Abstract
To compare gastrointestinal (GI) and genitourinary (GU) toxicities in patients with localized prostate cancer treated with ultrahypofractionated radiotherapy (UHF) or brachytherapy [BT; low dose rate, LDR or high dose rate (HDR) with or without external beam radiotherapy (EBRT)]. We compared 253 UHF and 1664 BT ± EBRT groups. The main outcomes were the incidence and severity of acute and late GU and GI toxicities. The secondary endpoint was biochemical control rate. Cumulative late actuarial GU toxicity did not differ for grade ≥ 2 (8.6% at 5-years in UHF and 13.3% in BT ± EBRT, hazard ratio [HR], 0.7066; 95% CI, 0.4093–1.22, p = 0.2127). Actuarial grade ≥ 2 late GI toxicity was higher in UHF (5.8% at 5-years, HR: 3.619; 95% CI, 1.774–7.383, p < 0.001) than in BT ± EBRT (1.1%). In detailed subgroup analyses, the high-dose UHF group (H-UHF) using BED ≥ 226 Gy1.5, showed higher GI toxicity profiles than the other subgroups (HDR + EBRT, LDR + EBRT, and LDR monotherapy, and L-UHF BED < 226 Gy1.5) with equivalent GU toxicity to other modalities. With a median follow-up period of 32 months and 75 months, the actuarial biochemical control rates were equivalent between the UHF and BT ± EBRT groups. UHF showed equivalent efficacy, higher GI and equivalent GU accumulated toxicity to BT ± EBRT, and the toxicity of UHF was largely dependent on the UHF schedule.
Similar content being viewed by others
Introduction
Following the trend of shortening the treatment period in radiotherapy, stereotactic ablative body radiotherapy (SBRT), and high-precision external beam radiotherapy using strict image-guidance1 enabled us to perform ultrahypofractionated radiotherapy (UHF) using a single fraction dose of 5 Gy or more, which could reduce the burden on healthcare resources1,2,3,4,5. The biological features of prostate cancer with a low α/β ratio also encouraged the adoption of these hypo-to ultra-hypofractionation worldwide1. The recent HYPO-RT-PC randomized control trial provided evidence that UHF is non-inferior to standard conventional fractionation of 78 Gy in 2 Gy fractions2. Long-term3 and large cohort outcomes including meta-analysis from Western countries confirmed the efficacy of UHF4,5. However, selection of the best treatment option for patients with localized prostate cancer remain difficult due to the many curative treatment options, such as surgery, external beam radiotherapy, and brachytherapy (BT)6. BT is an established treatment for localized prostate cancer with excellent dose distribution, including low-dose rate (LDR) BT and high dose rate (HDR) BT7. BT can be administered as monotherapy (usually for low or lower titer intermediate-risk prostate cancer) or as a boost (for higher titers intermediate or high-risk prostate cancer). There are concerns that for intermediate-and high-risk disease, BT alone may not adequately treat the peri-prostatic tissues; therefore, BT has been used as a boost in combination with external beam radiotherapy (EBRT) for high-intermediate or high-risk prostate cancer in general7. Although several randomized controlled trials indicated the superiority of BT boost over external beam radiotherapy alone8,9,10 not only in LDR8 but also in HDR9,10, there is a lack of conclusive data comparing BT ± EBRT and UHF11,12,13,14,15. Therefore, to compare the results of UHF to BT ± EBRT, we used open data constructed by multi-institution data accumulation in Japan16. In addition, as previous studies cited that a BED over 226 Gy1.5 might be a threshold to cause higher rates of grade > 2 toxicities16,17 in UHF, we divided the UHF group into L-UHF (BED < 226 Gy1.5) and H-UHF (BED ≥ 226 Gy1.5) groups using this threshold. Then, we performed a subgroup analysis (LDR monotherapy, LDR + EBRT, HDR + EBRT, L-UHF, H-UHF) compared to BT ± EBRT versus UHF. Thus, the aim of the present study was to compare the toxicity and preliminary PSA control of UHF and BT ± EBRT.
Methods
Patients
We retrospectively examined 253 patients treated with UHF (open data for public use)18 and 1664 patients treated with BT ± EBRT (1161 HDR + EBRT from open data and 477 LDR ± EBRT treated at Kyoto Prefectural University of Medicine) (Table 1). The patient eligibility criteria included treatment with UHF or BT ± EBRT, stage T1–T3, and N0M0 with histology-proven adenocarcinoma; the availability and accessibility of pretreatment data (initial prostate-specific antigen = iPSA) level, Gleason score sum (GS), and T classification to determine the stage according to the NCCN 2015 risk classification as follows: low (T1–T2a, GS 2–6, and iPSA < 10 ng/mL), intermediate (T2b–T2c, GS 7, or PSA 10–20 ng/mL), and high (T3, GS 8–10, or PSA > 20 ng/mL)19. We excluded (1) node-positive cases, (2) metastasis cases, and (3) follow-up period of less than 20 months.
The Common Terminology Criteria for Adverse Events version 4.0, was used for the toxicity analysis. Toxic effects occurring within 90 days after radiotherapy completion were considered acute, and toxic effects occurring after the 90-day period were considered late. Biochemical failure was defined as the time from the initiation of radiotherapy to the date of last follow-up and/or biochemical failure, whichever came first, according to the Phoenix definition (nadir, + 2 ng/ml)19.
All patients at Kyoto Prefectural University of Medicine provided written informed consent, and patients undergoing UFH (open data) and a part of those undergoing BT ± EBRT (open data) provided informed consent during the process of building public data. This study was conducted in accordance with the Declaration of Helsinki and with the permission of the Institutional Review Board (Kyoto Prefectural University of Medicine: ERB-C-1403).
Treatment planning
LDR-BT with or without EBRT
The implant technique has been described in detail previously20. All patients underwent transrectal ultrasound preplanning 3–4 weeks before implantation to determine the number of seeds. Permanent intraoperative Iodine-125 implantation using a modified peripheral loading method. We used combination therapy (LDR + EBRT) for T3 or Gleason score sum ≤ 8, or Gleason score sum 7 (4 + 3) cases (not for Gleason score sum 7 (3 + 4) cases) (Fig. 1). The prescription dose for the clinical target volume (prostate) was 145 Gy (LDR alone) or 110 Gy [LDR with 40 Gy/ 20 fractions EBRT by three-dimensional conformal radiotherapy (3D-CRT)].
Scheme of treatments according to National Comprehensive Cancer Network (NCCN) risk classification. Abbreviations; BT brachytherapy, HDR high-dose-rate, LDR low-dose-rate, EBRT external beam radiotherapy, UHF ultrahypofractionated radiotherapy, L-UHF low dose UHF EQD2 < 100 Gy1.5 (α/β = 1.5), H-UHF high dose UHF EQD2 ≥ 100 Gy1.5 (α/β = 1.5).
HDR-BT with EBRT
The multi-institution data were obtained from an open data source18, and the detailed method of applicator implantation has been described elsewhere21. All patients were treated with a combination of HDR and EBRT at various fractionations (Table 2). The median dose of HDR used was 31.5 Gy (10.5–31.5 Gy) and that of EBRT was 30 Gy (30–51 Gy). The median fraction size of HDR was 6.3 Gy (5–11 Gy) and that of EBRT was 3 Gy (2–3 Gy). Patients who were administered EBRT comprised 1166 (98.2%) on 3D-CRT and 21 (1.8%) on IMRT.
UHF
The detailed method of this study has been described elsewhere16,22. The median dose of UHF used was 36 Gy (32–36.25 Gy) and the median fraction size of UHF was 7.25 Gy (7–9 Gy) (Table 2).
Statistical analysis
The R stat package23 was used for the statistical analyses. We analyzed percentages using chi-square tests. To compare medians or means, we used Mann–Whitney U-tests for skewed data and Student’s t-tests for normally distributed data23. To analyze the biochemical control rate, overall survival, and toxicity, we used Kaplan–Meier method and log-rank tests including Bonferroni test in pos t-hoc p-value adjustment was used23. Univariate and multivariate analyses were made with Cox’s proportional hazards model23. All analysis used statistical significance level set at p < 0.05.
We divided the UHF group into two subgroups according to previous studies16,17: high (H-UHF) and low dose UHF (L-UHF) groups, using a cut-off value of BED of 226 Gy1.5; BED = n × d × (1 + d/[α/β]) where d = dose per fraction in Gy, n = number of treatment fractions, α/β = 1.5.
Since the included patients were not randomized, unbalanced patients baseline characteristics could influence on the selection bias and, hence, influence the decision to undergo BT ± EBRT or BT. The propensity score was defined as the probability of allocation to the BT ± EBRT or UHF group, given the patient characteristics23. We used logistic regression model in the calculation of the propensity scores using the baseline covariates shown in Table 2.
We used a propensity score-matched pair analysis to reduce the bias for choice of treatment; the UHF or BT ± EBRT groups (total population and HDR + EBRT group). Five factors prescribed before were selected as the variables that would be significantly related to the decision to choose UHF or BT ± EBRT, and a 1:1 matched cohort was made. Same procedure was applied in comparison between UHF and HDR + EBTT.
Results
Patient and tumor characteristics
The baseline patient characteristics of the UHF and BT ± EBRT groups are shown in Table 1. The 1921 patients with stage T1–T3 N0M0 prostate cancers were treated using UHF or BT ± EBRT. The median patient age was 70 years (range, 42–86 years). The median follow-up duration for the entire cohort was 70 months (range, 22–177 months). BT ± EBRT was used to treat patients with advanced disease and hormonal therapy history with longer follow-up periods than those in the UHF group.
Toxicity Comparison between UHF and BT ± EBRT
Table 3 shows the incidence of maximal grade of early and late gastrointestinal (GI) and genitourinary (GU) toxicities. UHF showed higher maximal grade GI and lower maximal grade GU toxicity than the BT ± EBRT group.
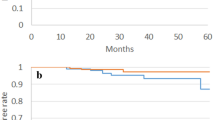
The 3- (and 5-year) cumulative incidence of grade ≥ 2 GI toxicities was 4.2% (5.8%) in the UHF group and 1.1% (1.8%) in the BT ± EBRT group (p < 0.0001; Fig. 2a), with a hazard ratio of 3.661 (95% CI: 1.799–7.454, p < 0.0001).
Comparison of accumulated incidence toxicity grade ≥ 2. (a) Accumulated incidence of grade ≥ 2 Gastrointestinal (GI) toxicity between BT ± EBRT and UHF. (b) Accumulated incidence of grade ≥ 2 Genitourinary (GU) toxicity between BT ± EBRT and UHF. (c) Accumulated incidence of grade ≥ 2 GI toxicity among subgroups. (HDR + EBRT vs. LDR + EBRT vs. DR monotherapy vs. L-UHF vs. H-UHF). (d) Accumulated incidence of grade ≥ 2 GU toxicity among subgroups. (HDR + EBRT vs. LDR + EBRT vs . LDR monotherapy vs. L-UHF vs. H-UHF).
The 3-year and 5-year cumulative incidence rates of grade ≥ 2 GU toxicities were 6.0% (8.6%) in the UHF group and 8.8% (13.3%) in the BT ± EBRT group (p = 0.205; Fig. 2b), with a hazard ratio of 0.7044 (95% CI: 0.408–1.216, p = 0.2085).
As shown in Table 4, the predictors of late GI toxicity grade ≥ 2 on the multivariate Cox regression analysis included modality (UHF worse than BT ± EBRT, hazard ratio 2.37, 95% CI = 1.04–5.39, p = 0.04), and acute GI toxicity grade ≥ 2 (hazard ratio 6.76, 95% CI = 1.94–23.59, p = 0.0027). For GU toxicity, only acute GU toxicity grade ≥ 2 (hazard ratio 2.19, 95% CI = 1.69–2.84, p < 0.0001) was identified as a statistically significant predictor of late GU toxicity grade ≥ 2.
Subgroup analysis for toxicity
In the detailed subgroup analysis, BT ± EBRT was divided into HDR + EBRT, LDR only, and LDR + EBRT, while UHF was divided into U-UHF and L-UHF (Table 2).
The 3-year and 5-year cumulative incidences of grade ≥ 2 late GI toxicities were 1.5% (2.6%), 0.3% (0.3%), 2.4% (2.4%), 1.2% (1.2%), and 9.3% (13%) in the HDR + EBRT, LDR only, LDR + EBRT, L-UHF, and H-UHF groups, respectively (p < 0.0001, Fig. 2c). H-UHF showed a higher cumulative incidence of GI toxicity than the other modalities (Table 5).
For GU toxicity, the 3-year and 5-year cumulative incidence rates of grade ≥ 2 late GU toxicities were 7.5% (13.5%) in the HDR + EBRT group, 9% (13.5% at 5 years) in the LDR group, 4.6% (13.4%) in the LDR + EBRT group, 3% (3%) in the L-UHF group, and 11.1% (17%) in the H-UHF group (p = 0.117; Fig. 2d). There were no statistically significant differences among the subgroups in terms of accumulated GU toxicity (Table 5).
Biochemical control and overall prostate cancer-specific survival
The number of patients with biochemical failure was 142 in the BT ± EBRT group (8.5%) and 10 in the UHF group (3.95%). The actuarial 3-year and 5-year biochemical control rates were 96.3% (95% CI: 92.7–98.2%) and 96.6% (95% CI: 95.6–97.4%, p = 0.766, Fig. 1) at 3-year, and 91.4% (95% CI: 78.8–96.6%) and 94.0% (95% CI: 92.6–95.1%) at 5-year in the UHF and BT ± EBRT groups, respectively (Fig. 3a).
Biochemical control rates between UHF and BT ± EBRT. (a) Comparison between UHF and BT ± EBRT in total population. (b) Comparison between UHF and BT ± EBRT in matched pair generated by propensity score matching. (c) Comparison between UHF and HDR + EBRT in matched pair generated by propensity score matching. (d) Comparison among five subgroups.
We generated a well-matched pair (228 and 228 patients; background comparison is shown in Supplemental Table 1) in each group using propensity score matching. The actuarial 3-year and 5-year biochemical control rates were 99.0% (95% CI: 96.0–99.8%) and 99.1% (95% CI: 96.3–99.8%, p = 0.164, Fig. 3b) at 3-year, and 92.9% (95% CI: 76.7–98.0%) and 96.1% (95% CI: 92.2–98.0%) at 5-year in the UHF and BT ± EBRT groups, respectively.
The 3-year and 5-year overall survival rates were 99.1% (95% CI: 96.5–99.8%) and 96.6% (95% CI: 90.1–98.8%) for UHF, and 99.4% (95% CI: 98.8–99.7%) and 98.0% (95% CI: 97.1–98.6%) and for the BT ± EBRT groups (p = 0.058), respectively. Seventeen and zero prostate cancer-related deaths were observed in the BT and UHF groups, respectively, in this cohort. The 5-year prostate cancer-specific survival rates were 100% and 99.5% (95% CI: 98.9–99.8%, p = 0.501) in the UHF and BT ± EBRT groups, respectively.
Subgroup analysis for Biochemical control and overall prostate cancer-specific survival
For comparison between UHF and LDR only (LDR monotherapy), we included patients with a lower titer intermediate-risk group and low-risk group (Fig. 1). The actuarial 3-year and 5-year biochemical control rates were 97.6% (95% CI: 92.6–99.2%) and 97.2% (95% CI: 95.1–98.5%, p = 0.632, supplemental Fig. 1a) at 3-year, and 89.7% (95% CI: 68.6–96.9%) and 95.9% (95% CI: 93.5%-97.5%) at 5-year in the UHF and LDR only groups, respectively.
For comparison between the UHF and LDR + EBRT groups, we included only patients with a high titer of intermediate-risk and high-risk groups. The actuarial 3-year and 5-year biochemical control rates were 94.8% (95% CI: 87.5–97.9%) and 95.2% (95% CI: 85.8–98.4%, p = 0.871, supplemental Fig. 1b) at 3-year, and 94.8% (95% CI: 87.5–97.9%) and 93.6% (95% CI: 83.8%-97.5%) at 5-year in the UHF and LDR + EBRT groups, respectively.
For comparison between UHF and HDR+EBRT, the actuarial 3-year and 5-year biochemical control rates were 96.3% (95% CI: 92.7–98.2%) and 96.5% (95% CI: 95.2–97.4%, p = 0.962, supplemental Fig. 1c) at 3-year, and 91.4% (95% CI: 78.8–96.6%) and 93.2% (95% CI: 91.4%-94.6%) at 5-year in the UHF and HDR+EBRT groups, respectively.
We generated well-matched pairs in the comparison between UHF and HDR + EBRT (169 patients each; background comparison is shown in Supplemental Table 2) using propensity score matching. The actuarial 3-year and 5-year biochemical control rates were 98.4% (95% CI: 95.0–99.5%) and 98.9% (95% CI: 95.7–99.7%, p = 0.522, Fig. 3c) at 3-year, and 97.3% (95% CI: 92.6–99.0%) and 97.3% (95% CI: 92.9%–99.0) at 5-year in the UHF and HDR + EBRT groups, respectively.
For comparison between L-UHF and U-UHF, the actuarial 3-year biochemical control rates were 98.1% (95% CI: 94.3–99.4%) and 93.6% (95% CI: 85.1–97.3%, p = 0.139) in the L-UHF and H-UHF groups, respectively (supplemental Fig. 1d). There were no statistically significant differences among the subgroups (Fig. 3d). Among the NCCN risk classifications, the actuarial 3-year biochemical control rates were 100% (L-UHF) and 100% (H-UHF, p = 1.0) in the low-risk group; 97.1% (95% CI: 91.2–99.0%), 95.6% (95% CI: 83.3–98.9%, p = 0.454) in the intermediate-risk group; and 100%, 88.3% (95% CI: 64.8–96.5%, p = 0.109) in the high-risk group. L-UHF showed equivalent outcomes compared with H-UHF.
Discussion
UHF showed higher GI and equivalent GU toxicity to BT ± EBRT and was largely dependent on the UHF schedule. Additionally, we found an equivalent PSA control rate between UHF and BT ± EBRT, although this was inconclusive due to short follow-up periods. To our knowledge, this is one of the largest cohorts to compare the toxicity of UHF and BT ± EBRT. To reduce bias and amend short follow-up periods, we used the propensity score matched pair analysis, which is the best achievable statistical method and provides a direct comparison of BT ± EBRT and UHF.
Recent advancements in radiotherapy for localized prostate cancer have enabled us to shorten the treatment period using hypofractionations and provide cost effectiveness and patient convenience. In addition to 2.3–3.4 Gy moderate hypofractionation, UHF gained attention for exploiting the low a/b ratio of this tumor and its high radiation fraction size sensitivity1,2,3,4,5,6. The recent HYPO-RT-PC phase 3 trial, which showed non-inferiority of ultrahypofractionation (42.7 Gy/7 fractions for 2.5 weeks) compared with conventional fractionation (78 Gy/39 fractions)2. It is anticipated that the efficacy of the UHF treatment schedule will be further validated when the PACE B trial outcome is consolidated and published24. Similarly, within our cohort of patients, an excellent biochemical control rate was achieved, which is comparable to HDR ± EBRT, although preliminary.
The HYPO-RT-PC phase 3 trial2 reported 28% acute RTOG grade ≥ 2 GU toxicity, and grade ≥ 2 RTOG late GU toxicity was 5% at 5 years, while bowel toxicity was 1% at 5-years. The PACE-B trial reported that the worst acute RTOG toxicity grade ≥ 2 was 23% in GU and 10% in GI24. In our UHF data, the worst acute toxicity grade ≥ 2 was 13% in GU and 5% in GI, and accumulated late toxicity grade ≥ 2 was 6% in GU and 5.8% in GI, which concurred with their data. Jackson et al. performed a systemic review and reported that the estimated late grade ≥ 3 GU and GI toxicity rates were 2.0% (95% CI, 1.4–2.8%) and 1.1% (95% CI, 0.6–2.0%) after UHF using SBRT, respectively4, which also concurred with our cohort.
In general, BT elevated GU toxicity and reduced GI toxicity compared to EBRT7. In addition, although the incidence of acute GU toxicity is tentatively elevated by BT, toxicity was ameliorated by time and cumulative late toxicity did not differ after a few years7. For GI toxicity, spacer (SpaceOAR etc.) insertion was found to reduce GI toxicity to almost negligible as no grade ≥ 2 GI events was found in spacer ( +) arms in a randomized controlled trial25. This technique could be applied not only in UHF but also in BT ± EBRT. Therefore, we hope that we could reduce GI toxicity in the near future, and the higher incidence of GI toxicity in H-UHF could be reduced with this technique.
As BT can achieve one of the best dose distributions among radiotherapy7,26, external beam radiotherapy has made efforts to improve dose distribution using SBRT, intensity-modulated radiotherapy, and image-guided radiotherapy techniques26. Several reviews 27,28 including three randomized controlled trials8,9,10 have already indicated superiority of BT boost than eternal beam radiotherapy alone. However, BT boost did not show superiority to UHF, and only indicated similarity of BT boost to UHF in low to intermediate risk groups11,12,13,14,15. So far, UHF could achieve equivocal outcomes without elevation of toxicity than BT boost in low-to intermediate-risk groups.
In 2018, the American Society for Radiation Oncology (ASTRO), ASCO, and American Urological Association (AUA) evidence-based guidelines stated that extreme hypofractionated 35–36,25 Gy in five fractions (BED 198–211.5 Gy1.5) may be offered to patients with low- and intermediate-risk prostate cancer29. Royce et al. found that in patients with low to intermediate risk disease treated with UHF, an equivalent dose of 2 Gy per fraction (EQD2) of 71 Gy (31.7 Gy in 5 fractions = BED: 165 Gy1.5) achieved a TCP of 90% and an EQD2 of 90 Gy (36.1 Gy in 5 fractions = BED: 209.8 Gy1.5) achieved a TCP of 95%30. Our data that L-UHF (BED = 198–226 Gy1.5) is in line with those of previous reports with a 3-years biochemical control rate of 97.7% (95% CI: 93–99.3%) in the low- to intermediate-risk group.
However, this does not apply for high risk and, most likely, a higher dose is needed3,31. Several groups seek better PSA control using higher prescribed doses, especially for intermediate- and high-risk groups31,32. In patients with high-risk disease, Royce et al. found that an EQD2 of 97 Gy (37.6 Gy in 5 fractions = 226 Gy1.5) can achieve a TCP of 90% and an EQD2 of 102 Gy (38.7 Gy in 5 fractions = 238.4 Gy1.5) can achieve a TCP of 95%3. Several studies used focal dose escalation with a boost of 38–50 Gy31,32. Although our cohort did not show the benefit of H-UHF (BED = 252 Gy1.5, with higher GI toxicity without improvement in biochemical control rate [88.3% at 3-years], although with short follow-up periods), further investigation could shed light on the dose escalation for high-risk prostate cancer.
Our study has several limitations. First, the lack of long-term follow-up and the small sample size limits its applicability, with only 25 (9.8%) patients with > 5 years of follow-up in the UHF group, especially in the high-risk group. Longer follow-up may reveal a divergence in toxicity or control rates in the UHF group. Next, the retrospective nature of this study led to an imbalance between the UHF and BT ± EBRT cohorts in terms of baseline characteristics. To mitigate this, we provided a comparative analysis and propensity score-matched analysis. Next, although using a free database is beneficial, its retrospective nature results in an ambiguous recording of the timing of toxicity and tumor control outcomes because of the heterogeneous follow-up periods depending on various institutions and physicians not restricted by protocol. Further studies should be conducted to validate our findings. Finally, for toxicity analysis, other predisposing factors are also important for prediction, including dosimetric factors for organs at risk33 and non-dosimetric factors (preexisting symptoms or surgery, transurethral resection of the prostate, anticoagulant use, diabetes mellitus, etc.)33.
Conclusions
UHF showed equivalent efficacy, higher GI and equivalent GU accumulated toxicity to BT ± EBRT, and the toxicity of UHF was largely dependent on the UHF schedule.
Data availability
The data of UHF for this manuscript can be obtained from the public data base on reasonable request [19] and LDR data can be obtained from the author upon reasonable request.
References
Gómez-Aparicio, M. A. et al. Extreme hypofractionation with SBRT in localized prostate cancer. Curr. Oncol. 28, 2933–2949 (2021).
Widmark, A. et al. Ultra-hypofractionated versus conventionally fractionated radiotherapy for prostate cancer: 5-year outcomes of the HYPO-RT-PC randomised, non-inferiority, phase 3 trial. Lancet 394, 385–395 (2019).
Katz, A. Stereotactic body radiotherapy for low-risk prostate cancer: A 10-year analysis. Cureus 9, e1668 (2019).
Jackson, W. C. et al. Stereotactic body radiation therapy for localized prostate cancer: A systematic review and meta-analysis of over 6000 patients treated on prospective studies. Int. J. Radiat. Oncol. Biol. Phys. 104, 778–789 (2019).
Kishan, A. U. et al. Long-term outcomes of stereotactic body radiotherapy for low-risk and intermediate-risk prostate cancer. JAMA Netw. Open 2, e188006 (2019).
Choudhury, A. et al. Photons, protons, SBRT, brachytherapy-what is leading the charge for the management of prostate cancer? a perspective from the GU editorial team. Int. J. Radiat. Oncol. Biol. Phys. 110, 1114–1121 (2021).
Chin, J. et al. Brachytherapy for patients with prostate cancer: American society of clinical oncology/cancer care ontario joint guideline update. J. Clin. Oncol. 35, 1737–1743 (2017).
Morris, W. J. et al. Androgen suppression combined with elective nodal and dose escalated radiation therapy (the ASCENDE-RT Trial): An analysis of survival endpoints for a randomized trial comparing a low-dose-rate brachytherapy boost to a dose-escalated external beam boost for high- and intermediate-risk prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 98, 275–285 (2017).
Sathya, J. R. et al. Randomized trial comparing iridium implant plus external-beam radiation therapy with external-beam radiation therapy alone in node-negative locally advanced cancer of the prostate. J. Clin. Oncol. 23, 1192–1199 (2005).
Hoskin, P. J. et al. Randomised trial of external-beam radiotherapy alone or with high-dose-rate brachytherapy for prostate cancer: Mature 12-year results. Radiother. Oncol. 154, 214–219 (2021).
Tsang, Y. M. et al. Ultra-hypofractionated radiotherapy for low- and intermediate risk prostate cancer: High-dose-rate brachytherapy vesus stereotactic ablative radiotherapy. Radiother. Oncol. 158, 184–190 (2021).
Loblaw, A. et al. Genitourinary radiation oncologists of Canada (GUROC). Stereotactic ablative radiotherapy versus low dose rate brachytherapy or external beam radiotherapy: Propensity score matched analyses of canadian data. Clin. Oncol. (R. Coll. Radiol.) 29, 161–170 (2017).
Hegde, J. V. et al. A pooled analysis of biochemical failure in intermediate-risk prostate cancer following definitive stereotactic body radiotherapy (SBRT) or high-dose-rate brachytherapy (HDR-B) monotherapy. Am. J. Clin. Oncol. 41, 502–507 (2018).
Gogineni, E. et al. Biochemical control and toxicity outcomes of stereotactic body radiation therapy versus low-dose-rate brachytherapy in the treatment of low- and intermediate-risk prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 109, 1232–1242 (2021).
Levin-Epstein, R. et al. Prostate-specific antigen kinetics and biochemical control following stereotactic body radiation therapy, high dose rate brachytherapy, and low dose rate brachytherapy: A multi-institutional analysis of 3502 patients. Radiother. Oncol. 151, 26–32 (2020).
Ishiyama, H. et al. Multi-institutional retrospective analysis of ultrahypofractionated radiotherapy for Japanese prostate cancer patients. Sci. Rep. 11, 13194 (2021).
Koontz, B. F. A systematic review of hypofractionation for primary management of prostate cancer. Eur. Urol. 68, 683–691 (2015).
An Open Data of Multicenter Data Collection: Outcome of Radiation Therapy for Prostate Cancer (BT ± EBRT : B16-143)(UFH:B20–118) Retrieved from August 30, 2021 https://www.khp.kitasato-u.ac.jp/ska/radiotherapy/arcivements/#results.
The National Comprehensive Cancer Network (NCCN), NCCN Clinical Practice Guidelines in Oncology. Prostate Cancer, 2015 version 4. https://www.nccn.org/store/login/login.aspx? ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf.
Yamada, Y. et al. Permanent prostate brachytherapy and short-term androgen deprivation for intermediate-risk prostate cancer in Japanese men: Outcome and toxicity. Brachytherapy 14, 118–123 (2015).
Ishiyama, H. et al. Nationwide multi-institutional retrospective analysis of high-dose-rate brachytherapy combined with external beam radiotherapy for localized prostate cancer: An Asian prostate HDR-BT consortium. Brachytherapy 16, 503–510 (2017).
Kainuma, T. et al. A phase I dose-escalation trial of stereotactic body radiotherapy using 4 fractions for patients with localized prostate cancer. Radiat. Oncol. 14, 158 (2019).
Kanda, Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. 48, 452–458 (2013).
Brand, D. H. et al. PACE trial investigators. Intensity-modulated fractionated radiotherapy versus stereotactic body radiotherapy for prostate cancer (PACE-B): Acute toxicity findings from an international, randomised, open-label, phase 3, non-inferiority trial. Lancet Oncol. 20, 1531–1543 (2019).
Mariados, N. et al. Hydrogel spacer prospective multicenter randomized controlled pivotal trial: Dosimetric and clinical effects of perirectal spacer application in men undergoing prostate image guided intensity modulated radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 92, 971–977 (2015).
Chatzikonstantinou, G. et al. Real-world dosimetric comparison between CyberKnife SBRT and HDR brachytherapy for the treatment of prostate cancer. Brachytherapy 20(1), 44–49 (2021).
Peyraga, G. et al. Brachytherapy boost (BT-boost) or stereotactic body radiation therapy boost (SBRT-boost) for high-risk prostate cancer (HR-PCa). Cancer Radiother. 25, 400–409 (2021).
Fischer-Valuck, B. W. et al. A brief review of low-dose rate (LDR) and high-dose rate (HDR) brachytherapy boost for high-risk prostate. Front. Oncol. 9, 1378 (2019).
Morgan, S. C. et al. Hypofractionated radiation therapy for localized prostate cancer: An ASTRO, ASCO, and AUA evidence-based guideline. J. Clin. Oncol. 36, JCO801097 (2018).
Royce, T. J. et al. Tumor control probability modeling and systematic review of the literature of stereotactic body radiation therapy for prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 110, 227–236 (2021).
Kerkmeijer, L. G. W. et al. Focal boost to the intraprostatic tumor in external beam radiotherapy for patients with localized prostate cancer: Results from the FLAME randomized phase III trial. J. Clin. Oncol. 39, 787–796 (2021).
van Dams, R. et al. Stereotactic body radiotherapy for high-risk localized carcinoma of the prostate (SHARP) consortium: Analysis of 344 prospectively treated patients. Int. J. Radiat. Oncol. Biol. Phys. 110, 731–737 (2021).
Wang, K. et al. Prostate stereotactic body radiation therapy: An overview of toxicity and dose response. Int. J. Radiat. Oncol. Biol. Phys. 110, 237–248 (2021).
Acknowledgements
We appreciate to participants and physicians for building big free data of treatment outcome [19].
Author information
Authors and Affiliations
Contributions
Author contributions H.Y. conceived study. K.M., G.S., N.A., D.S., T.K., K.Y. AU, TM, Y.Y., T.S., A.F., S.N., generated data. H.Y., G.S., performed analysis and interpreted results. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yamazaki, H., Masui, K., Suzuki, G. et al. Comparison of toxicities between ultrahypofractionated radiotherapy versus brachytherapy with or without external beam radiotherapy for clinically localized prostate cancer. Sci Rep 12, 5055 (2022). https://doi.org/10.1038/s41598-022-09120-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-09120-0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.