302 (2015).
Cong, W. et al. Prevalence, risk factors and genotype distribution of Toxoplasma gondii DNA in soil in China. Ecotoxicol. Environ. Saf. 189, 109999 (2020).
Lass, A., Pietkewicz, H., Szostakowska, B. & Myjak, P. The first detection of Toxoplasma gondii DNA in environmental fruits and vegetables samples. Eur. J. Clin. Microbiol. Infect. Dis. 31, 1101–1108 (2012).
Falk, C. C., Karanis, P., Schoenen, D. & Seitz, H. M. Bench scale experiments for the evaluation of a membrane filtration method for the recovery efficiency of Giardia and Cryptosporidium from water. Water Res. 32, 565–568 (1998).
Karanis, P. & Kimura, A. Evaluation of three flocculation methods for the purification of Cryptosporidium parvum oocysts from water samples. Lett. Appl. Microbiol. 34, 444–449 (2002).
Dumètre, A. & Dardé, M.-L. How to detect Toxoplasma gondii oocysts in environmental samples?. FEMS Microbiol. Rev. 27, 651–661 (2003).
Garcia, L. S. Diagnostic Medical Parasitology. in (ed. Garcia, L. S.) 123–141 (American Society for Microbiology, 2007).
Arkush, K. D. et al. Molecular and bioassay-based detection of Toxoplasma gondii oocyst uptake by mussels (Mytilus galloprovincialis). Int. J. Parasitol. 33, 1087–1097 (2003).
Burg, J. L., Grover, C. M., Pouletty, P. & Boothroyd, J. C. Direct and sensitive detection of a pathogenic protozoan, Toxoplasma gondii, by polymerase chain reaction. J. Clin. Microbiol. 27, 1787–1792 (1989).
Lass, A., Szostakowska, B., Korzeniewski, K. & Karanis, P. The first detection of Toxoplasma gondii DNA in environmental air samples using gelatine filters, real-time PCR and loop-mediated isothermal (LAMP) assays: qualitative and quantitative analysis. Parasitology 144, 1791–1801 (2017).
Ferreira, I. M. R. et al. Toxoplasma gondii isolates: Multilocus RFLP–PCR genotyping from human patients in Sao Paulo State, Brazil identified distinct genotypes. Exp. Parasitol. 129, 190–195 (2011).
Grigg, M. E., Ganatra, J., Boothroyd, J. C. & Margolis, T. P. Unusual abundance of atypical strains associated with human ocular toxoplasmosis. J. Infect. Dis. 184, 633–639 (2001).
Acknowledgements
Authors wish to thank to the Chinese Government for support of the project in the frame of Thousand Talents Plan Programme, Grant No. WQ2013630172 and High-end foreign experts Program No. Y20166300003.
Author information
Authors and Affiliations
Contributions
A.L.: conception of the study, study design, methodology selection, performance of real-time P.C.R., quantitative real-time P.C.R., genotyping, sequencing, microscopy, data analysis, interpretation of the results, writing manuscript; L.M.: collection of samples, contribution in laboratory work; I.K. – contribution in laboratory work (PCR), writing manuscript, data analysis, , graphical presentation of figures and tables, selection of references; X.Z. – collection of samples, contribution in P.C.R.; X.L. – collection of samples, flocculation; P.K. – conception of the study, financial support, contribution in data analysis, revision of manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lass, A., Kontogeorgos, I., Ma, L. et al. Investigation of Toxoplasma gondii in wastewater and surface water in the Qinghai-Tibet Plateau, China using real-time PCR and multilocus genotyping. Sci Rep 12, 5428 (2022). https://doi.org/10.1038/s41598-022-09166-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-09166-0
This article is cited by
-
PacBio next-generation sequencing uncovers Apicomplexa diversity in different habitats
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.