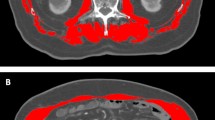
Abstract
While sarcopenia is associated with poor overall survival and cancer-specific survival in solid cancer patients, the impact of sarcopenia on clinicopathologic features that can influence conventional papillary thyroid cancer (PTC) prognosis remains unclear. To investigate the impact of sarcopenia on aggressive clinicopathologic features in PTC patients, prospectively collected data on 305 patients who underwent surgery for PTC with preoperative staging ultrasonography and bioelectrical impedance analysis were retrospectively analyzed. Nine sarcopenia patients with preoperative sarcopenia showed more patients aged 55 or older (p = 0.022), higher male proportion (p < 0.001), lower body-mass index (p = 0.015), higher incidence of major organ or vessel invasion (p = 0.001), higher T stage (p = 0.002), higher TNM stage (p = 0.007), and more tumor recurrence (p = 0.023) compared to the non-sarcopenia patients. Unadjusted and adjusted logistic regression analyses showed that sarcopenia (odds ratio (OR) 9.936, 95% confidence interval (CI) 2.052–48.111, p = 0.004), tumor size (OR 1.048, 95% CI 1.005–1.093, p = 0.027), and tumor multiplicity (OR 3.323, 95% CI 1.048–10.534, p = 0.041) significantly increased the risk of T4 cancer. Sarcopenia patients showed significantly lower disease-free survival probability compared to non-sarcopenia patients. Therefore, preoperative sarcopenia in PTC patients should raise clinical suspicion for a more locally advanced disease and direct appropriate management and careful follow-up.
Similar content being viewed by others
Introduction
Sarcopenia is defined as the age-related loss of skeletal muscle mass and function, and is associated with metabolic, physiologic, and functional impairments1,2. Clinically, sarcopenia is identified by low muscle strength as well as low muscle quantity and quality2. Such changes in body composition can impact an extensive variety of disease processes, such as cardiac disease, respiratory disease, as well as some malignancies3,4,5,6.
In oncology, severe sarcopenia may occur in cancer patients with profound weight loss, particularly of the skeletal muscle7,8. A high prevalence of sarcopenia has been reported in gastric cancer (57%), advanced hepatocellular carcinoma (HCC) (27.5%), and metastatic renal cell carcinoma (RCC) (29%)9,10,11. Sarcopenia has also been associated with poor overall survival and cancer-specific survival in breast cancer, HCC, RCC, and gastrointestinal tract cancer. In addition, sarcopenia has also been associated with increased chemotherapy toxicity and post-operative complications in various solid cancers4,12,13,14. However, sarcopenia has not been associated with poor survival in soft tissue sarcoma14, suggesting that these associations may vary according to the specific cancer type.
In thyroid cancers, only data on post-operative or post-treatment sarcopenia on patient prognosis have been reported. The DECISION trial showed a significant association between sorafenib and reduced muscle mass in advanced differentiated thyroid cancer15. In addition, a recent study showed that low thyroid stimulating hormone (TSH) levels after total thyroidectomy in PTC patients was associated with low grip strength, a parameter for assessing sarcopenia16. However, past studies have not explained whether preoperative sarcopenia can act as an aggressive clinicopathologic feature that may potentially affect the prognosis of conventional papillary thyroid cancer (PTC).
Therefore, this study aimed to investigate the potential implications of preoperative sarcopenia on aggressive clinicopathologic features in PTC patients.
Results
Baseline characteristics
Baseline clinicopathologic characteristics of the 305 patients with conventional PTC are summarized in Table 1. The median age was 43.0 years (interquartile range (IQR), 33.0–53.0 years) with 231 women (median age 43.0 years [IQR, 33.0–53.0 years]) and 74 men (median age, 41.0 years [IQR, 32.0–54.3 years])). Based on the bioelectrical impedance analysis (BIA) measurements of skeletal muscle index (SMI), nine patients (3.0%) were diagnosed with sarcopenia preoperatively. There were 234 patients (76.6%) with no or minimal extrathyroidal extension (ETE), 55 patients (18.0%) with gross strap muscle invasion, and 16 patients (5.3%) with major organ invasion involving the trachea, esophagus, recurrent laryngeal nerve, or major vessels. There were no cases of distant metastasis. The overall mean and standard deviation of body mass index (BMI) was 23.5 ± 3.4 kg/m2 [(range, 16.4–33.9 kg/m2) and there were 92 obese patients (30.2%). The median primary tumor size was 14.0 mm (IQR, 12.0–19.0 mm), ranging from 11.0 mm to 130.0 mm. The median disease-free survival was 63.4 months (IQR, 59.5–71.3 months), ranging from 3.1 months to 84.6 months. Tumor recurrence occurred in a total of five patients (1.65%).
Clinicopathologic features of PTC in the sarcopenia group
Compared to the non-sarcopenia group, the sarcopenia group showed more patients older than 55 years of age (p = 0.022), higher male proportion (p < 0.001), lower BMI (p = 0.015), and more tumor recurrence (p = 0.023) (Table 2). The sarcopenia group showed more frequent major organ or vessel invasion (p = 0.001), high T stage (p = 0.002), and high overall TNM stage (p = 0.007) than the non-sarcopenia group. There were no significant differences in LN metastasis between the two groups. There was no significant difference in median disease-free survival between the two groups (non-sarcopenia 63.3 months versus sarcopenia 68.1 months, p = 0.425). The disease-free survival probability at 6th year was significantly lower in the sarcopenia group compared to the non-sarcopenia group (0.833 versus 0.972, p = 0.017) (Fig. 1). The four tumor recurrences from the non-sarcopenia group occurred at a median of 70.5 months, and the recurrence in the sarcopenia group occurred at 65.5 months.
Unadjusted and adjusted logistic regression models between patient body composition and aggressive tumor features
Unadjusted logistic regression analysis between sarcopenia and various clinicopathologic features revealed that sarcopenia was significantly associated with major organ or vessel invasion (T4) (odds ratio (OR) 10.885, 95% confidence interval (CI) 2.445–48.453, p = 0.002) and high TNM stage (OR 12.208, 95% CI 1.142–130.552, p = 0.038) (Table 3). Sarcopenia was not significantly associated with high T stage (T3 or T4), lymph node (LN) metastasis, or tumor recurrence (Table 3).
On the unadjusted logistic regression analysis for clinicopathologic features and major organ invasion, sarcopenia, tumor size, tumor multiplicity, and LN metastasis showed significant associations with major organ or vessel invasion (T4) (Table 4). On adjusted logistic regression including these variables, patients with sarcopenia (OR 9.936, 95% CI 2.052–48.111, p = 0.004), larger tumor size (OR 1.048, 95% CI 1.005–1.093, p = 0.027), and tumor multiplicity (OR 3.323, 95% CI 1.048–10.534, p = 0.041) showed significantly higher risk of major organ or vessel invasion (T4).
Unadjusted logistic regression analysis for clinicopathologic features and high TNM stage showed that age (OR 1.157, 95% CI 1.032–1.299, p = 0.013) and sarcopenia (OR 12.208, 95% CI 1.142–130.552, p = 0.038) were significantly associated with high TNM stage. However, adjusted regression analysis revealed that only older age (OR 1.148, 95% CI 1.018–1.294, p = 0.024) and not sarcopenia (OR 6.099, 95% CI 0.478–77.874, p = 0.164) was a significant risk factor for high TNM stage (Supplementary Information 1).
Discussion
In this study, sarcopenia was a significant risk factor for major organ or vessel invasion (or T4 cancer) in PTC, and consequently a significant risk factor for more locally advanced disease. Preoperative sarcopenia significantly decreased the disease-free survival probability in PTC.
Although significant associations between sarcopenia and adverse cancer outcomes have been suggested4, published literature on the link between sarcopenia and PTC have not been clearly elucidated. Available previous studies suggest a major role of pro-inflammatory and anti-inflammatory cytokines in sarcopenia which may also influence the aggressiveness of PTC17. Serum interleukin 6 (IL-6), which is increased in cancer-caused sarcopenia, is found in high levels in PTC patients and showed a significant positive correlation with larger tumor size and ETE18. In addition, IL-6 mRNA was a significant prognostic factor for overall survival but not disease-free survival in PTC patients18. In addition, another study suggested that some PTC cells may be able to escape the suppression of cellular proliferation and invasiveness of IL-1β, a cytokine which is increased in sarcopenia19. This suggests that escaping the antitumor effect of IL-1β may be a step towards anaplastic change that could result in a more aggressive thyroid cancer19,20. These findings suggest an immunologic basis for sarcopenia and PTC aggressiveness20.
Preoperative sarcopenia significantly lowered disease-free survival probability, but it did not significantly increase the risk of tumor recurrence. This apparent discrepancy may indicate that sarcopenia is a risk factor for earlier tumor recurrence rather than the risk of tumor recurrence itself. Indeed, the median disease-free survival of tumor recurrence in the sarcopenia patient occurred earlier than the that of the non-sarcopenia patients, but without statistical significance due to the low number of tumor recurrences. This inference may be further supported by the fact that the prognostic impact of gross strap muscle invasion in PTC has been challenged in recent years21,22,23,24. Despite the AJCC 8th TNM staging criteria, these findings may be attributed to advancements in surgical techniques as well as complete en bloc resection, even in cases with advanced ETE25,26,27. These findings may indicate that at-risk PTC patients for tumor recurrence diagnosed with preoperative sarcopenia may require a more continuous post-operative follow-up to detect earlier tumor recurrence.
Preoperative sarcopenia was not significantly associated with LN metastasis, which may be the reason for the lack of significant association between high TNM stage on adjusted logistic regression analysis, despite a significant association on unadjusted analysis. In addition, the association between sarcopenia and LN metastasis seems to vary according to the type of cancer28,29,30,31. Sarcopenia has been shown to increase the risk of LN metastasis in colorectal cancer and advanced urothelial carcinoma, but not in resectable bile duct cancer or recurrent pancreas adenocarcinoma28,29,30,31. Our study suggests that sarcopenia is not significantly associated with the risk of LN metastasis in PTC.
In our study, body composition analysis and identification of preoperative sarcopenia was done using the BIA, an affordable, widely available, and portable instrument with reproducible results2,32. Unlike other malignancies in which abdomen and pelvic CT scans are often included during routine staging work-up, measuring SMI on CT scans to assess sarcopenia in thyroid cancer may unnecessarily burden patients with more radiation exposure. Alternatively, BIA may be an easier, more affordable tool to diagnose sarcopenia in PTC, as demonstrated in our study. In addition, the assessment of sarcopenia may be of importance in treatment decisions for advanced or refractory thyroid cancer because sarcopenia is considered a contraindication or discouraging factor in tyrosine kinase inhibitor treatment15,33.
There are several limitations to this study. First, an inherent bias owing to the retrospective analysis of prospectively obtained data was inevitable. Second, the very low incidence of not only preoperative sarcopenia, but also of tumor recurrence in our study is a critical limitation. In addition, the impact of sarcopenia on the overall survival of PTC patients could not be evaluated due to no deaths during follow-up of our study population. Lastly, body composition analysis by BIA may yield inconsistent or discrepant results depending on different instrument brands, as well as different population characteristics2. Therefore, we utilized cutoff values previously obtained with an identical equipment in young, healthy Korean subjects34. However, the findings of this study may be limited to Korean patients. Further research that includes patients from more diverse populations may help confirm the impact of sarcopenia on the outcomes and clinicopathologic features of PTC patients.
In conclusion, preoperative sarcopenia is significantly associated with a higher risk of major organ invasion in conventional PTC, which significantly decreased disease-free survival probability. Preoperative diagnosis of sarcopenia in PTC patients should raise clinical, radiological, and surgical suspicion for a more locally advanced disease and direct appropriate management and follow-up.
Methods
This prospective study was approved by the Severance Hospital Institutional Review Board, and was conducted in accordance with the Declaration of Helsinki as revised in 2013. Informed consent was obtained from all patients enrolled in this study.
Study population
From February 2014 to October 2015, a total of 2,717 patients aged 19 years or older underwent preoperative staging ultrasonography (US) at our institution. Patients who did not undergo preoperative BIA (n = 1,284) or thyroid surgery (n = 283) were excluded. Patients with benign pathology (including diffuse hyperplasia, adenomatous hyperplasia, follicular adenoma, Hurthle cell adenoma, Hashimoto’s thyroiditis, lymphocytic thyroiditis, Grave’s disease, hyalinizing trabecular tumor and branchial cleft cyst anomaly type II) (n = 98), PTC variants (follicular variant, oncocytic variant, diffuse sclerosing variant, and solid variant PTC) (n = 68), follicular carcinoma (n = 4), medullary carcinoma (n = 1), neuroendocrine tumor (n = 1), metastatic adenocarcinoma (n = 2), as well as papillary microcarcinoma (n = 671) were excluded. A total of 305 patients with a pathologically confirmed diagnosis of conventional PTC were eligible for final analysis (Fig. 2). Disease-free survival was defined as the time interval from the date of diagnosis to the last follow-up visit or the date of tumor recurrence.
Patient selection diagram. A total of 2,717 patients were enrolled. Patients who did not undergo preoperative bioelectrical impedance analysis (BIA) or thyroid surgery were excluded. Only pathologic diagnosis of conventional PTC were included, resulting in a total of 305 patients eligible for final analysis.
Preoperative staging ultrasonography (US)
Preoperative staging US was performed by one of 17 radiologists (12 fellows with 1 or 2 years of experience and 5 faculties with 6 to 18 years of experience in thyroid US) using a 5–12 MHz linear transducer (iU22, Philips Medical Systems, Bothell, WA). Primary tumor and cervical LNs were evaluated and reviewed based on the 8th AJCC TNM classification35. Any LN with at least one suspicious US feature (focal or diffuse hyperechogenicity, presence of internal calcification, cystic change, round shape, and chaotic or peripheral vascularity on Doppler US) was considered pathologic and was subsequently confirmed by either US-FNA or surgery.
Bioelectrical impedance analysis (BIA)
All included patients underwent preoperative BIA. Multifrequency bioimpedance data was measured using In-Body 720 (Biospace, Seoul, South Korea) in a standardized manner for all patients who underwent thyroid surgery. In-Body 720 employs a direct segmental multi-frequency bioelectrical impedance (MF-BIA) analysis method using a tetrapolar 8-point tactile electrode system with 30 impedance measurements taken at 6 frequencies (1 kHz, 5 kHz, 50 kHz, 250 kHz, 500 kHz, 1000 kHz) and reactance evaluated by 15 impedance measurements at 3 frequencies (5 kHz, 50 kHz, 250 kHz) for each of the 5 body segments (right arm, left arm, trunk, right leg, and left leg). All measurements were carried out prior to surgery in all patients. Body fat mass, body fat percentage, muscle mass, skeletal muscle mass, visceral fat surface, waist-hip ratio, and basal metabolic rate were measured to assess body composition. Furthermore, the outer circumference and fat thickness of various body compartments (neck, chest, abdomen, hip, arm, and thigh) were measured. Finally, the SMI (skeletal muscle mass/height2) and BMI of each patient was analyzed using the aforementioned parameters. Sarcopenia was diagnosed according to sex-specific cutoff values (two standard deviations below the mean) for SMI that were suggested in a previous study of a healthy Korean population34. Obesity was categorized according to the BMI as follows: underweight (< 18.5 kg/m2), normal weight (18.5–22.9 kg/m2), overweight (23.0–24.9 kg/m2), obesity (25.0–29.9 kg/m2) and severe obesity (≥ 30 kg/m2) according to the World Health Organization guidelines for Asians36.
Thyroid surgery and pathologic diagnosis
All total thyroidectomy at our institution routinely included bilateral central compartment LN dissection (CCND), which included the paratracheal, pretracheal, and prelaryngeal LNs. All hemithyroidectomy surgeries routinely included unilateral CCND. Selective lateral compartment LN dissection was performed only when lateral LN metastasis was suspected on either preoperative US and US-FNA, or during intraoperative observations. The lateral compartment included level II, III, IV, and V LNs. Pathologic tumor size, tumor multiplicity, ETE, and the presence of central and/or lateral LN metastasis on final surgical pathology reports were reviewed for analysis. TNM staging was performed according to the AJCC 8th staging criteria35. T staging was done as follows: (1) T1, tumor size smaller than 20 mm in greatest dimension limited to the thyroid; (2) T2, tumor size larger than 20 mm but equal to or smaller than 40 mm in greatest dimension limited to the thyroid; (3) T3a, tumor size larger than 40 mm but limited to the thyroid; (4) T3b, tumor of any size with gross strap muscle invasion; (5) T4, tumor of any size with invasion of major neck structures such as the larynx, trachea, esophagus, recurrent laryngeal nerve, prevertebral fascia or encasing major vessels. N staging was done as follow: (1) N0, no evidence of regional LN metastasis; (2) N1a, metastasis to level VI or VII (pretracheal, paratracheal, or prelaryngeal/Delphian, or upper mediastinal); (3) N1b, metastasis to unilateral, bilateral, or contralateral lateral neck LNs (levels I, II, III, IV, or V) or retropharyngeal LNs. T3 and T4 were considered high T stages, and the overall TNM stages III and IV were considered as high TNM stages.
Statistical analysis
All statistical analyses were performed with commercial software (IBM SPSS Statistics, version 25.0; IBM Corp., Armonk, NY). The independent t-test or Mann–Whitney U test was performed to compare clinical and pathological variables for parametric and non-parametric continuous variables, respectively. Categorical variables were compared using the chi square test or Fisher’s exact test. Logistic regression analyses were performed to estimate unadjusted ORs with 95% CIs to elucidate the association between sarcopenia and aggressive tumor features (such as tumor multiplicity, ETE, LN metastasis, T stage, TNM stage, and tumor recurrence). Unadjusted logistic regression analysis was performed to explore the potential implications of sarcopenia on those aggressive tumor features. For variables that were significantly associated with sarcopenia on unadjusted analysis, a subsequent adjusted logistic regression analysis was performed that included the other clinicopathologic variables to obtain ORs and 95% CIs. The Kaplan–Meier analysis with log-rank test was performed to compare the disease-free survival between the sarcopenia group and non-sarcopenia group. A p-value of less than 0.05 was considered statistically significant.
References
Baumgartner, R. N. et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am. J. Epidemiol. 147, 755–763 (1998).
Cruz-Jentoft, A. J. et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 48, 16–31 (2019).
Waters, D. L. & Baumgartner, R. N. Sarcopenia and obesity. Clin. Geriatr. Med. 27, 401–421 (2011).
Shachar, S. S., Williams, G. R., Muss, H. B. & Nishijima, T. F. Prognostic value of sarcopenia in adults with solid tumours: A meta-analysis and systematic review. Eur. J. Cancer 57, 58–67 (2016).
Bahat, G. & Ilhan, B. Sarcopenia and the cardiometabolic syndrome: A narrative review. Eur. Geriatr. Med. 7, 220–223 (2016).
Bone, A. E., Hepgul, N., Kon, S. & Maddocks, M. Sarcopenia and frailty in chronic respiratory disease: Lessons from gerontology. Chron. Respir. Dis. 14, 85–99 (2017).
Irwin, M. L. et al. Changes in body fat and weight after a breast cancer diagnosis: Influence of demographic, prognostic and lifestyle factors. J. Clin. Oncol. 23, 774 (2005).
Tan, B. H., Birdsell, L. A., Martin, L., Baracos, V. E. & Fearon, K. C. Sarcopenia in an overweight or obese patient is an adverse prognostic factor in pancreatic cancer. Clin. Cancer Res., 1078-0432. CCR-1009-1525 (2009).
Mir, O. et al. Sarcopenia predicts early dose-limiting toxicities and pharmacokinetics of sorafenib in patients with hepatocellular carcinoma. PLoS ONE 7, e37563 (2012).
Tegels, J. J. et al. Sarcopenia is highly prevalent in patients undergoing surgery for gastric cancer but not associated with worse outcomes. J. Surg. Oncol. 112, 403–407 (2015).
Sharma, P. et al. Sarcopenia as a predictor of overall survival after cytoreductive nephrectomy for metastatic renal cell carcinoma. Urol. Oncol. 33, 339.e17-339.e23 (2015).
Kazemi-Bajestani, S. M. R., Mazurak, V. C. & Baracos, V. Computed tomography-defined muscle and fat wasting are associated with cancer clinical outcomes. Semin. Cell Dev. Biol. 54, 2–10 (2016).
Caan, B. J. et al. Association of muscle and adiposity measured by computed tomography with survival in patients with nonmetastatic breast cancer. JAMA oncol 4, 798–804 (2018).
Wilson, R. J. et al. Sarcopenia does not affect survival or outcomes in soft-tissue sarcoma. Sarcoma 2015, 1–6 (2015).
Huillard, O. et al. Body composition in patients with radioactive iodine-refractory, advanced differentiated thyroid cancer treated with sorafenib or placebo: A retrospective analysis of the phase III DECISION trial. Thyroid 29, 1820–1827 (2019).
Lee, J. C. et al. Effect of thyroid-stimulating hormone suppression on muscle function after total thyroidectomy in patients with thyroid cancer. Front. Endocrinol. (Lausanne) 12, 769074 (2021).
Melillo, R. M. et al. The RET/PTC-RAS-BRAF linear signaling cascade mediates the motile and mitogenic phenotype of thyroid cancer cells. J. Clin. Investig. 115, 1068–1081 (2005).
Kobawala, T. P. et al. Significance of interleukin-6 in papillary thyroid carcinoma. J. Thyroid Res. 2016, 1–12 (2016).
Ohta, K., Pang, X.-P., Berg, L. & Hershman, J. M. Antitumor actions of cytokines on new human papillary thyroid carcinoma cell lines. J. Clin. Endocrinol. 81, 2607–2612 (1996).
Xi, C. et al. Interleukins in thyroid cancer: From basic researches to applications in clinical practice. Front. Immunol. 11, 1124 (2020).
Yoon, J. K. et al. Strap muscle invasion in differentiated thyroid cancer does not impact disease-specific survival: A population-based study. Sci. Rep. 10, 1–9 (2020).
Song, E. et al. A relook at the T stage of differentiated thyroid carcinoma with a focus on gross extrathyroidal extension. Thyroid 29, 202–208 (2019).
Amit, M. et al. Extrathyroidal extension: Does strap muscle invasion alone influence recurrence and survival in patients with differentiated thyroid cancer?. Ann. Surg. Oncol. 25, 3380–3388 (2018).
Park, S. et al. Prognostic significance of gross extrathyroidal extension invading only strap muscles in differentiated thyroid carcinoma. J. Brit. Surg. 105, 1155–1162 (2018).
Mete, O., Rotstein, L. & Asa, S. L. Controversies in thyroid pathology: Thyroid capsule invasion and extrathyroidal extension. Ann. Surg. Oncol. 17, 386–391 (2010).
Gaissert, H. A. et al. Segmental laryngotracheal and tracheal resection for invasive thyroid carcinoma. Ann. Thorac. Surg. 83, 1952–1959 (2007).
Tsukahara, K., Sugitani, I. & Kawabata, K. Surgical management of tracheal shaving for papillary thyroid carcinoma with tracheal invasion. Acta Otolaryngol. 129, 1498–1502 (2009).
Sakamoto, T. et al. Sarcopenia as a prognostic factor in patients with recurrent pancreatic cancer: A retrospective study. World J. Surg. Oncol. 18, 1–7 (2020).
Kim, H. J., Park, M.-S., Kim, B.-S. & Lee, S.-M. Relationship of sarcopenia with the outcomes of patients who underwent surgery for bile duct cancer. Surg. Metab. Nutr. 10, 54–58 (2019).
Okugawa, Y. et al. Prognostic impact of sarcopenia and its correlation with circulating miR-21 in colorectal cancer patients. Oncol. Rep. 39, 1555–1564 (2018).
Fukushima, H., Yokoyama, M., Nakanishi, Y., Tobisu, K.-I. & Koga, F. Sarcopenia as a prognostic biomarker of advanced urothelial carcinoma. PLoS ONE 10, e0115895 (2015).
Buchholz, A. C., Bartok, C. & Schoeller, D. A. The validity of bioelectrical impedance models in clinical populations. Nutr. Clin. Pract. 19, 433–446 (2004).
Berdelou, A., Lamartina, L., Klain, M., Leboulleux, S. & Schlumberger, M. Treatment of refractory thyroid cancer. Endocr. Relat. Cancer 25, R209–R223 (2018).
Lee, J., Hong, Y. P., Shin, H. J. & Lee, W. Associations of sarcopenia and sarcopenic obesity with metabolic syndrome considering both muscle mass and muscle strength. J. Prev. Med. Public Health 49, 35 (2016).
Tuttle, M. et al. Thyroid-differentiated and anaplastic carcinoma (Chapter 73). In AJCC Cancer Staging Manual 8th edn (eds Amin, M. et al.) 873–890 (Springer, 2017).
World Health Organization, The Asia-Pacific perspective: Redefining obesity and its treatment (2000).
Author information
Authors and Affiliations
Contributions
Conception or design of the study was carried out by J.Y.K. and Y.J.K. All authors were responsible for data acquisition, analysis, interpretation as well as drafting or revising of the work. Y.J.K. and J.Y.K approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yoon, J.K., Yoon, J.H., Park, V.Y. et al. Sarcopenia increases the risk of major organ or vessel invasion in patients with papillary thyroid cancer. Sci Rep 12, 4233 (2022). https://doi.org/10.1038/s41598-022-08224-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-08224-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.