Abstract
We studied the alpha-globin gene genotypes, hematologic values, and transfusion-dependence of patients with Hb H disease. Molecular characterization of alpha-thalassemia was performed. We identified 120 patients with Hb H disease. Of these patients, 35 (29.16%) had deletional form of Hb H disease, and 85 (70.83%) had different form of non-deletional Hb H disease. The most frequently observed Hb H genotypes were --Med/–α3.7 in 33 patients (27.5%), αCD19(-G) α/αCD19(-G) α in 25 cases (20.83%), αpolyA2α/αpolyA2α in 15 (12.5%), and αpolyA1α/αpolyA1α in 13 (10.83%) respectively. The probability of receiving at least one transfusion blood in deletional form was observed in 3 of 35 (8.57%) patients which just seen in 3 of 33 (9%) patients with --Med/–α3.7 genotype. This form was also observed in 8 of 85 (9.4%) patients in non-deletional Hb H diseases which five of them had Med deletion in compound with alpha globin point mutations. Nondeletional Hb H disease was more severe than deletional Hb H disease requiring more blood transfusions. We can recommend that Med deletion in compound with alpha-globin point mutations, polyA1 and constant spring in homozygous form needs to be taken into consideration when offering counseling to high-risk couples.
Similar content being viewed by others
Introduction
Alpha thalassemia is one of the most common monogenic disorders in the Mediterranean region, Middle East and East and Southeast Asia, as well as in countries with migration from these regions1,2. The clinical manifestations of alpha globin abnormalities vary from the silent carrier state, in which only one α-globin gene is deleted, to fatal hydrops fetalis, in which all four α-globin genes are missing3. Hemoglobin H (Hb H) disease is caused by the loss of three α-globin genes, when there is only one functional alpha globin gene the patient produce a form of hemoglobin (Hb) composed of four β-chain (β4) called Hb H4. The clinical severity of patients with Hb H disease is variable, even in the presence of similar genotype, which is probably due to genetic and environmental modifiers5. More than 95% of α-thalassemia syndromes are caused by deletional abnormalities while the rest result from point mutations6.
Hb H patients also have been classified as mild, intermediate or severe phenotypes. Mild phenotype included the patients, who diagnosed above 4 years of age, Hb levels above 9.0 g/dl, transfusion-independent, normal growth, minimal bone changes and slight splenomegaly. Intermediate phenotype included patients diagnosed between 2 ± 4 years, Hb more than 8.0 g/dl, transfusion-independent, with mildly impaired growth, some bone abnormalities and moderate splenomegaly. Severe phenotype included patients with severe anemia, Hb less than, 8.0 g/l, needing frequent or occasional transfusions. Hb H Patients with severe phenotype had impaired growth, moderate to severe bone changes and splenomegaly7.
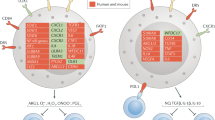
There are two types of Hb H disease, deletional and non-deletional. The first type is the most common form of Hb H disease, is caused by compound heterozygosity with a double α-globin gene deletion on one allele and a single α-globin gene deletion on the other allele (16p13.3). Second type is the non-deletional form of Hb H disease which at least one of the genetic abnormalities was non-deletional8. Non-deletional Hb H disease is relatively rare, usually caused by homozygosity for non-deletional alleles, and sometimes by compound heterozygosity for a double α-globin gene deletion on one chromosome and a point mutation of either the α1 or α2 globin gene on the other chromosome8. In general, the clinical phenotypes of Hb H disease are variable, ranging from asymptomatic to harsh forms as in some patients on regular/irregular blood transfusion4.
Non-deletional Hb H disease has more severe clinical symptoms and they are more anemic, prone to hepatosplenomegaly and transfusion dependency7,9. Because of high number of α-thalassemia carriers and consanguineous marriages in Iranian families, the prevalence of individuals with Hb H or even hydrops fetalis is increased10.
We evaluated Hb H disease in Iranian patients in Khuzestan Province in order to arrange a sensible prevention and management approach for the disease. Consanguineous and ethnic marriages in this region makes the controlling of disease more complicated and bring the necessity of clinical follow-up and routine screening for anemia at birth, during infancy and childhood.
Methods
Patients
We followed medical records of 120 patients with Hb H disease. These patients referred to the Narges Prenatal Diagnostics and Medical Genetics Laboratory as part of a national program for the prevention of thalassemia. Informed consent was obtained from the parents or the patients participating in this study. The red blood cell indices were automatically measured on a Coulter Counter ABX Micros 60 (Helena Laboratories, Beaumont, TX, USA). Hemoglobin H value was measured by high performance liquid chromatography (HPLC) using the VARIANT ™ HPLC system (Bio-Rad Laboratories, Hercules, CA, USA). The HbA2 band was measured by column chromatography (Beta-Thal HbA2 Quik Column Kit, Helena Laboratories) although HbF was performed by hemoglobin electrophoresis on cellulose acetate. Patients came from different cities of Khuzestan province with different ethnics. Phenotypic analysis was performed based on some routine analysis of polar, splenomegaly, Hepatomegaly, transfusion histories, and whether the patient had undergone splenectomy. This study was also reviewed and approved by the Ethics Committee of Pasteur Institute of Iran. All methods were carried out in accordance with relevant guidelines and regulations.
Genotypic analysis
Molecular studies were conducted on genomic DNA isolated from peripheral blood cells by salting-out procedure11. For identifying α-thalassemia genotype, investigation of common mediterranean -globin gene deletions (-3.7, -4.2 -20.5 and --MED) was performed by multiplex gap polymerase chain reaction as described previously;12 the entire α and β-globin genes was amplified and DNA sequenced, ABI -3130 (Applied Biosystems, Foster City, CA, USA). In order to detect non common alpha deletions, multiplex ligation-dependent probe amplification (MLPA assay) was performed using the SALSA MLPA kit (MRC-Holland, Amsterdam, Netherlands). Then amplified fragments were separated by capillary electrophoresis, on an ABI PRISM 3130 Genetic Analyzer (Applied Biosystems, Foster city, CA, USA) and analysis was performed by gene marker software v.1.6 (Soft Genetics, State College, PA, USA).
Results
The cohort
Of the 120 patients with Hb H disease, 50 were male and 70 were female. The average age was 23.0 ± 7 years.
Hb H genotypes
Different genotype of alpha globin genes was detected among 120 patients in Khuzestan province of Iran. Of these patients, 35 (29.16%) had deletional form of Hb H disease, and 85 (70.83%) had different form of deletional/nondeletional and nondeletional Hb H diseases. The most frequently observed Hb H genotypes were --Med/–α3.7 in 33 patients (27.5%), αCD19(-G) α/αCD19(-G) α in 25 cases (20.83%), αpolyA2α/αpolyA2α in 15 (12.5%), and αpolyA1α/αpolyA1α in 13 (10.83%) respectively. The probability of receiving at least one transfusion blood in deletional form was observed in 3 of 35(8.57%) patients which just seen in 3 of 33(9%) patients with --Med/–α3.7 genotype. In deletional/nondeletional and nondeletional forms of Hb H disease blood transfusions was observed in 8 of 85 (9.4%) which included following genotypes, 2 of 2 (100%) --Med/αpolyA1α, 1 of 1 (100%) --Med/αInitiation CD (T>G)α, 2 of 3 (66.7%) --Med/αCD19(-G)α , 1 of 2 (50%) αConstant springα/αConstant springα, and 2 of 13 (15.38% ) αpolyA1α/αpolyA1α (Table 1).
Due to multiethnic nature of khuzestan province like Arabs, lor, Shoshtari, Dezfuli, and Fars, the ethnic background was assessed. The parental ethnic background was Arab in 88 patients (73.33%), Lor in 22 (18.33%), Fars in 9 (7.5%), and Shoshtari in 1 (0.83%).
Arabs in deletional and non-deletional Hb H disease mutations were the dominant ethnic group. The most frequent types of Hb H disease mutations among Arabs was Med/–α3.7 in 28 patients (23.3%), αCD19(-G) α/αCD19(-G) α in 12 cases (10%), αpolyA1α/αpolyA1α in 12 (10%), and αpolyA2α/αpolyA2α in 11 (9.16%) respectively (Table 1).
In deletional form of Hb H disease, three patients were received blood who inherited --Med/–α3.7 genotype. The first patient was a 60 year old woman who was receiving blood every month and who had a hemoglobin level (Hb) of 7.1 g per deciliter (g/dl). She underwent splenectomy because of the need for frequent blood transfusion. Second patient was a 28 year old woman who had Hb of 7.8 g/dl receiving blood for two times during pregnancy and also underwent splenectomy. Third patient was a 24 year old woman who had Hb of 7.9 g/dl receiving blood just once during pregnancy.
In non- deletional form of Hb H disease, eight patients were received blood which five of them had Med deletion in compound with alpha globin point mutations, especially during pregnancy. Interestingly, two patients with the --Med/α polyA1α genotype received once transfusion had undergone splenectomy (Table 1).
Hematological parameters were evaluated following genotypes -(20.5)/α5ntα, --Med/–α4.2, --Med/–α3.7, --Med/α poly A4 α and --Med/α poly A6α were associated with more severe anemia having low hemoglobin level (Hb), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH) respectively. The average hematological indices for each genotype are shown in Table 1.
Discussion
Hb H disease displayed by a varied clinical and hematologic phenotypic heterogeneity which is mostly seen in some regions of Southeast Asia, the Middle East and the Mediterranean countries 13. The phenotypic variability of Hb H disease is ranging from asymptomatic, to need for periodic transfusions, to severe anemia with hemolysis4.
Of the 120 patients we studied, 29.16% had the deletional genotype and 70.83% had the non-deletional genotype. As in many other genetic diseases, the incidence of Hb H disease varies in different ethnic groups. By contrast with our patients from Khuzestan province, which more ethnic background was Arab (73.33%), the most common α-globin genotype in our Hb H patients was --Med/–α3.7 (27.5%) that was similar to other reports of Iran and other populations with different ethnic backgrounds14,15,16,17,18,19. This genotype frequency followed by αCD19(-G) α/αCD19(-G) α (20.83%), αpolyA2α/αpolyA2α (12.5%), and αpolyA1α/αpolyA1α (10.83%) genotypes which is almost similar to previous reported study from Khuzestan province16. Although, this frequencies of Hb H genotypes are in contrast to Arabian Peninsula countries where the majority of Hb H disease cases are actually due to homozygosity for polyA1 mutation20 but of the 13 αpolyA1α/αpolyA1α mutations detected 12 were from Arab ethnic group.
In this study we reviewed 12 Hb H genotypes of patients for Hb (g/dl), Hb H (%), transfusion-dependent that presented in our study, Iranian population and other countries. We just selected articles that reported transfusion dependent or independent of Hb H patients. Here, we classified genotypes causing Hb H disease into three Hb H categories: deletional, non-deletional and non deletional/deletional. Of 189 deletional Hb H diseases including -Med/–α3.7, --Med/–α4.2 and –(α)20.5/–α3.7 genotypes, 9 (4.76%) were transfusion-dependent with Hb 9.6 ± 1.8 g/dl and Hb H 8.6 ± 3.5% . In the compound heterozygosity of the deletion and non-deletion genotypes or non deletional/deletional Hb H , seven genotypes as follows: --Med/αCSα, –(α)20.5/α–5ntα, –(α)20.5/αpolyA2α, --Med/αpolyA1α, --Med/αpolyA2α, --Med/α–5ntα, αCD19(-G) α/--Med were reviewed. Of 93 non deletional/deletional Hb H, 24 (25.8%) were transfusion-dependent, had Hb 8.9 ± 1.1 g/dl and Hb H 14.4 ± 6.2%. In non-deletional Hb H category with αpolyA1α/αpolyA1α, αpolyA2α/αpolyA2α and αCSα/αCSα genotypes, 283 Hb H diseases reported, among them 54 (19.8%) had been transfused, had Hb 9.0 ± 1.7 g/dl and Hb H 11.2 ± 4.9%. The most common Hb H genotypes that associated with regular transfusions were --Med/αCSα and αCD19(-G)α/--Med genotypes respectively which is related to non deletional/deletional category (Table 2). Because of large sample size of Med/ –α3.7 and αpolyA1α/αpolyA1α genotypes, we can accurately judge the association of these genotypes with blood receiving. As we showed in Table 2, of 147 Med/–α3.7 genotype, 8(5.4%), and of 257 αpolyA1α/αpolyA1α genotype, 51(19.8%) had been transfused.
It seems that the clinical phenotype of Med/–α3.7 genotype usually presents with mild or moderate thalassemia. The probable cause of blood transfusion receiving in some patients might be a consequence of circumstantial factors or other modifying factors that play a role in the proteolytic capacities of the erythroid cells21.
On the other hand, red cells hemolysis in Hb Constant Spring is maybe because of precipitation and aggregation of mRNAs that affecting the red cell membrane and producing visible basophilic stippling30.
According to this study, patients with non deletional/deletional and nondeletional Hb H disease usually are more anemic, and more likely to require transfusions suggested that Hb H disease is not as benign a disorder.
The diagnosis of Hb H disease at the molecular level is important for genetic counseling and the identification of families at risk for having pregnancies affected with Hb H disease. Regarding the need for blood transfusion in deletional and non- deletional Hb H disease, most of deletional Hb H cases were managed without blood transfusion. Nondeletional Hb H disease was more severe than deletional Hb H disease, with patients undergoing lower Hb levels and higher HB H percentage, requiring more blood transfusions and should be monitored closely. Therefore, we can recommend that Med deletion in compound with alpha-globin point mutations, polyA1 and constant spring in homozygous form needs to be taken into consideration when offering counseling to high-risk couples.
Data availability
All data generated during and/or analyzed during the current study are available upon request by contact the corresponding author.
References
Vichinsky, E. Advances in the treatment of alpha-thalassemia. Blood Rev. 26, S31–S34 (2012).
Vichinsky, E. P. Alpha thalassemia major—New mutations, intrauterine management, and outcomes. ASH. Edu. Pro. Book. 2009, 35–41 (2009).
Galanello, R. & Cao, A. Alpha-thalassemia. Genet. Med. 13, 83–88 (2011).
Chui, D. H., Fucharoen, S. & Chan, V. Hemoglobin H disease: Not necessarily a benign disorder. Blood 101, 791–800 (2003).
Piel, F. B. & Weatherall, D. J. The α-thalassemias. N. Engl. J. Med. 371, 1908–1916 (2014).
Pornprasert, S. et al. Hematological analysis in Thai samples with deletional and nondeletional HbH diseases. Lab. Med. 49, 154–159 (2018).
Kanavakis, E. et al. Phenotypic and molecular diversity of haemoglobin H disease: a Greek experience. Br. J. Haematol. 111, 915–923 (2000).
Origa, R. et al. Clinical and molecular analysis of haemoglobin H disease in Sardinia: Haematological, obstetric and cardiac aspects in patients with different genotypes. Br. J. Haematol. 136, 326–332 (2007).
Fucharoen, S. & Viprakasit, V. Hb H disease: Clinical course and disease modifiers. Hematol. Am. Soc. Hematol. Educ. Program. 2009, 26–34 (2009).
Hamid, M. et al. A novel alpha-thalassemia nonsense mutation in HBA2: C.382 A > T globin gene. Arch. Iran. Med. 17, 475–476 (2014).
Miller, S. A., Dykes, D. D. & Polesky, H. F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 16, 1215 (1988).
Bowden, D. K., Vickers, M. A. & Higgs, D. R. A PCR-based strategy to detect the common severe determinants of alpha thalassaemia. Br. J. Haematol. 81, 104–108 (1992).
Somervaille, T. Disorders of Hemoglobin: Genetics, Pathophysiology, and Clinical Management (SAGE Publications Sage, 2001).
Ebrahimkhani, S. et al. Genotype-phenotype correlation in Iranian patients with Hb H disease. Hemoglobin 35, 40–46 (2011).
Farashi, S. et al. Point mutations which should not be overlooked in Hb H disease. Expert Rev. Hematol. 9, 107–113 (2016).
Paridar, M. et al. Iranian patients with hemoglobin H disease: Genotype–phenotype correlation. Mol. Biol. Rep. 46, 5041–5048 (2019).
Baysal, E. et al. α-Thalassaemia in the population of Cyprus. Br. J. Haematol. 89, 496–499 (1995).
Shamoon, R. P., Yassin, A. K., Polus, R. K. & Ali, M. D. Genotype-phenotype correlation of HbH disease in northern Iraq. BMC Med. Genet. 21, 1–6 (2020).
Kountouris, P. et al. The molecular spectrum and distribution of haemoglobinopathies in Cyprus: A 20-year retrospective study. Sci. Rep. 6, 1–10 (2016).
Al-Riyami, A. Z. et al. α-Globin genotypes associated with Hb H disease: A report from Oman and a review of the literature from the Eastern Mediterranean Region. Hemoglobin 44, 20–26 (2020).
Lal, A. et al. Heterogeneity of hemoglobin H disease in childhood. N. Engl. J. Med. 364, 2069–2070 (2011).
El-Kalla, S. & Baysal, E. α-thalassemia in the United Arab Emirates. Acta Haematol. 100, 49–53 (1998).
Adekile, A., Sukumaran, J., Thomas, D., D’Souza, T. & Haider, M. Alpha thalassemia genotypes in Kuwait. BMC Med. Genet. 21, 1–5 (2020).
Siala, H., Ouali, F., Messaoud, T., Bibi, A. & Fattoum, S. α-Thalassaemia in Tunisia: Some epidemiological and molecular data. J. Genet. 87, 229 (2008).
Öner, C. et al. The molecular basis of Hb H disease in Turkey. Hemoglobin 21, 41–51 (1997).
Traeger-Synodinos, J., Kanavakis, E., Tzetis, M., Kattamis, A. & Kattamis, C. Characterization of nondeletion α-thalassemia mutations in the Greek population. Am. J. Hematol. 44, 162–167 (1993).
Adekile, A. D. et al. Clinical and molecular characteristics of non-transfusion-dependent thalassemia in Kuwait. Hemoglobin 39, 320–326 (2015).
Al Moamen, N. J., Thabet, A., Mahdi, F., Newton, H. & Salman, E. Various α-thalassemia genotype combinations of the Saudi-type polyadenylation signal mutation (αT-Saudiα) in the population of Bahrain: An update of genotype-phenotype analyses. Hemoglobin 42, 166–170 (2018).
Fei, Y.-J. et al. Hb H disease caused by a homozygosity for the AATAAA→ AATAAG mutation in the polyadenylation site of the α2-globin gene: Hematological observations. Acta Haematol. 88, 82–85 (1992).
Komvilaisak, P. et al. Clinical course of homozygous hemoglobin constant spring in pediatric patients. J. Pediatr. Hematol. Oncol. 40, 409–412 (2018).
Acknowledgements
We would like to thank all patients for their contributions to the study.
Funding
This study was supported by Grant Number 687 from the Pasteur Institute of Iran, Tehran, Iran and Narges Prenatal Diagnostics and Medical Genetics Laboratory.
Author information
Authors and Affiliations
Contributions
M.H. directed the project, collected data, performed analysis and wrote the manuscript. B.K., A.S., G.S., H.G., M.M. provided the samples and clinical data. All of authors reviewed and gave the final approval for the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hamid, M., keikhaei, B., Galehdari, H. et al. Genotype–phenotype correlation in patients with deletional and nondeletional mutations of Hb H disease in Southwest of Iran. Sci Rep 12, 4856 (2022). https://doi.org/10.1038/s41598-022-08986-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-08986-4
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.