Abstract
The onset of both chronic pain and insomnia is high during adolescence. Although a bidirectional relationship between pain and insomnia has support, how pain and sleep co-develop throughout adolescence remains unknown. Sleep–wake patterns, pre-sleep behavior and pre-sleep arousal may influence the co-development of pain and insomnia. Four waves of longitudinal self-report data were used (Nbaseline = 2767, Agebaseline M = 13.65 years, SD = 0.65). Multidimensional growth mixture modeling was used to identify four subgroups of adolescents with different concurrent trajectories of pain and insomnia. The trajectories followed each other across time in all classes: one class of consistently low pain and insomnia (68.7%), one class with persistent high symptoms (4.9%), as well as one class of increasing (13.9%), and one of decreasing (12.5%), trajectories. Later sleep–wake patterns and more pre-sleep cognitive-emotional arousal predicted both increasing and decreasing trajectories of concurrent pain and insomnia. The current study showed that developmental trajectories of pain and insomnia follow each other within adolescents and across adolescence. Both sleep-phase focused interventions as well as psychological interventions that focus on pre-sleep cognitive-emotional arousal may prove beneficial for adolescents with comorbid pain and insomnia.
Similar content being viewed by others
Introduction
Chronic pain is a leading cause of years lived with disability worldwide1. Insomnia appears to be a considerable driver of pain2,3, also during adolescence4, which has been identified as a critical period for their co-development5. The incidence and onset of both chronic pain6 and insomnia7 is high across adolescence. About 50% of adolescents who suffer from chronic pain also have insomnia8,9, and this comorbidity may be more disabling than each condition on its own9.
Evidence supporting an association between pain and insomnia continues to grow. Yet, little is known about how pain and insomnia co-develop over time. Which co-developmental trajectories of pain and insomnia can be distinguished, and what factors predict these different patterns? It is known that adolescents who develop chronic pain10 or insomnia11 are at high risk of maintaining these conditions into adulthood. A better understanding of how pain and insomnia co-develop throughout adolescence may not only reveal specific targets for intervention for adolescents with comorbid insomnia and chronic pain but is also needed to develop effective strategies to prevent the maintenance and exacerbation of chronic pain and sleep conditions.
We are unaware of studies that have specifically addressed individual differences in longitudinal co-development of insomnia and pain. A recent review established that a change towards poorer sleep can predict a future increase in pain, but it remains unclear whether an improvement in sleep quality can predict a decrease in pain12. Can these mixed findings on the reciprocal relationship between sleep disturbance and pain2 be attributed to (unobserved) subgroups in the general population of adolescents who show differentiated sleep-pain relationships? Are there subgroups of adolescents with only insomnia or pain problems? Such subgroups may explain heterogeneity of results in previous studies.
Relatively little is known of risk factors for concurrent insomnia and pain, but potential candidates include female gender, older age, depression and anxiety, short sleep duration, stress, low socioeconomic status (SES), and immigrant background8,13,14. Since insomnia has been found to be a stronger predictor of future pain than vice versa, a focus on sleep-related risk factors has been proposed when examining comorbid pain and insomnia8. Although such risk factors have the clearest association with insomnia, it is not known if they predict how both pain and insomnia co-develop over time.
The increasing prevalence of pain and insomnia during adolescence temporally coincides with both pubertal development and a pronounced delay in sleep–wake patterns15, the latter mainly driven by a reduced build-up of sleep pressure and a delayed circadian rhythm5. Therefore, the concurrent increases of pain and insomnia in adolescents have been explained in terms of developmental changes associated with puberty or by factors specifically related to the delay in sleep–wake patterns5,15, such as melatonin. For example, delayed dim light melatonin onset is associated with delayed sleep–wake patterns in adolescents7,16 as well as with pain17. Emerging evidence also suggests that both pain and sleep are regulated by circadian rhythm; the suprachiasmatic nucleus synchronizes the circadian clocks in peripheral tissues through secretion of, for example, dopamine and cytokines, which are each involved in the regulation of both pain and sleep18. In cross-sectional studies, a late sleep-phase has been associated with insomnia and depressed mood in adolescents19,20 and pain in adults21,22. However, no longitudinal studies to date have explored the effect of a late sleep phase on concurrent pain and insomnia.
The increase in pain and insomnia during adolescence may not only be due to physiological factors, since adolescents also show a distinct increase in maladaptive late-night behavior concurrently with the advancement in sleep phase23. Pre-sleep arousal and poor sleep hygiene habits have been shown to predict insomnia in adolescents with comorbid pain9,24. Moreover, cognitive and physiological hyperarousal has been found to be a main etiological factor in the pathogenesis of insomnia7, and has been associated both with pain generally17, as well as the interaction between sleep and pain specifically5. The question of whether and how these risk factors simultaneously influence pain, beyond the indirect effect through insomnia, remains unclear. As with a late sleep phase25, pre-sleep arousal predicts depressed mood26, which, in turn, is established as an important mediator in the insomnia-pain relationship27. Depressed mood may therefore be an underlying mechanism linking a late sleep phase and pre-sleep arousal to insomnia and pain.
The purpose of the current study was threefold: First, to identify subgroups of adolescents with unique co-developmental patterns of pain and insomnia by using a person-oriented multidimensional growth mixture modeling approach in a large longitudinal community sample of adolescents (N = 2767) spanning from 13/14 to 16/17 years old. Second, to describe the subgroups on demographic and mental health variables. Third, to explore the predictive effects of sleep phase and pre-sleep cognitive-emotional arousal (e.g., worry, rumination) or pre-sleep behavior (e.g., chatting with friends), on longitudinal growth trajectories of concurrent pain and insomnia. The resulting insights may prove useful in uniquely adapting interventions for adolescents with comorbid chronic pain and insomnia.
Method
Participants and procedure
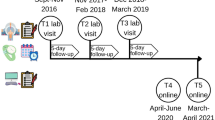
This study is part of the Three Cities Study, a 5-year longitudinal study in Sweden that sought to better understand risk factors and mechanisms underlying mental health problems among adolescents. The program targeted adolescents in 7th and 8th grade (13 and 14 years old; N = 3 336) in 18 public upper secondary schools in three cities in central Sweden and data was acquired for four consecutive years after the baseline measurement (2014–2018). These participants filled out a battery of self-report questionnaires (paper-and-pencil format) each spring, in their classroom during school hours. Teachers left the classroom while trained test leaders supervised the procedure. The adolescents had 90 min to complete the questionnaires, including a short snack break. Each class received 300 SEK for participating. To reduce risks for sampling bias, the Three Cities Study used passive consent from the parents and active consent from adolescents28. The parents of 122 adolescents (3.7%) returned a prepaid letter where they declined participation, and, additionally, 447 adolescents (13.4%) either declined participation or were absent on the day of the first data collection. This resulted in a baseline sample of N = 2767 (82.9% of the N = 3336 target population: Mage = 13.65 (SD = 0.65). The analyses reported in this article included the first four data waves since data on the predictor variables were not collected in the fifth wave of data collection. All cases that had any data on the main pain and insomnia variables at baseline were included in the analyses. This resulted in an analytic sample of N = 2755 participants (82.6% of baseline target population, 99.6% of baseline sample). 1384 adolescents (50% of baseline sample) participated in all four measurement occasions, 2167 (78.3%) adolescents participated in at least three, 2619 (94.7%) in at least two, and 148 (5.3%) only participated at baseline. The study was approved by the regional Ethical Board in Uppsala (No 2013/384).
To investigate whether attrition across the four yearly measurement occasions (T1 to T4) was influenced by any systematic bias, we regressed attrition (number of missed measurement occasions, ranging from 1 to 3) on the baseline demographic characteristics of the adolescents (gender, age, SES and immigrant background) and all the other study variables (insomnia, sleep duration, sleep phase, depression, anxiety, pain frequency, pain intensity, pain interference, sleep-related behavior). Older adolescents (Wald χ2(1) = 28.57, p < 0.001), adolescents not born in Sweden or whose parents were not born in Sweden (Wald χ2(1) = 23.31, p < 0.001), or with a later sleep phase (Wald χ2(1) = 8.23, p = 0.004) were more likely to drop out from the study.
Measures
Descriptive measures at baseline
Age, gender, SES, immigrant background, stress, depression, anxiety, sleep duration, insomnia, pain intensity, pain frequency, and pain interference were assessed at baseline (T1). Age was measured by asking the respondents to indicate their chronological age in years. Gender was measured by the respondent indicating “boy” or “girl” on one dichotomous item. SES was assessed using the four items of the Family Affluence Scale, version 2 (FAS-II), from WHO’s Health Behavior in School-aged Children (HBSC)—survey29. The respondent was instructed to indicate whether they had their own bedroom in the household, how many cars the household had, how many computers the household currently owned, and how many times the household went on a holiday during the last 12 months. The mean on FAS-II in the Swedish HBSC-survey (N = 23,088) was M = 6.28 (SD = 1.67). The cut-off for low SES was set at 4.61, one standard deviation below the population mean29. Immigrant status was assessed by the respondent indicating whether they were born in Sweden and where either of their parents were born (Sweden, Scandinavia, Europe or outside of Europe). Immigrant background was defined as being born in Sweden or having one parent being born in Sweden (i.e., non-immigrant background), or being born outside of Sweden or having both parents being born outside of Sweden (i.e. immigrant background), according to the official definition of Statistics Sweden30. Depressive symptoms were assessed with the Center for Epidemiology Studies Depression Scale for Children (CES-DC). CES-DC is a 20-item Likert scale with possible responses ranging from 0 (“not at all”) to 3 (“a lot”), resulting in a total score ranging from 0 to 6031 At T1, possible responses ranged from 1 to 5 and were adjusted to the original scaling using a POMP method32. CES-DC has shown good reliability and validity when applied on a sample of Swedish adolescents31, and the cut-off for clinical depression was set at a total score of at least 2431. In the current sample, the internal consistency of the scale at T1 was Cronbach’s α = 0.84. Anxiety was assessed with the Overall Anxiety Severity and Impairment Scale (OASIS). OASIS includes five Likert scale items assessing the degree of anxiety and impairment due to anxiety experienced during the last week. Possible responses range from 0 (“no”/”none”/”not at all”) to 4 (“constant”/”extreme”/”all the time”), resulting in a total score ranging from 0 to 2033. The cutoff for clinical anxiety problems is 833, and the scale has previously shown good psychometric properties33. Cronbach’s alpha at T1 in the current study was α = 0.87. Stress was assessed with the Adolescent Stress Questionnaire, Short version34. Adolescents indicated how much stress from various social domains they have experienced in the past 6 months, on a 27 item, five-point Likert scale, ranging from 0 = not at all stressful to 4 = very stressful. The internal consistency at timepoint 1 was α = 0.87.
Pain measures
Pain intensity, pain frequency, and pain interference were measured separately for the three most common pain types in adolescents6: abdominal pain, headache, and musculoskeletal pain (i.e., pain in the neck, shoulders or back).
Pain intensity was measured by one item where the participants rated their average pain intensity during the last 6 months on a 10-point scale ranging from 0 (“not at all”) to 9 (“very painful”)35. In T3, the intensity measures for headache and abdominal pain asked for the average pain during the last 2 months instead for the last 6 months. To assess the potential impact of this, we fitted a longitudinal confirmatory factor analysis including the pain intensity measures for the three pain types across the four measurement occasions and tested for measurement invariance. The model met the assumption of strong measurement invariance, indicating that the meaning of the measures remained constant across the four measurement occasions, and that the deviation in T3 was inconsequential. For more details on these analyses, see the Supplementary Information.
Pain frequency; how often the participant had experienced pain, on average, during the last 6 months, was assessed with one item from the HBSC Symptom Checklist36, with the possible answers on a five-point Likert scale being “rarely or never” (0), “about every month” (1), “about every week” (2), “more than once per week” (3), and “about every day” (4).
Pain interference: how much the pain severely interfered with three life domains—school work, friend relationships and leisure activities. Possible responses were “no”, scored as 2, “yes, a bit”, scored as 1, and “yes, definitely”, scored as 0. The scores were reversed and summed across the three domains into a total score, ranging from 0 to 6. The measure was adapted from the Social Phobia Screening Questionnaire37 for the purpose of the Three Cities Study and has been used in previously published studies4,38.
Pain grades. A composite “pain grade” was constructed based on the above-mentioned facets of the pain experience (pain intensity, pain frequency, and pain interference)14,38, as described in Table 1. We calculated separate pain grades for musculoskeletal pain, abdominal pain, and headache. An average pain grade was calculated by averaging the pain grades across these three pain types, for each individual and per measurement occasion. Higher average pain grades indicate more intense and/or more widespread pain. In the rest of this paper, pain grade refers to this average pain grade.
The pain grade measure can be taken to capture the degree of problematic pain, which is defined as having a high pain intensity associated with either significant distress or disability, or being experienced as multiple types of pain39. A pain grade of at least 2 on all three pain types can be taken to reflect generalized, problematic pain39.
Sleep
Insomnia symptoms were measured with the Insomnia Severity Index (ISI)40, from T1 to T4. The ISI consists of seven items regarding sleep problems during the last 6 months, each with ratings from 0 (“no problems at all”) to 4 (“very much”). Total scores range from 0 to 28. It has been used on a sample of Swedish youths with chronic pain41, and it has shown significant correlations with other measures of sleep quality, such as the Pittsburgh Sleep Quality Index, sleep diaries, and polysomnography42. A total score of 9 has been suggested as an appropriate cut-off for insomnia in adolescents43. The internal consistency in the current sample was α = 0.85 for T1, α = 0.84 for T2, α = 0.86 for T3, α = 0.87 for T4.
Pre-sleep cognitive-emotional arousal and Pre-sleep behavior were measured from T1 to T4 by using two subscales of the Adolescent Sleep Hygiene Scale, revised version (ASHS-R)44. ASHS is a questionnaire including 28 items with possible responses on Likert scales ranging from 0 (“Always”) to 5 (“Never”). The 6-item cognitive-emotional sub-scale [0–30] measures pre-sleep cognitive-emotional arousal (PSCEA) and includes items assessing worrying or ruminating in bed or feeling strong emotions in proximity to bedtime. The 3-item behavioral arousal subscale [0–15] measures pre-sleep behavior (PSB) and includes items assessing behavior before going to bed, or in bed, that increase physiological arousal and keeps you awake, such as talking on the phone or playing video games (3 items). The internal consistency in the current sample for the cognitive-emotional subscale was: 0.83 for T1, 0.83 for T2, 0.85 for T3, and 0.85 for T4; for the behavioral arousal subscale, the internal consistency was: 0.63 for T1 (mean inter-item correlation = 0.361), 0.67 for T2 (mean inter-item correlation = 0.403), 0.67 for T3 (mean inter-item correlation = 0.414), and 0.65 for T4 (mean inter-item correlation = 0.512). To represent change, we calculated the difference between pre-sleep behavior at T1 and T4 (T4-value minus T1-value) and used it as a predictor of the trajectories of pain grade and insomnia symptoms.
Sleep duration was measured by using the School Sleep Habits Survey for adolescents (SSHS)45, where the respondent is asked to estimate when they usually went to bed, how long time they usually took to fall asleep, and at what time they usually woke up, on average weekdays and average weekend days, during the last two weeks. In the current study, we included the average sleep time on weekdays and the average sleep time on weekends separately. Sleep duration was measured by calculating the time from bedtime to wake time, subtracting the reported time it took to fall asleep.
Sleep phase was also assessed with the SSHS45, and operationalized as hours and minutes between midnight and sleep midpoint (the mid-point between sleep onset and wake time) on weekend days46. However, most adolescents’ biological clocks are out of sync with school times, resulting in a general lack of sleep and accumulated sleep debt during weekdays for which they then compensate by sleeping in on weekends47. To adjust sleep phase for this confounder sleep debt, we utilized the correction that is used in the Munich Chronotype Questionnaire: In individuals who sleep longer on weekends than on weekdays, half the difference of sleep time during weekdays and sleep time during weekends is subtracted from the sleep phase-value46.
Shift in sleep phase was operationalized as change in adjusted sleep phase between T1 and T4 (T4-value minus T1-value), measured in minutes.
Statistical analyses
Management of the raw data was handled in SPSS, version 24, and all subsequent analyses were conducted in Mplus, version 8.1. We applied multidimensional growth mixture modelling (GMM) following previously proposed guidelines47,48,49. GMM is a person-oriented analysis that extends latent growth curve modelling by incorporating a latent class variable that accounts for between-individual heterogeneity48. It assigns individuals to latent classes based on similar patterns of growth curves. A probability distribution decides what class the individual is most likely to belong to49.
To assess model fit, we put substantive value on meaningful, theoretical interpretability of the class solution47,48. We also used two types of statistical indices: the sample size-adjusted Bayesian Information Criteria (SSA-BIC) and the adjusted Lo-Mendell-Rubin likelihood ratio test (LMR-LRT)48. In addition, we considered entropy, as opted for the model with the least restrictions on model parameters, while also encountering the least convergence problems50: First, separate growth models were constructed on insomnia symptoms and pain grade. Low model fit and significant variance factors indicate heterogeneity in growth trajectories48. Secondly, a multidimensional growth mixture model was constructed on these two growth models simultaneously. We opted for an explorative approach49 and fitted latent class growth analyses (LCGA) with 2 to 7 classes, to establish the optimal number of classes. Age and gender were included as covariates in the analysis. We then utilized Wald χ2-tests to compare a zero-variance model (LCGA) to a model with class-invariant variances (GMM-CI), as well as to explore whether equal variances across the classes fitted the data as well as class-varying variances (GMM-CV). As a general rule, the preferred model is as freely estimated as possible while not encountering convergence problems48. Convergence problems indicate that the model is inadequate, that it may be overparameterized, and that a more parsimonious model is preferred49. Thereafter, we successively freed parameters—first class-invariant parameters, then class-varying parameters, until the optimal model was established. Since freeing parameters may change what number of classes is optimal48, we continuously tested 2 to 7 classes for both the class-invariant, and the class-varying models.
For incorporating the predictors (sleep phase, pre-sleep cognitive-emotional arousal and pre-sleep behavior, as well as change scores of those measures) into the GMM, we utilized the “three-step approach”, consisting of: (1) estimating an unconditional GMM with covariates included, (2) assigning individuals to latent classes while accounting for uncertainty of class membership, and (3) including predictors of the class-variable and within-class growth factors. Benefits of this approach is that the parameters of the mixture model are not influenced by the auxiliary predictor variable, while still accounting for uncertainty rate in class membership47. Three sets of analyses were done: First, differences among the classes on selected baseline variables were compared via Wald χ2-tests. Secondly, a multinomial regression analysis was done to assess if baseline sleep phase, pre-sleep cognitive-emotional arousal and pre-sleep behavior could predict class membership. Third, we examined the effect of sleep-related predictors on within-class growth factors (intercept and slope) of insomnia symptoms and pain grade. More information on statistical indices and criteria can be found in the Supplementary Information.
For handling missing data, we used full information maximum likelihood estimation (FIML)51.
Results
Estimation of the best fitting multidimensional growth mixture model
First, we established that latent basis growth curves fitted the data best. Second, we opted for a CI-GMM as the best-fitting model, since it fitted the data better than the LCGA while not encountering convergence problems (which the GMM-CV did), and it yielded theoretically meaningful classes that facilitated interpretation. Third, we found that a four-class solution of a GMM with class-invariant variances fitted the data best, when comparing theoretical meaningfulness and model fit indices of 2 to 7 classes. Details on model fit indices are described in Table 2. For more detailed information on these analyses, see the Supplementary information.
Class characteristics
Class descriptions
Figure 1 shows the conjoint trajectories of pain grade and insomnia symptoms of the four classes. Class 1 (n = 1893; 68.7% of total sample) was primarily characterized by consistently low levels of pain grade and insomnia, although both trajectories increased slightly. This class was labeled “Low pain and insomnia”. Class 2 (n = 134; 4.9%) was characterized by high levels of pain grade and insomnia symptoms throughout the measured period, although the trajectory of insomnia symptoms increased slightly. This class was labeled “High pain and insomnia”. Class 3 (n = 383; 13.9%) was characterized by increasing trajectories of both pain grade and insomnia symptoms. We labeled this class “Increasing pain and insomnia”. Class 4 (n = 345; 12.5%) was characterized by decreasing trajectories of pain grade and insomnia symptoms, although the decrease in pain grade was modest. This class was labeled “Decreasing pain and insomnia”.
Longitudinal class solution of conjoint pain and insomnia development. The trajectories of pain are in red color (dotted line), and the trajectories of insomnia are in blue (solid line). The y-axis in the diagrams, which ranges from 0 to 100, represents percentage of maximum score of both insomnia symptoms and pain grade to facilitate comparisons of the trajectories. The x-axis represents measurement occasion, ranging from T1 to T4, and the numbers within parentheses are the age cohorts (in years) at each time point.
Baseline characteristics of classes
Table 3 compares the classes on baseline variables. All variables, except for low SES and sleep phase shift, differed depending on class. Class 2 (high pain and insomnia) consistently reported a more severe profile than the other classes. About two thirds (68.7%) of the class-members were girls. This class reported the highest levels of pre-sleep cognitive-emotional arousal and pre-sleep behavior. About half of the class-members fulfilled the criteria for clinical anxiety and clinical depression. Furthermore, class 2 reported the shortest average sleep times and the latest sleep phase. Compared to class 1 (low pain and insomnia), class 2 reported a more than 13-fold prevalence of generalized problematic pain. Class 3 (increasing pain and insomnia) generally showed low symptoms at baseline, but reported a large shift towards a later sleep phase and the most profound deterioration of pre-sleep cognitive-emotional arousal. Class 4 (decreasing pain and insomnia) showed the second highest symptom levels on most variables at baseline, but also the smallest shift in sleep phase and was the only class to show an improvement in pre-sleep cognitive-emotional arousal.
Sleep-related predictors of longitudinal trajectories and change
Sleep phase and pre-sleep cognitive-emotional arousal predict diverging trajectories of pain grade and insomnia symptoms
To explore whether sleep phase, pre-sleep cognitive-emotional arousal and pre-sleep behavior at baseline could predict diverging concurrent trajectories of pain grade and insomnia symptoms, we conducted a multinomial regression analysis and focused on comparing the “Low pain and insomnia” class (1) with the “Increasing pain and insomnia” class (2), and the “High pain and insomnia” class (3) with the “Decreasing pain and insomnia” class (4). Note that all classes were compared, but that for clarity only the above-mentioned comparisons are presented here. See the Supplementary Information for the full set of analyses. As can be seen in Table 4, a later sleep phase and higher ratings on pre-sleep cognitive-emotional arousal (but not pre-sleep behavior) at baseline predicted increased insomnia symptoms and pain grade. An earlier sleep phase and less pre-sleep cognitive-emotional arousal at baseline predicted reduced pain grade and insomnia symptoms.
Sleep phase, pre-sleep cognitive-emotional arousal and pre-sleep behavior predict within-class change of insomnia symptoms and pain grade
To assess whether sleep phase, pre-sleep cognitive-emotional arousal and pre-sleep behavior could predict initial levels and longitudinal change in pain grade and insomnia symptoms, we regressed the intercepts and slopes of pain grade and insomnia symptoms on the predictors at baseline, as well as on their change scores, in a multivariate regression analysis. Table 5 depicts the results of these analyses. More pre-sleep cognitive-emotional arousal predicted higher levels of both pain grade and insomnia symptoms, a later sleep phase predicted more insomnia symptoms, and higher levels of pre-sleep behavior predicted more insomnia symptoms as well as a slighter increase or a decrease in insomnia symptoms. A greater shift to a later sleep phase and a larger increase in reported pre-sleep cognitive-emotional arousal predicted a steeper longitudinal increase in insomnia symptoms, whereas only a greater increase of pre-sleep cognitive-emotional arousal predicted a steeper longitudinal increase of pain grade.
Discussion
The current study examined the co-development of pain and insomnia throughout adolescence. We identified four subgroups with different concurrent developmental trajectories of pain and insomnia symptoms. The results suggest that adolescents with consistently high pain and insomnia may constitute a unique subgroup that is particularly vulnerable. Furthermore, the study established that both a late sleep phase and pre-sleep cognitive-emotional arousal (but not pre-sleep behavior) predicted pain and insomnia symptoms.
The current results show that trajectories of pain and insomnia co-occur to a high degree in all subgroups, regardless of the direction of the trajectories. The relationship between pain and insomnia is in line with previous work2,3,52. Given the substantial variability in the trajectories, however, there may be adolescents with high symptoms levels of either pain or insomnia included. This finding also indicates that lack of such relationship in previous study samples may be due to a mix of subgroups with both increasing and decreasing trajectories, resulting in a null result when looking at grand means. We are not aware of any previous study that has explored individual differences in longitudinal trajectories of co-developmental pain and insomnia. Previous studies have primarily focused on associations between variable means, for example that mean levels of insomnia symptoms at one timepoint can predict mean levels of pain at a later timepoint. Such an approach fails to capture continuity of trajectories over time and cannot demonstrate that pain and insomnia continuously co-change within individuals.
The four classes identified in the current study correspond to previous studies on longitudinal pain trajectories in adolescents. Most commonly, four or five classes have been identified as the optimal solution in studies on pain, typically including one class with persistent low pain, one with persistent high pain, as well as two or three classes of fluctuating, increasing or decreasing pain53. Few studies have included insomnia trajectories in adolescents, but one study identified trajectories similar to those found in regard to pain54. In the current study, the yearly measurements and the 6-month coverage of the measures likely means that the trajectories primarily represent stable aspects of pain and insomnia. Future work with more frequent testing, more transient measures or longer measurement periods could reveal long-term trajectories of change that we are unable to show with the current study design. Such study designs could possibly help characterizing dynamic and complementary trajectories, as well as identifying more state-like aspects of pain and insomnia. Most of the adolescents in the current sample had consistently low pain and insomnia levels throughout the measured period (Class 1, 68.7%), which is to be expected in a sample derived from the general population. About 14 percent of the sample (Class 3, 13.9%) were characterized by increasing levels of both pain and insomnia symptoms. This subgroup showed the largest shift towards a later sleep phase and the largest increase in pre-sleep cognitive-emotional arousal. A third subgroup showed decreasing levels of pain and insomnia symptoms (Class 4, 12.5%). Earlier sleep phases and less pre-sleep cognitive-emotional arousal could predict these decreasing trajectories, compared to the high stable trajectories. It may be that early, proactive strategies that focus on late sleep phases and pre-sleep cognitive-emotional arousal help in preventing the concurrent development of pain and insomnia. This may be further investigated in experimental studies that manipulate sleep–wake patterns and pre-sleep habits directly in order to demonstrate their causal effect on pain and insomnia.
A subgroup of consistently high pain and insomnia symptoms was identified (Class 2.4, 9%), constituting about 5 percent of the overall sample. Girls made up two thirds of this subgroup. Adolescents in this subgroup showed higher levels of symptoms on most of the baseline characteristics examined, including stress, depression, and anxiety. They also reported the shortest sleep times, the latest average sleep phase, as well as the most problematic pre-sleep behavior. Compared to Class 1 (low pain and insomnia), they reported a more than 13-fold rate of generalized problematic pain. This indicates that the subpopulation of adolescents with comorbid pain and insomnia is associated with a particularly complex illness-profile, and indeed may benefit from specialized interventions. However, future studies should thoroughly explore potential differences between comorbid pain and insomnia and subgroups of adolescents with only pain or insomnia problems.
Pre-sleep cognitive-emotional arousal was the most consistent predictor of change in both pain and insomnia symptoms. This is in line with previous research suggesting that cognitive-emotional arousal may delay sleep onset and lower sleep quality24, and that worry, rumination and emotional distress are associated with the sleep-pain relationship3,55. Additionally, stress responses or arousal has been proposed to explain the sleep-pain relationship5, as well as influencing melatonin secretion56. Pre-sleep arousal may have both a moderating and a mediating effect on comorbid pain and insomnia. We encourage future studies to explore the efficacy of hybrid interventions for comorbid pain and insomnia that incorporate a focus on pre-sleep cognitive-emotional arousal. Notably, sleep hygiene practices (including pre-sleep behavior) are often a main focus in insomnia-interventions for adolescents7.
Pre-sleep behavior did not consistently predict conjoint trajectories of pain and insomnia. However, pre-sleep behavior and somatic pre-sleep arousal have previously been associated with insomnia in adolescents suffering from chronic pain24. Plausible explanations for our null finding that require further investigation lie either in that this finding is specific to conjoint pain and insomnia symptoms, that the internal consistency of the behavioral arousal subscale was insufficient in the current study, or that the items in this subscale are relatively positive in their valence.
The association between sleep phase and trajectories of pain and insomnia symptoms may help explain inconsistent results of non-pharmacological treatments for comorbid pain and insomnia57,58. Social and behavioral factors related to adolescent sleep–wake patterns, which are not a target in psychological treatments, may impede or confound treatment effects. In addition, pharmacological, social or psychological interventions may be effective in treating the conjoint development of pain and insomnia, including melatonin7, synchronizing adolescents’ school time with their biological clock59, and CBT60. The current results may indicate that the effect of melatonin on pain is mediated through insomnia symptoms, practically meaning that advancing adolescents’ sleep phase may lead to a reduction in insomnia symptoms, which, in turn, may reduce pain56.
A strength of the current study is the large population sample of adolescents followed over four yearly measurement occasions. This large set of data allowed us to use class-invariant GMM and account for potential sub-distributions via the class-variable, as well as between-individual differences and within-individual temporal change via the growth factors. Accounting for within-class variance often decreases the risk of identifying spurious classes48 and reduces the risk of bias in the trajectories61. The use of latent basis and unspecified growth curves is also a strength of the study, since it does not impose any restrictions to the shape of longitudinal change32.
Some limitations need to be considered. First, for current purposes, we summarized the experience of three common types of pain (musculoskeletal pain, headache and abdominal pain) in an average pain grade, taking into account the intensity, frequency, and interference of the experience. Although this composite measure captures covariance across different pain types with insomnia5, future studies might want to focus on co-developmental patterns in specific pain types, which would also allow interpretation of the pain grades in terms of pain intensity and pain-related disability only14,62. Indeed, the co-development of pain and insomnia might turn out to be different for specific pain types63. A second limitation is that only self-report measures of complex constructs were used. Since objective and subjective sleep measures appear to partly measure different aspects of sleep9, future studies should include also objective sleep-related measures. While the ISI has been used in a few studies in adolescents43,64,65, it has not been extensively validated in this population. A third limitation is that diverging trajectories were not perfectly overlapping at baseline, which may (although unlikely) have confounded the multinomial analysis of diverging trajectories.
In conclusion, the current study brings novel insights into how pain and insomnia symptoms longitudinally co-develop in adolescents. We identified four unique subgroups, showing that longitudinal trajectories of pain and insomnia closely follow each other, and that sleep phase and pre-sleep cognitive-emotional arousal and behavior could predict the trajectories. A considerable portion of adolescents have either high persistent or increasing levels of problematic pain and insomnia. Adolescence constitute a melting pot of factors contributing to the development of both insomnia and pain5, and therefore appears to be an important developmental stage for preventing these conditions from becoming chronic sources of disability and suffering. A sleep-phase focus may be relevant in interventions for comorbid pain and insomnia in adolescents, and pre-sleep cognitive-emotional arousal seems to be of particular importance to focus on in psychological interventions.
Data availability
The syntax code and the variance–covariance matrix that the study’s analyses are based on are provided in the Supplementary information, which allows for replication of the analyses. The raw dataset may be made available after permission from the Three Cities Study PI Katja Boersma (katja.boersma@oru.se) upon request.
References
James, S. L. et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 392, 1789–1858. https://doi.org/10.1016/S0140-6736(18)32279-7 (2018).
Andersen, M. L., Araujo, P., Frange, C. & Tufik, S. Sleep disturbance and pain: A tale of two common problems. Chest 154, 1249–1259. https://doi.org/10.1016/j.chest.2018.07.019 (2018).
Finan, P. H., Goodin, B. R. & Smith, M. T. The association of sleep and pain: An update and a path forward. J. Pain 14, 1539–1552. https://doi.org/10.1016/j.jpain.2013.08.007 (2013).
Arnison, T., Schrooten, M. G. S., Hesser, H., Jansson-Frojmark, M. & Persson, J. Longitudinal, bidirectional relationships of insomnia symptoms and musculoskeletal pain across adolescence: The mediating role of mood. Pain 163, 287-298. https://doi.org/10.1097/j.pain.0000000000002334 (2022).
Christensen, J., Noel, M. & Mychasiuk, R. Neurobiological mechanisms underlying the sleep-pain relationship in adolescence: A review. Neurosci. Biobehav. Rev. 96, 401–413. https://doi.org/10.1016/j.neubiorev.2018.11.006 (2019).
King, S. et al. The epidemiology of chronic pain in children and adolescents revisited: A systematic review. Pain 152, 2729–2738. https://doi.org/10.1016/j.pain.2011.07.016 (2011).
de Zambotti, M., Goldstone, A., Colrain, I. M. & Baker, F. C. Insomnia disorder in adolescence: Diagnosis, impact, and treatment. Sleep Med. Rev. 39, 12–24. https://doi.org/10.1016/j.smrv.2017.06.009 (2018).
Allen, J. M., Graef, D. M., Ehrentraut, J. H., Tynes, B. L. & Crabtree, V. M. Sleep and pain in pediatric illness: A conceptual review. CNS Neurosci. Ther. 22, 880–893. https://doi.org/10.1111/cns.12583 (2016).
Palermo, T. M., Law, E., Churchill, S. S. & Walker, A. Longitudinal course and impact of insomnia symptoms in adolescents with and without chronic pain. J. Pain 13, 1099–1106. https://doi.org/10.1016/j.jpain.2012.08.003 (2012).
Walker, L. S., Sherman, A. L., Bruehl, S., Garber, J. & Smith, C. A. Functional abdominal pain patient subtypes in childhood predict functional gastrointestinal disorders with chronic pain and psychiatric comorbidities in adolescence and adulthood. Pain 153, 1798–1806. https://doi.org/10.1016/j.pain.2012.03.026 (2012).
Hysing, M. et al. Trajectories of sleep problems from adolescence to adulthood. Linking two population-based studies from Norway. Sleep Med. 75, 411–417. https://doi.org/10.1016/j.sleep.2020.08.035 (2020).
Afolalu, E. F., Ramlee, F. & Tang, N. K. Y. Effects of sleep changes on pain-related health outcomes in the general population: A systematic review of longitudinal studies with exploratory meta-analysis. Sleep Med. Rev. 39, 82–97. https://doi.org/10.1016/j.smrv.2017.08.001 (2018).
Valrie, C. R., Bromberg, M. H., Palermo, T. & Schanberg, L. E. A systematic review of sleep in pediatric pain populations. J. Dev. Behav. Pediatr. 34, 120–128. https://doi.org/10.1097/DBP.0b013e31827d5848 (2013).
Konning, A., Rosenthal, N., Brown, D., Stahlschmidt, L. & Wager, J. Severity of chronic pain in German adolescent school students: A cross-sectional study. Clin. J. Pain 37, 118–125. https://doi.org/10.1097/AJP.0000000000000898 (2021).
Roenneberg, T. et al. A marker for the end of adolescence. Curr. Biol. 14, R1038-1039. https://doi.org/10.1016/j.cub.2004.11.039 (2004).
Boafo, A. et al. Could long-term administration of melatonin to prepubertal children affect timing of puberty? A clinician’s perspective. Nat. Sci. Sleep 11, 1–10. https://doi.org/10.2147/NSS.S181365 (2019).
Herrero Babiloni, A. et al. Sleep and pain: Recent insights, mechanisms, and future directions in the investigation of this relationship. J. Neural Transm. 127, 647–660. https://doi.org/10.1007/s00702-019-02067-z (2020).
Palada, V., Gilron, I., Canlon, B., Svensson, C. I. & Kalso, E. The circadian clock at the intercept of sleep and pain. Pain 161, 894–900. https://doi.org/10.1097/j.pain.0000000000001786 (2020).
Ksinan Jiskrova, G., Vazsonyi, A. T., Klanova, J. & Dusek, L. Sleep quantity and problems as mediators of the eveningness-adjustment link during childhood and adolescence. J. Youth Adolesc. 48, 620–634. https://doi.org/10.1007/s10964-018-0965-8 (2019).
Li, S. X. et al. Eveningness chronotype, insomnia symptoms, and emotional and behavioural problems in adolescents. Sleep Med. 47, 93–99. https://doi.org/10.1016/j.sleep.2018.03.025 (2018).
Zhang, Y., Duffy, J. F., de Castillero, E. R. & Wang, K. Chronotype, sleep characteristics, and musculoskeletal disorders among hospital nurses. Workplace Health Saf. 66, 8–15. https://doi.org/10.1177/2165079917704671 (2018).
Jankowski, K. S. Morning types are less sensitive to pain than evening types all day long. Eur. J. Pain 17, 1068–1073. https://doi.org/10.1002/j.1532-2149.2012.00274.x (2013).
Bartel, K. A., Gradisar, M. & Williamson, P. Protective and risk factors for adolescent sleep: A meta-analytic review. Sleep Med. Rev. 21, 72–85. https://doi.org/10.1016/j.smrv.2014.08.002 (2015).
Palermo, T. M., Wilson, A. C., Lewandowski, A. S., Toliver-Sokol, M. & Murray, C. B. Behavioral and psychosocial factors associated with insomnia in adolescents with chronic pain. Pain 152, 89–94. https://doi.org/10.1016/j.pain.2010.09.035 (2011).
Bauducco, S., Richardson, C. & Gradisar, M. Chronotype, circadian rhythms and mood. Curr. Opin. Psychol. 34, 77–83. https://doi.org/10.1016/j.copsyc.2019.09.002 (2020).
Bean, D. J., Horne, J., Lee, A. C. & Johnson, M. H. Pre-sleep cognitive arousal exacerbates sleep disturbance in chronic pain: An exploratory daily diary and actigraphy study. Scand. J. Pain 21, 724–731. https://doi.org/10.1515/sjpain-2020-0185 (2021).
Whibley, D. et al. Sleep and pain: A systematic review of studies of mediation. Clin. J. Pain 35, 544–558. https://doi.org/10.1097/AJP.0000000000000697 (2019).
Shaw, T., Cross, D., Thomas, L. T. & Zubrick, S. R. Bias in student survey findings from active parental consent procedures. Br. Educ. Res. J. 41, 229–243 (2014).
Ahlborg, M., Svedberg, P., Nyholm, M., Morgan, A. & Nygren, J. M. Socioeconomic inequalities in health among Swedish adolescents—adding the subjective perspective. BMC Public Health 17, 838. https://doi.org/10.1186/s12889-017-4863-x (2017).
Official Statistics of Sweden. Reports on Statistical Co-ordination for the Official Statistics of Sweden (SCB-Tryck, 2002).
Olsson, G. & von Knorring, A.-L. Depression among Swedish adolescents measured by the self-rating scale Center for Epidemiology Studies-Depression Child (CES-DC). Eur. Child Adolesc. Psychiatry 6, 81–87 (1997).
Little, T. D. Longitudinal Structural Equation Modeling (The Guilford Press, ], 2013).
Campbell-Sills, L. et al. Validation of a brief measure of anxiety-related severity and impairment: The Overall Anxiety Severity and Impairment Scale (OASIS). J. Affect. Disord. 112, 92–101. https://doi.org/10.1016/j.jad.2008.03.014 (2009).
Anniko, M. K., Boersma, K., van Wijk, N. P. L., Byrne, D. & Tillfors, M. Development of a Shortened Version of the Adolescent Stress Questionnaire (ASQ-S): Construct validity and sex invariance in a large sample of Swedish adolescents. Scand. J. Child Adolesc. Psychiatr. Psychol. 6, 4–15. https://doi.org/10.21307/sjcapp-2018-001 (2018).
von Baeyer, C. L. Numerical rating scale for self-report of pain intensity in children and adolescents: Recent progress and further questions. Eur. J. Pain 13, 1005–1007. https://doi.org/10.1016/j.ejpain.2009.08.006 (2009).
Haugland, S. & Wold, B. Subjective health complaints in adolescence–reliability and validity of survey methods. J. Adolesc. 24, 611–624. https://doi.org/10.1006/jado.2000.0393 (2001).
Furmark, T. et al. Social phobia in the general population: Prevalence and sociodemographic profile. Soc. Psychiatry Psychiatr. Epidemiol. 34, 416–424. https://doi.org/10.1007/s001270050163 (1999).
Wurm, M., Anniko, M., Tillfors, M., Flink, I. & Boersma, K. Musculoskeletal pain in early adolescence: A longitudinal examination of pain prevalence and the role of peer-related stress, worry, and gender. J. Psychosom. Res. 111, 76–82. https://doi.org/10.1016/j.jpsychores.2018.05.016 (2018).
Barker, C., Taylor, A. & Johnson, M. Problematic pain—Redefining how we view pain?. Br. J. Pain 8, 9–15. https://doi.org/10.1177/2049463713512618 (2014).
Bastien, C. H., Vallières, A. & Morin, C. M. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2, 297–307. https://doi.org/10.1016/s1389-9457(00)00065-4 (2001).
Kanstrup, M., Holmstrom, L., Ringstrom, R. & Wicksell, R. K. Insomnia in paediatric chronic pain and its impact on depression and functional disability. Eur. J. Pain 18, 1094–1102. https://doi.org/10.1002/j.1532-2149.2013.00450.x (2014).
Morin, C. M., Belleville, G., Bélanger, L. & Ivers, H. The Insomnia Severity Index: Psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep Med. 34, 601–608. https://doi.org/10.1093/sleep/34.5.601 (2011).
Chung, K. F., Kan, K. K. & Yeung, W. F. Assessing insomnia in adolescents: Comparison of Insomnia Severity Index, Athens Insomnia Scale and Sleep Quality Index. Sleep Med. 12, 463–470. https://doi.org/10.1016/j.sleep.2010.09.019 (2011).
Storfer-Isser, A., Lebourgeois, M. K., Harsh, J., Tompsett, C. J. & Redline, S. Psychometric properties of the Adolescent Sleep Hygiene Scale. J. Sleep Res. 22, 707–716. https://doi.org/10.1111/jsr.12059 (2013).
Wolfson, A. R. et al. Evidence for the validity of a sleep habits survey for adolescents. Sleep 26, 213–216. https://doi.org/10.1093/sleep/26.2.213 (2003).
Roenneberg, T., Pilz, L. K., Zerbini, G. & Winnebeck, E. C. Chronotype and social jetlag: A (self-) critical review. Biology 8, 54. https://doi.org/10.3390/biology8030054 (2019).
Nylund-Gibson, K., Gruimm, R., Quirk, M. & Furlong, M. A latent transition mixture model using the three-step specification. Struct. Equ. Model. 21, 1–16 (2014).
Wickrama, K. A. S., Lee, T. K., Walker O’Neal, C. & Lorenz, F. O. Higher-order Growth Curves and Mixture Modeling with Mplus: A Practical Guide (Routledge, 2016).
Berlin, K. S., Parra, G. R. & Williams, N. A. An introduction to latent variable mixture modeling (part 2): Longitudinal latent class growth analysis and growth mixture models. J. Pediatr. Psychol. 39, 188–203. https://doi.org/10.1093/jpepsy/jst085 (2014).
Jung, T. & Wickrama, K. A. S. An introduction to latent class growth analysis and growth mixture modeling. Soc. Pers. Psychol. Compass 2, 302–317 (2008).
Enders, C. K. Applied Missing Data Analysis (The Guilford Press, 2010).
McBeth, J., Wilkie, R., Bedson, J., Chew-Graham, C. & Lacey, R. J. Sleep disturbance and chronic widespread pain. Curr. Rheumatol. Rep. 17, 469. https://doi.org/10.1007/s11926-014-0469-9 (2015).
Junge, T., Wedderkopp, N., Boyle, E. & Kjaer, P. The natural course of low back pain from childhood to young adulthood—A systematic review. Chiropr. Man. Ther. 27, 10. https://doi.org/10.1186/s12998-018-0231-x (2019).
Sivertsen, B., Harvey, A. G., Pallesen, S. & Hysing, M. Trajectories of sleep problems from childhood to adolescence: A population-based longitudinal study from Norway. J. Sleep Res. 26, 55–63. https://doi.org/10.1111/jsr.12443 (2017).
Buenaver, L. F. et al. Evidence for indirect effects of pain catastrophizing on clinical pain among myofascial temporomandibular disorder participants: The mediating role of sleep disturbance. Pain 153, 1159–1166. https://doi.org/10.1016/j.pain.2012.01.023 (2012).
Warfield, A. E., Prather, J. F. & Todd, W. D. Systems and circuits linking chronic pain and circadian rhythms. Front. Neurosci. 15, 705173. https://doi.org/10.3389/fnins.2021.705173 (2021).
Finan, P. H., Buenaver, L. F., Coryell, V. T. & Smith, M. T. Cognitive-behavioral therapy for comorbid insomnia and chronic pain. Sleep Med. Clin. 9, 261–274. https://doi.org/10.1016/j.jsmc.2014.02.007 (2014).
Tang, N. K. et al. Nonpharmacological treatments of insomnia for long-term painful conditions: A systematic review and meta-analysis of patient-reported outcomes in randomized controlled trials. Sleep 38, 1751–1764. https://doi.org/10.5665/sleep.5158 (2015).
Zerbini, G. & Merrow, M. Time to learn: How chronotype impacts education. PsyCh J. 6, 263–276. https://doi.org/10.1002/pchj.178 (2017).
Jansson-Frojmark, M., Danielsson, K., Markstrom, A. & Broman, J. E. Developing a cognitive behavioral therapy manual for delayed sleep-wake phase disorder. Cogn. Behav. Ther. 45, 518–532. https://doi.org/10.1080/16506073.2016.1207096 (2016).
Sijbrandij, J. J. et al. Variance constraints strongly influenced model performance in growth mixture modeling: A simulation and empirical study. BMC Med. Res. Methodol. 20, 276. https://doi.org/10.1186/s12874-020-01154-0 (2020).
Huguet, A. & Miro, J. The severity of chronic pediatric pain: An epidemiological study. J. Pain 9, 226–236. https://doi.org/10.1016/j.jpain.2007.10.015 (2008).
Bonvanie, I. J., Oldehinkel, A. J., Rosmalen, J. G. & Janssens, K. A. Sleep problems and pain: A longitudinal cohort study in emerging adults. Pain 157, 957–963. https://doi.org/10.1097/j.pain.0000000000000466 (2016).
Chehri, A., Goldaste, N., Ahmadi, S., Khazaie, H. & Jalali, A. Psychometric properties of insomnia severity index in Iranian adolescents. Sleep Sci. 14, 101–106. https://doi.org/10.5935/1984-0063.20200045 (2021).
Gerber, M. et al. Validation of the German version of the insomnia severity index in adolescents, young adults and adult workers: Results from three cross-sectional studies. BMC Psychiatry 16, 174. https://doi.org/10.1186/s12888-016-0876-8 (2016).
Acknowledgements
This study was made possible by access to data from the Three Cities Study, a longitudinal research program at the department of Law, Psychology and Social work at Örebro University, Sweden. The research program was supported by a grant from the Swedish research council for sustainable development (Formas), the Swedish research council for health, work life and welfare (Forte), The Swedish Research Council (VR) and Sweden’s innovation agency (Vinnova) (grant number 2012-65). The program was approved by the regional ethics board of Uppsala (nr. 2013/384). All data collection was carried out in accordance with the ethical principles of the Declaration of Helsinki. Special thanks the schools and adolescents who participated in the project. Additional thanks to Katja Boersma, PhD, project principal investigator, and Liesbet Goubert, PhD, for initial input on the study’s focus. Additional thanks also to Brittany Evans, PhD, for input and help with methodological and statistical queries.
Funding
Open access funding provided by Örebro University.
Author information
Authors and Affiliations
Contributions
All authors made a substantial, direct, and intellectual contribution to the manuscript. T.A. conceived the idea behind the study. All authors contributed to the conceptual development of the study. T.A. and S.B. handled the raw dataset. Statistical analyses were performed by T.A. All authors contributed to the interpretation of the results. T.A. wrote the manuscript and prepared figures and tables, with substantive input from M.S., S.B., M.J.F. and J.P. All authors reviewed the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Arnison, T., Schrooten, M.G.S., Bauducco, S. et al. Sleep phase and pre-sleep arousal predicted co-developmental trajectories of pain and insomnia within adolescence. Sci Rep 12, 4480 (2022). https://doi.org/10.1038/s41598-022-08207-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-08207-y
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.