Abstract
Epidemiological studies reveal a link between osteoporosis and the risk of ischemic cardiovascular disease. We illustrate an association between coronary calcification and bone microarchitecture in older adults based on the SPAH study. This cross-sectional research comprised 256 individuals subjected to cardiac coronary computed tomography angiography (CCTA) for coronary artery calcification (CAC), high-resolution peripheral quantitative computed tomography (HR-pQCT) at the tibia and radius with standardized z score parameters, and dual-energy X-ray absorptiometry (DXA) to evaluate bone status. We used Student’s t test and the Mann–Whitney and Chi-squared tests for comparison of basal measurements. Association analysis was performed using the Poisson regression model with adjustment for CAC and sex. Multivariate analysis revealed different bone variables for predicting CAC in DXA and HR-pQCT scenarios. Although most of the bone parameters are related to vascular calcification, only cortical porosity (Ct.Po) remained uniform by HR-pQCT. Results for were as follows: the tibia—women (exp β = 1.12 (95% CI 1.10–1.13, p < 0.001) and men (exp β = 1.44, 95% CI 1.42–1.46, p < 0.001); the radius—women (exp β = 1.07 (95% CI 1.07–1.08, p < 0.001) and men (exp β = 1.33 (95% CI 1.30–1.37, p < 0.001). These findings suggest an inverse relationship between CAC and cortical bone content, as assessed by HR-pQCT, with higher coronary calcification in individuals older than 65 years.
Similar content being viewed by others
Introduction
Atherosclerotic disease and osteoporosis are highly prevalent pathologies in older adults1, 2. In addition to sharing risk factors3, evidence suggests that the diseases have common pathophysiological mechanisms4–6.
Furthermore, bone loss is associated with cardiovascular disease severity at the time of diagnosis, and the degree of vascular calcification is a significant predictor of bone loss and vertebral and nonvertebral fractures7, 8.
In this scenario, coronary artery calcification (CAC), as calculated by the Agatston score9, has proven to be a powerful tool in personalized cardiovascular event risk assessment10. Its negative predictive value is reported to be 87% using a cutoff point of 10011. Additionally, the score is an independent predictor of cardiac outcomes up to 10 years, better than other combined risk factors that individualize atherosclerotic burden in a clinical form (ethnicity, age, and sex)12.
Areal bone mineral density (aBMD) assessed by dual-energy X-ray absorptiometry (DXA) is the most used exam for osteoporosis diagnosis and fracture risk assessment. It is a reproducible method with a low radiation dose. The main parameter is the T-score, which is considered normal when greater than − 1.0 standard deviation (SD); osteopenia is considered at − 1 to − 2.5 SD and osteoporosis at less than − 2.5 SD13. A reduction in aBMD has been independently associated with a higher number and greater severity of vertebral and hip fractures13, 14. Nevertheless, the Rotterdam study15 revealed a need for a more predictive tool based on results indicating that more than 60% of fractures occur in individuals considered to have a low risk. With its routine use worldwide, some authors have specified key limitations with regard to pitfalls in measuring and interpreting bone mineral density (BMD)16. For example, the need for internal rotation of the femur in 15–20% of cases favors an anteverted position, with a further 2.8% increase in BMD17. Other authors recommend avoiding Ward’s area18, 19, in which osteoporosis can be overestimated20.
High-resolution peripheral quantitative computed tomography (HR-pQCT) is a method that can address the deficiencies of DXA. It allows 3-D evaluation of trabecular and cortical bone compartments separately, as well as volumetric bone mineral density and structural parameters. In addition, HR-pQCT provides data on biomechanical characteristics through finite element software21, 22, offering better predictors of fracture risk than DXA23.
To the best of our knowledge, only one study has shown an inverse association between trabecular bone volume (measured with HR-pQCT) and CACs, though only end-stage dialysis chronic kidney disease (CKD) patients were included24. Hence, the relationship in asymptomatic individuals with subclinical pathologies according to HR-pQCT remains unclearknown using HR-pQCT. This study aimed to investigate whether there is an association between coronary artery calcification and density and BMD (obtained by HR-pQCT) in a cohort of community-dwelling older adults.
Materials and methods
Population of study
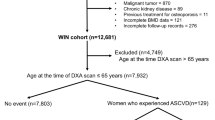
This was a cross-sectional study of 256 individuals older than 65 years. The data belonged to a third party (a cohort of community-dwelling older adults living in São Paulo city and participating in the São Paulo Ageing and Health Study [SPAH] study)25 (Fig. 1). Contact with the participants was initially achieved by phone, after which a physician explained the study aims and logistics. Recruitment was carried out from 4/30/2014 to 4/13/2015. Exclusion criteria were individuals with a history of liver failure, creatinine clearance (CrCl) < 30 mL/min/1.73 m2, hyperthyroidism, hypothyroidism, uncontrolled diabetes, malabsorption, other bone metabolic diseases (primary hyperparathyroidism, osteomalacia) based on previous laboratory results concerning bone metabolism, surgical myocardial revascularization, percutaneous coronary intervention, and chronic steroid use. The research was approved by the Ethics Committee of the University of São Paulo School of Medicine (CAPPesq # 522.369), as based on the World Medical Association’s guidance and principles, and subject to the Declaration of Helsinki, including its last amendment by the General Assembly, Fortaleza, Brazil, October 201326.
All participants agreed to and signed the written informed consent form prior to enrollment into the study, thereafter, they were asked to fill out a questionnaire, which captured personal pathological background, current medications, and health behaviors. Specially trained examiners performed anthropometric measurements. Height and weight were measured using a digital scale with a stadiometer (Mettler Toledo, mod. 2096PP, Toledo do Brasil Indústria de Balanças Ltda); all measurements were taken closest to 0.1 kg and 0.1 cm, and each participant’s weight in kilograms was divided by the square of their height in meters to calculate body mass index (BMI). Waist circumference measurement was also performed.
Systemic arterial hypertension (SHT) was defined as documented history or treatment with antihypertensive medications; diabetes mellitus (DM) was defined as physician diagnosis and/or use of insulin or oral hypoglycemic agents and dyslipidemia (DLP) as a known but untreated disease or by current use of hypolipidemic drugs. Metabolic syndrome was defined using the recommendations of the third NCEP panel report27. A positive smoking history was considered current or previous smoking. A history of acute myocardial infarction (AMI), angina, stroke, carotid disease, osteoporosis, and coffee consumption was self-reported. The degree of physical activity was classified as either low (no housework), moderate (housework, gardening, nonregular walking), or high (regular physical activity outside the usual routine, e.g., walking or dancing at least twice a week for 30 min28). Coffee consumption data collection was based on a previously validated questionnaire29: participants were asked if they consumed at least one cup daily (50 mL = 1 cup). Alcohol intake was split into four groups: current drinker, intake in the last 3–4 years, never, or in a social manner. Bone T-score (BT score) ≤ − 2.5 was considered the smallest measurement acquired by DXA for the lumbar spine, total hip, or femoral neck. New fracture was self-reported and considered if present in the last 5 years after the initial entry-study date. To calculate creatinine clearance (CrCl), we used the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation30.
Laboratory parameters
Blood samples were collected to assess the lipid profile, creatinine, glycemia, insulin, and biochemical bone metabolism profile (calcium, phosphorus, alkaline phosphatase, PTH, and 25-hydroxyvitamin D) and perform ultrasensitive PCR.
Bone remodeling markers were analyzed in 10 mL of fasting blood stored at − 80 °C. The following markers of serum bone metabolism were examined: CTX (C-terminal telopeptide type I collagen) and P1NP (aminoterminal propeptide of type I procollagen) by electrochemiluminescence (Elecsys systems, Roche Diagnostics, Mannheim, Germany). The intra-assay coefficients of variation (CVs) for CTX and P1NP were 2.5 and 2.2%, respectively.
Evaluation of coronary calcification
Image acquisition for coronary calcium evaluation was performed using a 320-detector CT system (Aquilion One ViSION, Toshiba Medical System, Japan), with cardiac synchronization using prospective triggering with a maximum temporal resolution of 125–250 ms as referenced in the literature31. The produced slice thickness of 0.5 mm was reconstructed to a 3 mm slice thickness for analysis, X-ray tube peak voltage was fixed at 120 kV, and the tube current was adjusted to the patient's body size, with a range of 250–450 mA. The adaptive iterative dose reduction 3-dimensional algorithm (AIDR 3D) was used for iterative reconstruction32, resulting in a radiation dose ranging from 2.6 to 4.0 mSv. CAC values were calculated using the Agatston score9 at a dedicated workstation (Aquarius workstation, TeraRecon, Inc, San Mateo, CA). The nonenhanced scan protocol was used to analyze the calcium score evaluation. The images were evaluated by one specialist medical doctor with at least 2 years of specific training who was unaware of the patient’s clinical and tomographic information.
Evaluation of the distal tibia and radius using HR-pQCT
The distal segment of the tibia and radius were evaluated. The measurement included 110 slices, corresponding to a 9.02 mm section along the axial direction. For the distal radius and tibia, the first slice of the scan was positioned at fixed distances from the reference line of 9.5 and 22.5 mm, respectively33. Microarchitecture was measured using a three-dimensional HR-pQCT system (Xtreme CT; Scanco Medical AG). This system allowed simultaneous acquisition of a stack of parallel slices with a resolution of 82 µm (voxel size). The following imaging parameters were used: 60 kVp effective energy, 95 mA X-ray tube current, and 1536 × 1536 matrix. Quality control was monitored through daily examinations of a phantom (Phanton) containing hydroxyapatite rods (HA) incorporated in an equivalent resin fabric (QRM, Moehrendorf, Germany). Daily device calibration was performed to obtain images with good technical quality; parameters were kept stable throughout the study period.
All measurements were obtained using the same scanner, and one biomedical specialist analyzed the data according to guidelines21. The quality of the scan image was categorized as follows: perfect (G1), showing a slight (G2); moderate (G3); or unacceptable (G4) degree of movement artifact34. As the presence of movement artifacts was unacceptable, for the distal tibia, only 11 exams were repeated due to movement during the exam (talking, coughing).
The following trabecular parameters were analyzed: Tb.vBMD, trabecular volumetric density (mg HA/cm3); Tb.N, number of trabeculae (1 mm, SD); Tb.Th, trabecular thickness (mm); Tb.Sp, trabecular separation (mm); and cortical parameters: Ct.vBMD, cortical volumetric density (mg HA/cm3); Ct.Th, mean cortical bone thickness (mm); and Ct.Po, cortical porosity.
In addition to standard morphological analysis, automatic segmentation software called “advanced cortical analysis” was used to determine cortical parameters35, 36.
The HR-pQCT precision measurement from our laboratory, expressed as the coefficient of variation, ranged from 0.25 to 1.16% for the density parameters at the tibia site37.
Areal bone mineral density
Areal bone mineral density at the lumbar spine, femoral neck, and total hip were measured using DXA (Hologic QDR 4500, Inc. Bedford, MA, USA), and the results are expressed in g/cm2. The T-score was applied as a noninvasive tool for osteoporosis diagnosis and as an addendum to the original description by the WHO criteria definition threshold30, 38. The same DXA scanner was used for all single exams, and the results were evaluated by one biomedical specialist. The least significant changes (LSCs) for BMD measurement were 0.033 g/cm2 at the lumbar spine, 0.047 g/cm2 at the femoral neck, and 0.039 g/cm2 at the total hip.
Statistical analysis
Statistical analysis was performed using STATA statistical software, version 12.0 (Stata Corp., College Station, USA). Continuous variables are expressed as medians and interquartile ranges or means ± standard deviations depending on the Kolmogorov–Smirnov test for normality. Continuous variables in the total sample were separated by sex and analyzed for differences with Student’s t test if they showed a parametric pattern or the Mann–Whitney test if they did not (as with insulin and RCP values). Linkert scales such as for alcohol consumption and degree of physical activity were also evaluated by the Mann–Whitney test. In the case of binomial variables, a Chi-squared test was performed, and Fisher’s adaptation was used as needed. The study’s response variable was the calcium score, as expressed by its integer value. This variable was grouped by sex, assumed nonnegative integer values, with an asymmetric distribution on the left. Variables related to HR-pQCT were standardized in z scores using the Formula: z = (observed value − sample mean)/sample standard deviation39. T-scores were used to evaluate bone density compared to normal values in a young adult and formed part of the regression model. In univariate analysis, variables with p < 0.20 were used in the regression model with a stepwise variable selection procedure. Analysis of association was performed using a generalized linear regression model with a Poisson probability distribution because of the nonparametric distribution of calcium content. In addition, a layered stratification approach was taken: (1) by sex; and (2) by anatomical site. Thus, there were four groups (radio men, tibia men, radio women and tibia women). All tests were two-tailed, and p values < 0.05 were considered statistically significant.
Results
Anthropometric and clinical characteristics
The baseline patient characteristics regarding anthropometric and clinical variables are shown in Table 1. The mean age was 79.5 ± 4.24 years without sex differences. Women represented the largest group (65%). In them was observed a higher percentage of non-white women compared to men. Waist circumference was similar in both groups. BMI, hypertension, and diabetes were significantly higher in women, and consequently, women had a higher prevalence of metabolic syndrome. Stroke and osteoporosis prevalence was also higher in women. In contrast, alcohol consumption was higher in men. Different grades of physical activity from low to high were similar in both groups. Although the women had more fall histories and worse BT scores, they had similar new fracture events when compared to men.
Regarding coronary calcium, median CAC values did not differ significantly between the groups (Fig. 2).
Laboratory characteristics
Lipid indices (CT, HDL, and LDL) and phosphorus were higher in women; carbohydrate metabolism, as represented by glucose and insulin, was similar for both groups. Although plasma creatinine was different between the groups, renal function, as measured by clearance, was similar for men and women (Table 2).
Medication use
Table 3 shows that thiazide diuretics were the most used medication (and more frequently in women), followed by statins and beta-blockers. Regarding osteoporosis, specific treatment was used more by women than men: almost twice as much for calcium and 25(OH)D and with a higher proportion for bisphosphonate. Unfortunately, neither the specific kind of bisphosphonate nor the dosage of vitamin D was recorded.
Bone mineral density and bone microarchitecture based on CAC
The median CAC value was 54.5 (IQR 1–290.75) for the general population, with no difference observed between women [33 (IQR 0.25–236.5) and men (80.5 (IQR 1.25–450.25)], p = 0.06 (supplementary information).
In univariate analysis, total hip aBMD in both sexes and femoral neck aBMD in men were associated with lower CAC. Inversely, lumbar spine aBMD in both sex, and femoral neck aBMD in women, were associated with higher coronary calcification. Also, we found that T-score ≤ − 2.5 SD, was associated with higher coronary calcification in women; however, the association was the opposite in men.
Regarding HR-pQCT parameters, Ct.vBMD and Ct.Po in women at the tibia was negatively and positively associated with CAC, respectively. In men, more variables were associated with higher CAC, namely, Ct.vBMD, CT.Po, Tb.N, and Ct.Th. Overall, trabecular parameters were positively associated with CAC, with a 31–47% chance for each standard deviation of the z score. For the radius, only CT.Po had a positive association with CAC in women, and the other parameters followed an opposite trend. In men, Ct.vBMD, Ct.Th, and CT.Po were positively associated with CAC (Tables 4 and 5).
Multivariate analysis was adjusted for CAC and sex, the dependent variables were DXA and HR-pQCT parameters. Surprisingly, some tibia and radius DXA/HR-pQCT results in multivariate analysis were different between the sexes (Table 6), with paradoxical results, as described below.
Tibia measurements
In women, regarding DXA results, a greater quantity of CACs was associated with BT score ≤ − 2.5 SD. Concerning HR-pQCT, CT.Po showed a positive relationship. In contrast, Ct.vBMD had an inverse relation of 21% for each unit in the standard deviation of the z score.
In men, BT score ≤ − 2.5 SD was significantly associated with lower CACs. Concerning HR-pQCT parameters, only CT.Po was associated with higher CAC, with a positive 44% chance for a unit in the calcium score, Ct.vBMD presented a coefficient opposite to what was expected compared to women.
Radius measurements
In women, BT score ≤ − 2.5 SD was also significantly associated with higher CACs. About HR-pQCT parameters, CT.Po and Ct.vBMD were associated with greater coronary calcification.
In men, vascular calcification was not related to osteoporosis based only BT score ≤ − 2.5 SD. Concerning CT.Po, it was congruent not only with the radius measurements in women but also with the tibial measurements. However, in men, Ct.vBMD showed an inverse relationship with coronary calcification (Table 6).
Discussion
In this cross-sectional study involving healthy older adults, we observed an association between CAC and HR-pQCT parameters. Our main findings suggest an inverse relationship between calcium content in the coronary arteries and bone microarchitecture.
Previous studies have revealed a relationship between CAC and bone density. Using DXA and multidetector tomography in postmenopausal women, Lee et al.40 reported an associated odds ratio of 2.79, 95% CI 1.05–6.90) for osteoporosis, with a CAC score above 100 and higher odds of multivessel disease (8.91, 95% CI 1.93–41.22), as adjusted for cardiovascular risk factors and age. Nonetheless, that study did not address intake of bone metabolism drugs.
Later, a large cohort study with an 8-year follow-up41 found increased mortality risk and CAC related to BMD. The study used the trabecular thoracic spine as a reference for BMD levels, and after stratifying CAC into four categories, the observed association was more robust in postmenopausal women than men. Interestingly, the authors also explored ethnicity and found a weaker link for Asians in the CAC 1–100 category. We were unable to investigate this link because our population was not ethnically diverse.
Cortical and trabecular bone loss exhibited different behaviors in renal patients. Costa et al.42 used vertebral tomography and observed that cortical bone loss in mid-term follow-up (24 months), unlike trabecular bone loss, was not associated with aortic calcification. One of the hypotheses to explain this lack of association was that cortical bone has lower porosity and turnover rates than trabecular bone. The study found no effect of bone-affecting medications (diuretics, calcium base phosphate binders, and calcitriol).
Another study evaluated the relationship between BMD (assessed by HR-pQCT) and ischemic heart disease (myocardial infarction and angina)43. Interestingly, lower cortical volumetric BMD in the distal radius but not in the tibia was found; the study did not estimate coronary calcium content or medications, which can modify bone turnover.
Other studies, however, showed inverse results between vessel calcification and BMD. Aoyagi et al.44 evaluated aortic calcification contrasting with different skeletal sites (calcaneus, proximal and distal radius) and failed to observe a relationship in women. Their rationale revolved around hormonal imbalances and a lack of knowledge about vitamin D intake.
To the best of our knowledge, only one study in the literature has compared these two biological parameters using highly accurate imaging methods. However, this study was performed in a specific population with chronic kidney disease on dialysis, in whom lower trabecular and cortical parameters were associated with higher coronary calcification24.
Our results showed that higher CT.Po in both sexes and lower Ct.vBMD in women were associated with higher CACs. Furthermore, BT score ≤ − 2.5 SD, as evaluated by DXA, was associated with higher CACs in women.
Regarding HR-pQCT analysis, changes in cortical bone parameters were more greatly influenced by age and sex45, 46. Mc Donald et al.47 showed a decline in Ct. BMD with age in both women and men (14% and 17%, respectively), and the increase in CT.Po was three times higher in women than in men. Whitmarsh et al.48 observed a significant decrease in Tb. BMD (38.1%) and Ct.Th (13%) using micro-CT on femoral head specimens from women undergoing hemiarthroplasty from the 9th to the 10th decade of life. The difference between the sexes likely involves the influence of testosterone supporting periosteal bone expansion and estrogen preventing bone loss49. This might explain why our findings were more evident in women.
Moreover, there is evidence of a different response to exogenous and endogenous factors, in addition to the metabolic and structural differences between cortical and trabecular tissue50. A study analyzing HR-pQCT reference curves in 450 healthy women aged 20–85 years observed an age-related increase in CT.Po in women, though trabecular parameters remained relatively stable35.
A hypothesis that might explain this antagonistic pattern is the existence of an intracortical remodeling transition area that increases porosity by the process of trabecularization of the internal cortex, which may cause underestimation in the measurement of cortical bone and overestimation in that of trabecular tissue51. This phenomenon is subject to ex vivo analysis using computed microtomography (μCT), HR-pQCT images from cadaveric specimens, or electron microscopy52. A study of postmortem femur specimens obtained from 24 white women aged 29–99 years (mean age 69 years) suggested that cortical thinning occurs owing to the coalescence of cortical pores rather than endocortical expansion53.
This behavior is likely amplified by interaction of the similar pathophysiological processes of the two illnesses. Because of the high accuracy of both methods in diagnosis and risk stratification, it may be relevant to continue investigating this association to obtain new prevention and treatment strategies that involve both pathologies, especially for females.
Furthermore, our results reinforce knowledge of the relationship between VC and decreased bone remodeling and have important clinical implications in the design of more specific diagnostic and treatment strategies for both pathologies.
We recognize the limitations of our study, as a cross-sectional design does not allow for inference of cause and effect in the association. The sample size might be considered small regarding the initial referential study, which confers less statistical power. The small number of participants in the group of men may explain those results, and the differences in bone-health medications were lower in this group.
In conclusion, we observed that cortical bone impairment at the tibia and radius, as assessed by HR-pQCT, is associated with increased coronary calcification in older people from the São Paulo Ageing and Health Study cohort. Otherwise, bone mineral density by DXA showed the opposite trend when related to coronary calcification depending on sex.
References
Farhat, G. N. et al. Volumetric and areal bone mineral density measures are associated with cardiovascular disease in older men and women: The health, aging and body composition study. Calcif. Tissue Int. 79(2), 102–111. https://doi.org/10.1007/s00223-006-0052-0 (2006).
Wade, S. W., Strader, C., Fitzpatrick, L. A., Anthony, M. S. & O’Malley, C. D. Estimating prevalence of osteoporosis: Examples from industrialized countries. Arch. Osteoporos. 9, 182. https://doi.org/10.1007/s11657-014-0182-3 (2014).
Crepaldi, G. & Maggi, S. Epidemiologic link between osteoporosis and cardiovascular disease. J. Endocrinol. Investig. 32, 2–5 (2009).
Anagnostis, P. et al. Atherosclerosis and osteoporosis: Age-dependent degenerative processes or related entities?. Osteoporos. Int. 20, 197–207. https://doi.org/10.1007/s00198-008-0648-5 (2009).
Reiss, A. B. et al. CKD, arterial calcification, atherosclerosis and bone health: Inter-relationships and controversies. Atherosclerosis 278, 49–59. https://doi.org/10.1016/j.atherosclerosis.2018.08.046 (2018).
Escobar Guzman, L. F., Escobar Guzman, C. A. & Lopes, N. H. M. Pathophysiological and genetic aspects of vascular calcification. Cardiol. Res. Pract. 2020, 5169069. https://doi.org/10.1155/2020/5169069 (2020).
Tankó, L. B. et al. Relationship between osteoporosis and cardiovascular disease in postmenopausal women. J. Bone Miner Res. 20, 1912–1920. https://doi.org/10.1359/JBMR.050711 (2005).
Zhou, R., Zhou, H., Cui, M., Chen, L. & Xu, J. The association between aortic calcification and fracture risk in postmenopausal women in China: The prospective Chongqing osteoporosis study. PLoS One 9, 1–6. https://doi.org/10.1371/journal.pone.0093882 (2014).
Agatston, A. S. et al. Quantification of coronary artery calcium using ultrafast computed tomography. J. Am. Coll. Cardiol. 15(4), 827–832. https://doi.org/10.1016/0735-1097(90)90282-t (1990).
Kapoor, K., Cainzos-Achirica, M. & Nasir, K. The evolving role of coronary artery calcium in preventive cardiology 30 years after the Agatston score. Curr. Opin. Cardiol. 35(5), 500–507. https://doi.org/10.1097/HCO.0000000000000771 (2020).
Hanifehpour, R., Motevalli, M., Ghanaati, H., Shahriari, M. & Aliyari Ghasabeh, M. Diagnostic accuracy of coronary calcium score less than 100 in excluding coronary artery disease. Iran. J. Radiol. 13(2), e16705. https://doi.org/10.5812/iranjradiol.16705 (2016).
Budoff, M. J. et al. Coronary calcium predicts events better with absolute calcium scores than age-sex-race/ethnicity percentiles. MESA (Multi-Ethnic Study of Atherosclerosis). J. Am. Coll. Cardiol. 53, 345–352. https://doi.org/10.1016/j.jacc.2008.07.072 (2009).
Lorente-Ramos, R. et al. Dual-energy X-ray absorptiometry in the diagnosis of osteoporosis: A practical guide. Am. J. Roentgenol. 196, 897–904 (2011).
Bouxsein, M. L. et al. Change in bone density and reduction in fracture risk: A meta-regression of published trials. J. Bone Miner. Res. 34, 632–642 (2019).
Schuit, S. C. et al. Fracture incidence and association with bone mineral density in elderly men and women: The Rotterdam Study. Bone 34(1), 195–202 (2004).
Garg, M. K. & Kharb, S. Dual energy X-ray absorptiometry: Pitfalls in measurement and interpretation of bone mineral density. Indian J. Endocrinol. Metab. 17(2), 203–210 (2013).
Cheng, X. G. et al. Effects of anteversion on femoral bone mineral density and geometry measured by dual energy X-ray absorptiometry: A cadaver study. Bone 21, 113–117 (1997).
Watts, N. B. et al. Osteoporosis in men: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 97(6), 1802–1822 (2012).
Doroudinia, A. & Colletti, P. M. Bone mineral measurements. Clin. Nucl. Med. 40(8), 647–657 (2015).
Fogelman, I. & Blake, G. M. Different approaches to bone densitometry. J. Nucl. Med. 41(12), 2015–2025 (2000).
Fuller, H., Fuller, R. & Pereira, R. M. R. High resolution peripheral quantitative computed tomography for the assessment of morphological and mechanical bone parameters. Rev. Bras. Reumatol. Rev. Bras. Reumatol. 55(4), 352–362 (2015).
Macneil, J. A. & Boyd, S. K. Improved reproducibility of high-resolution peripheral quantitative computed tomography for measurement of bone quality. Med. Eng. Phys. 29, 1096–1105 (2007).
Biver, E. et al. Evaluation of radius microstructure and areal bone mineral density improves fracture prediction in postmenopausal women. J. Bone Miner. Res. 33, 328–337 (2018).
Cejka, D. et al. Inverse association between bone microarchitecture assessed by HR-pQCT and coronary artery calcification in patients with end-stage renal disease. Bone 64, 33–38 (2014).
Domiciano, D. S. et al. Incidence and risk factors for osteoporotic vertebral fracture in low-income community-dwelling elderly: A population-based prospective cohort study in Brazil. The Sao Paulo Ageing & Health (SPAH) Study. Osteoporos. Int. 25(12), 2805–2815 (2014).
World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 310(20), 2191–2194 (2013).
National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 106(25), 3143–3421 (2002).
Fitti, J. E. & Kovar, M. The supplement on aging to the 1984 National Health Interview Survey. Vital Health Stat. 1, 1e115 (1987).
Molina, M. C. et al. Reproducibility and relative validity of the Food Frequency Questionnaire used in the ELSA-Brasil. Cad Saude Publica 29(2), 379–389 (2013).
Levey, A. S. et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 150, 604–612 (2009).
Mühlenbruch, G. et al. The accuracy of 1- and 3-mm slices in coronary calcium scoring using multi-slice CT in vitro and in vivo. Eur. Radiol. 17, 321–329. https://doi.org/10.1007/s00330-006-0332-0 (2007).
Blobel, J., Mews, J., Schuijf, J. D. & Overlaet, W. Determining the radiation dose reduction potential for coronary calcium scanning with computed tomography: An anthropomorphic phantom study comparing filtered backprojection and the adaptive iterative dose reduction algorithm for image reconstruction. Investig. Radiol. 48(12), 857–862. https://doi.org/10.1097/RLI.0b013e31829e3932 (2013).
Boutroy, S., Bouxsein, M. L., Munoz, F. & Delmas, P. D. In vivo assessment of trabecular bone microarchitecture by high-resolution peripheral quantitative computed tomography. J. Clin. Endocrinol. Metab. 90, 6508–6515 (2005).
Paggiosi, M. A., Eastell, R. & Walsh, J. S. Precision of high-resolution peripheral quantitative computed tomography measurement variables: Influence of gender, examination site, and age. Calcif. Tissue Int. 94(2), 191–201. https://doi.org/10.1007/s00223-013-9798-3 (2014).
Alvarenga, J. C., Fuller, H., Pasoto, S. G. & Pereira, R. M. Age-related reference curves of volumetric bone density, structure, and biomechanical parameters adjusted for weight and height in a population of healthy women: An HR-pQCT study. Osteoporos. Int. 28, 1335. https://doi.org/10.1007/s00198-016-3876-0 (2017).
Torres, G. H. F., Guzman, L. F. E., Alvarenga, J. C., Lopes, N. H. M. & Pereira, R. M. R. Association of moderate/severe vertebral fractures with reduced trabecular volumetric bone density in older women and reduced areal femoral neck bone density in older men from the community: A cross-sectional study (SPAH). Maturitas 120, 61–67. https://doi.org/10.1016/j.maturitas.2018.11.019 (2019).
Paupitz, J. A. et al. Bone impairment assessed by HR-pQCT in juvenile-onset systemic lupus erythematosus. Osteoporos. Int. 27(5), 1839–1848. https://doi.org/10.1007/s00198-015-3461-y (2016).
Dimai, H. P. Use of dual-energy X-ray absorptiometry (DXA) for diagnosis and fracture risk assessment; WHO-criteria, t- and z-score, and reference databases. Bone 104, 39–43. https://doi.org/10.1016/j.bone.2016.12.016 (2017).
Wang, Y. & Chen, H. J. Use of percentiles and Z-scores in anthropometry. In Handbook of Anthropometry (ed. Preedy, V.) 28–48 (Springer, 2012). https://doi.org/10.1007/978-1-4419-1788-1_2.
Lee, S. N., Cho, J. Y., Eun, Y. M., Song, S. W. & Moon, K. W. Associations between osteoporosis and coronary artery disease in postmenopausal women. Climacteric 19, 458–462. https://doi.org/10.1080/13697137.2016.1200550 (2016).
Ahmadi, N. et al. The relation of low levels of bone mineral density with coronary artery calcium and mortality. Osteoporos. Int. 29, 1609–1616. https://doi.org/10.1007/s00198-018-4524-7 (2018).
Costa, L. R., Carvalho, A. B., Bittencourt, A. L., Rochitte, C. E. & Canziani, M. E. F. Cortical unlike trabecular bone loss is not associated with vascular calcification progression in CKD patients. BMC Nephrol. 21, 121. https://doi.org/10.1186/s12882-020-01756-2 (2020).
Paccou, J. et al. Ischemic heart disease is associated with lower cortical volumetric bone mineral density of distal radius. Osteoporos. Int. 26, 1893–1901. https://doi.org/10.1007/s00198-015-3132-z (2015).
Aoyagi, K. et al. Low bone density is not associated with aortic calcification. Calcif. Tissue Int. 69, 20–24. https://doi.org/10.1007/s002230020003 (2001).
Vilayphiou, N., Boutroy, S., Sornay-Rendu, E., Van Rietbergen, B. & Chapurlat, R. Age-related changes in bone strength from HR-pQCT derived microarchitectural parameters with an emphasis on the role of cortical porosity. Bone 83, 233–240. https://doi.org/10.1016/j.bone.2015.10.012 (2016).
Fink, H. A. et al. Association of high-resolution peripheral quantitative computed tomography (HR-pQCT) bone microarchitectural parameters with previous clinical fracture in older men: The osteoporotic fractures in men (MrOS) study. Bone 113, 49–56. https://doi.org/10.1016/j.bone.2018.05.005 (2018).
MacDonald, H. M., Nishiyama, K. K., Kang, J., Hanley, D. A. & Boyd, S. K. Age-related patterns of trabecular and cortical bone loss differ between sexes and skeletal sites: A population-based HR-pQCT study. J. Bone Miner. Res. 26, 50–62. https://doi.org/10.1002/jbmr.171 (2011).
Whitmarsh, T. et al. A cross-sectional study on the age-related cortical and trabecular bone changes at the femoral head in elderly female hip fracture patients. Sci. Rep. 9, 1–8. https://doi.org/10.1038/s41598-018-36299-y (2019).
Ott, S. M. Cortical or trabecular bone: What’s the difference?. Am. J. Nephrol. 47, 373–375. https://doi.org/10.1159/000489672 (2018).
Nickolas, T. L. et al. Rapid cortical bone loss in patients with chronic kidney disease. J. Bone Miner. Res. 28, 1811–1820. https://doi.org/10.1002/jbmr.1916 (2013).
Zebaze, R. & Seeman, E. Cortical bone: A challenging geography. J. Bone Miner. Res. 30, 24–29. https://doi.org/10.1002/jbmr.2419 (2015).
Zebaze, R., Ghasem-Zadeh, A., Mbala, A. & Seeman, E. A new method of segmentation of compact-appearing, transitional and trabecular compartments and quantification of cortical porosity from high resolution peripheral quantitative computed tomographic images. Bone 54(1), 8–20. https://doi.org/10.1016/j.bone.2013.01.007 (2013).
Zebaze, R. M. et al. Intracortical remodelling and porosity in the distal radius and post-mortem femurs of women: A cross-sectional study. Lancet 375(9727), 1729–1736. https://doi.org/10.1016/S0140-6736(10)60320-0 (2010).
Funding
The SPAH project was supported by grants from the National Council of Science and Technology (CNPQ) 2013/457590-2013-0.
Author information
Authors and Affiliations
Contributions
L.F.E.G. contributed to the study design, execution of the study, data collection, analysis and interpretation and writing of the manuscript. N.H.M.L. contributed to the study design, execution of the study, analysis and interpretation of data, and writing of the manuscript. G.H.F.T. contributed to the execution of the study and the data collection and interpretation. L.T. contributed to the data collection and analysis. S.S.A. contributed to the study design, execution of the study, analysis, interpretation of data, and writing of the manuscript. J.R.L.-L. contributed to the data analysis, interpretation of the data and writing of the manuscript. R.M.R.P. contributed to the study design, execution of the study, analysis, interpretation of data, and writing of the manuscript. C.E.R. contributed to the analysis and interpretation of the data and writing of the manuscript. All authors helped revise the manuscript and have seen and approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Guzman, L.F.E., Lopes, N.H.M., Torres, G.H.F. et al. Coronary calcification and bone microarchitecture by high-resolution peripheral quantitative computed tomography from the São Paulo Ageing and Health (SPAH) Study. Sci Rep 12, 5282 (2022). https://doi.org/10.1038/s41598-022-08839-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-08839-0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.