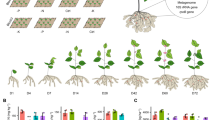
Abstract
Flooding is one of the major abiotic stresses for vegetable production in Florida. Hydroponic and pot trials were conducted with snap bean to evaluate the effects of oxygen fertilization on the biochemical and physiological status of flooded snap bean plants. There were three treatments in the hydroponic trials were: (1) flooded (control), (2) bubble aeration with ambient air, and (3) hydrogen peroxide (H2O2) applied at the beginning of the trial. Plant health was evaluated by determining nitrogen (N) and phosphorus (P) uptake rates. The greenhouse pot trials were used to quantify the effects of three different application rates of solid oxygen fertilizers as calcium peroxide (CaO2) and magnesium peroxide (MgO2). The results showed that plant N and P uptake rates were significantly greater (p < 0.05) with H2O2 than without H2O2. The N uptake rates with H2O2 were like that of those with bubbling. The uptake rate of NH4+ was significantly greater than that of NO3− with the bubbling and H2O2 conditions, but the uptake rate of NO3− was significantly greater than that of NH4+ in the flooding condition. The plant height, leaf greenness, shoot biomass, and yield were all significantly greater with CaO2 or MgO2 than without either solid oxygen fertilizer. The minimum damage of flooded snap bean was found with 2 g CaO2 or 4 g MgO2 per pot. These results indicated that oxygen fertilization may potentially improve yield of flooded snap bean plants.
Similar content being viewed by others
Introduction
Florida ranks first in acreage and total value of snap beans (Phaseolus vulgaris) production, but snap bean productivity is often greatly reduced by heavy rain events resulting from hurricanes and tropical storms. Snap bean is susceptible to flooding and suffers from hypoxic stresses, which results in growth suppression and yield reduction1. According to Holbrook and Zwieniecki2, the primary cause of hypoxic stress is oxygen deficiency in the soil; the oxygen diffusion coefficient is 10,000 times greater in the air (2.14 × 10–1 cm2 s−1) than in water (1.97 × 10–5 cm2 s−1). For most plant species3, oxygen deficiency occurs in the root zone soil with dissolved O2 concentrations less than 2 mg L−1. Thus, snap bean plants in flooded fields receive insufficient bioavailable O2 for normal root metabolism and suffer from waterlogging damage.
In hypoxia, anaerobic metabolism is activated, and oxidative phosphorylation is stopped. The ATP biosynthetic rate derived from glycolysis is low4,5,6,7. In the Krebs cycle, due to the lack of bioavailable O2 as the final electron acceptor, the intermediates build-up, NAD(P)+ levels decrease, pyruvate accumulates, and ATP concentrations decline. These changes negatively affect plant metabolism, including uptake of nutrients such as nitrogen (N) and phosphorus (P) and their assimilation3,8. In addition, plants suffer from ethanol accumulation and toxins formed in anaerobic metabolism9.
Alcohol dehydrogenase (ADH) is a well-studied enzyme in plants and is inducible in roots upon exposure to hypoxic or anaerobic conditions. When the oxygen bioavailability in the root zone is low, ADH activity increases significantly, improving the plant’s tolerance to hypoxia or anoxia10,11. ADH activity is considered essential for the survival of plants in hypoxic or anaerobic conditions12. Thus, ADH activity in root tips of flooded plants is used as an indicator of potential tolerance to flood and flooding severity.
Oxygen fertilization of the root zone is a potential method to mitigate the damage from flooding stress. There are two types of oxygen fertilizers: solid oxygen fertilizers (SOF), such as CaO2 and MgO2, and liquid oxygen fertilizers, such as hydrogen peroxide (H2O2)13,14. The solid oxygen fertilizers listed here release bioavailable oxygen slowly, i.e., slow-release oxygen fertilizer, whereas the liquid oxygen fertilizer, H2O2 liberates bioavailable oxygen rapidly and is considered as a fast oxygen fertilizer. Hydrogen peroxide can be decomposed biologically by the enzyme catalase (EC 1.11.1.16). The decomposition of H2O2 releases 0.5 mol of bioavailable O2 per mole H2O2 as shown in the equation15:
In flooded soil, solid oxygen compounds (e.g., CaO2, MgO2) break down to H2O2, which then provides bioavailable oxygen to the rhizosphere, as shown in the equation1,13:
Thus, soil amended with insoluble solid oxygen fertilizers may potentially reduce hypoxic stress in the root zone caused by flooding.
The objectives of this study were to (1) quantify the oxygen bioavailability dynamics of hydroponic solution with H2O2 application, (2) determine the effects of oxygen fertilizer application on N and P uptake by flooded snap bean plants, and (3) evaluate the effect of slow-release solid oxygen fertilizers at different rates on growth and yield of flooded snap bean.
Results
Effects of H2O2 application on NH4 +, NO3, and P uptake by flooded snap beans in hydroponic solution
In the hydroponic trial 1–1, “flooding” is defined as plants grown in a solution that receives neither aeration nor application of H2O2. In all the treatments, the concentrations of NO3− and P in the nutrient solution declined over time (Fig. 1), which indicated NO3− and P were taken up by the plant. There were differences in the nutrient uptake rates between the control and either aeration or H2O2 application. However, there was not any significant difference in the uptake rate of NO3− between the treatments of H2O2 and aeration (Fig. 2). The NO3 uptake rate was approximately 50% lower with flooding than with the aeration or H2O2 treatment. Phosphorus showed a similar trend as NO3−; there was no significant difference between the H2O2 and aeration treatments, but the flooding control had significantly lower P uptake rate (Fig. 2) than each of the H2O2 and aeration treatments. The concentration of dissolved oxygen (DO) was not significantly different between the H2O2 and aeration treatments in the 10 h experiment (Fig. 3). The DO level of the control was significantly lower than each of the H2O2 and aeration treatments after 2 h. This result also indicated that the H2O2 application had a slightly better effect than aeration in oxygenating the hydroponic solution.
The dynamic changes of NO3− (I) and P (II) concentrations in the measuring solution with three treatments over time: (1) flood without bubbling or H2O2 application after pre-set time for the measurement (approximately 100 μM DO level); (2) aeration (bubbling, 250 μM DO); and (3) application of 529 μM H2O2. Different letters at the same time-point indicate significant differences based on the Tukey’s Honest Significant Difference (HSD) test (p ≤ 0.05).
The cumulative uptake of NO3− (I) and P (II) with three treatments for 10 h: (1) flood (approximately 100 μM DO level at 0 h); (2) aeration (bubbling, 250 μM DO); and (3) application of 529 μM H2O2. The bars with different letters are significantly different based on the Tukey’s Honest Significant Difference (HSD) test (p ≤ 0.05).
The dissolved oxygen (DO) concentrations with three treatments for 10 h: (1) flood without aeration or H2O2 application); (2) aeration (bubbling, 250 μM DO); and (3) application of 529 μM H2O2. The bars with different letters are significantly different based on the Tukey’s Honest Significant Difference (HSD) test (p ≤ 0.05).
In trial 1–2, the plant took up NH4+ first in the H2O2 and aeration treatments, whereas it took up NO3− first in the flooding treatment. For the flooding treatment, the DO levels declined from 3 mg L−1 to approximately 0.01 mg L−1 from 0 to 10 h, the NO3− and NH4+ concentrations reduced with time (Fig. 4). The depletion of NO3− was faster than that of NH4+ from 0 to 8 h, and the NH4+ concentration remained greater than that of NO3−. After 10 h, the NO3− and NH4+ concentrations were not significantly different. However, in the H2O2 and aeration treatments (Fig. 4), the concentration of NH4+ decreased faster than that of NO3− during 0 to 8 h, and the concentration of NO3− remained greater than that of NH4+. The DO levels of the H2O2 treatment remained greater than 8 mg L−1 during 0 to 10 h and declined to 3 mg L−1 at 20 h, which indicated that H2O2 had to be applied every 20 h to maintain a satisfactory DO concentration.
The dynamic changes for NO3−, NH4+, and DO concentrations in containers with three conditions. I = aeration condition, II = flooding conditions (starting at 3 mg L−1 DO level, without any oxygen supplement) for 20 h, and III = flooding conditions with 529 μM H2O2 at 0 h. Ammonium = NH4+ concentration, Nitrate = NO3− concentration, Oxygen = DO concentration. The concentration readings were taken every 10 min.
For trial 1–3, the DO level in the solution was 8 mg L−1 at the beginning, which was air saturated. The DO levels were consistently and significantly greater with H2O2 (Fig. 5) than with aeration (8 mg L−1) during 0 to 20 h. However, the DO level dropped to approximately 0.01 mg L−1 after 20 h, which indicated a hypoxic stress occurred before the beginning of day 2. At the beginning of day 2 before H2O2 was applied, the uptake rate of NO3− was significantly greater than that of NH4+ which was also observed in the flooding treatment in trial 1–2 (Fig. 4). Nitrate contains 3 atoms of oxygen and can provide oxygen served as the terminal electron acceptor in cellular respiration, but ammonium does not contain any oxygen. The reversal of this trend occurred 1 h after the H2O2 was applied at the beginning of day 2 and occurred 3 h after the H2O2 was applied on day 3.
The dynamic changes of NO3−, NH4+, and DO concentrations in the container for 96 h. The solution in the container was O2 aerated at 0 h, and the 529 μM H2O2 was applied at 48 h. The 5 mg L−1 of NO3− and NH4+ were applied every 24 h. Ammonium = NH4+ concentrations, Nitrate = NO3− concentrations, Oxygen = DO concentrations. The concentration readings were taken once every 10 min.
Effects of SOF application on flooded plants in containers
For trial 2, the seedlings with SOF application had significantly more units of soil plant analysis development (SPAD) readings than those without SOF (Table 1) 2 days after flooding. The SPAD reading is an indicator of leaf greenness related to chlorophyll content in leaves. Across the different CaO2 rates, the SPAD readings were lower with 1 g CaO2 than with higher CaO2 rates. Application rates of MgO2 were not significant in leaf greenness. Furthermore, the differences in the SPAD readings of the flooded plants were also significant greater with each of 2 g, 4 g CaO2, and 8 g MgO2 than without applying peroxide. However, the plants without flooding had significantly greater SPAD readings with each of 2 g, 4 g CaO2, and 4 g, 8 g MgO2 than with either of 1 g CaO2 or no peroxide. The readings with 1 g CaO2 were significantly greater than the control without oxygen fertilization. No significant difference was found among 2 g and 4 g CaO2 and all rates of MgO2 (Table 1).
The flooded plants were significantly taller with CaO2 or MgO2 than without peroxide application. Plant height without flooding was significantly taller with oxygen fertilizer than without oxygen fertilizer. The non-flooded plants with 4 g CaO2 and 4 g MgO2 had the highest height (Table 1).
Shoot biomass of flooded plants was greater with CaO2 or MgO2 than without SOF (Table 1). In the non-flooding treatment, only those with 2 g MgO2 and without SOF had the lowest plant biomass (Table 1).
The pod yields of flooded snap bean were greater with SOF treatments than the control (Fig. 6). The 2 g CaO2 and 8 g MgO2 treatments had almost 50% greater yield than the control, which was significantly greater than that of rest of the treatments (Fig. 6).
Effects of flooding and applications of CaO2 and MgO2 on the yields (I) and ADH (II) of snap bean in pots. The horizontal axis shows different amount of CaO2 and MgO2 applied. CK1 represents the group without CaO2 and MgO2 with flooding, CK2 represents the group without CaO2 and MgO2 and without flooding. The bins with different letters are significantly different at p ≤ 0.05.
As a bio-indicator, ADH activity is used to evaluate the effects of different amounts of SOF on the growth and development of flooded plants. The ADH activities were significantly lower with all the SOF treatments than with the flooding treatment. For example, ADH activity was in a range from 44 nmol NADH per minute per mg protein for the flooded snap bean plants without oxygen fertilization to 5 nmol NADH per minute per mg protein for those flooded plants with 4 g CaO2 (Fig. 6). The lower ADH activities indicated the SOF treatments had much less flooding stress compared to the flooding treatment. the treatments of 2 g CaO2, 4 g CaO2, and 8 g MgO2 had significantly lower ADH activities than the other treatments (Fig. 6), which indicated those treatments had even less or no flooding stress compared to the other treatments. This result provided physiologic evidence for evaluation of the potential of SOF on mitigating the flooding stress.
Discussion
In trial 1, the H2O2 treatment had a positive effect on the N and P uptake of the plants in hypoxic stress. The lower uptake rate of flooded plants was possibly caused by ATP shortage, resulting from flooding-induced oxygen deficiency and anaerobic respiration. Thus, the application of H2O2, supplying O2 directly, was a potential method to alleviate the hypoxia damage. This finding is supported by other studies. Jampeetong and Brix16 found that Salvinia natans plants supplied with NO3− grew normally in a hypoxic solution but were greatly inhibited when only NH4+ was supplied as the nitrogen source.
In trial 2, the results showed that the application of SOFs enhanced the growth and development of snap bean plants in flooding stress. Lakitan17 reported that the total yield and survival of snap beans were susceptible to flooding imposed after reproductive development had begun (28 DAP), and flooding imposed at later growth (> 28 DAP) significantly reduced the total yields. Few plants survived, and there was no measurable yield when flooding was imposed at 36 DAP. More trials using SOF with different flood durations need to be conducted at various plants growth stages in the future.
After uptake, nitrogen assimilation follows a few steps including from the reduction of NO3− to NH4+ and the subsequent incorporation of NH4+ into amino acids18. NO3− reduction is catalyzed by the enzyme nitrate reductase (NR, EC 1.6.6.1-3). Nitrate reductase reduces NO3− to NO2−, which is then transferred to the chloroplast to be reduced to NH4+ by nitrite reductase (NiR, EC 1.6.6.4). After that, NH4+ is used to biosynthesize glutamine by glutamine synthase (GS, EC 6.3.5.1)19. Nitrate reductase is a key enzyme tightly regulated by many environmental factors, such as flooding20,21. Anoxia and hypoxia increase NR activity, and NO3− without available molecular oxygen is used to oxidize organic molecules to obtain energy, a phenomenon called “nitrate respiration”22. That study may support the result that snap bean absorbs more NO3− than NH4+ in hypoxia because the increased NR and NiR activity would promote NO3− uptake and assimilation. Early trials used labeled15 NO3− to monitor nitrate assimilation during anaerobic germination of rice and showed that 15N was incorporated into amino acids23. The results confirmed that exogenous NO3− supplied during oxygen deficiency had been assimilated by plants23. Even if NO3− supply to flooded crops increases the percentage of survival, the biochemical and molecular mechanisms underneath its positive effects are not fully understood. Thus, future research will be needed to invest the mechanisms for plant surviving from flooding with NO3− amendment.
The results of this study showed the trend that the supplement of NO3− may enhance the NH4+ uptake (Fig. 4). For example, ample supply of NO3− was able to alleviate the consequences of root anoxia or hypoxia in barley9. It has been hypothesized that NO3− can serve as an alternative terminal electron acceptor in hypoxic or anoxic conditions8. Nitrate may alleviate the consequences of anoxia in other ways. As an ample supply of NO3− can directly induce nitrate reductase activity24, whereby NADH is diverted from the reduction of acetaldehyde to ethanol. The accumulation of injurious ethanol concentrations in the cells is delayed. Also, NO3− may influence rhizosphere redox potential and may act as an oxygen source through influx into O2-deficient roots or by uptake in partially aerated shallow roots9.
The results of this study indicated that the peroxides, CaO2 and MgO2 have the potential to mitigate the flooding damage in snap beans. Several researchers have observed that prolonged flooding causes a cessation of root and shoot growth, wilting, decreased nutrient uptake, and often plant death25. According to Lakitan17 flooding on different growth stages of snap bean had different effects on growth and yield, and there is evidence that longer flooding duration would result in lower survival and yields17. Thus, further studies are warranted to assess the effects of solid oxygen fertilizations on reducing flooding stress, and survival rates of snap beans at different growth stages and with different duration of flooding treatments.
Conclusions
Snap bean is susceptible to hypoxic stress and often suffers from flooding, particularly in the hurricane and tropical storm seasons. We conducted both hydroponic and pot trials to investigate how oxygen fertilization affects uptake rates of NH4− and NO3− and P, growth, and yields of flooded snap bean. Three peroxides, including H2O2, CaO2, and MgO2, were evaluated. The findings from this study indicated oxygen fertilization had the potential to minimize flooding damage to flooded snap bean. Hydrogen peroxide application significantly increased the dissolved oxygen level and the uptake rate of NH4+, NO3− and P by snap bean. Snap bean had an uptake preference for of NH4+-N with sufficient bioavailable oxygen. However, NO3-N is preferred in flooding conditions. The right dosage of solid oxygen fertilizer of 2 g CaO2 or 8 g MgO2 had the greatest yield among all flooded snap bean plants. Thus, both CaO2 and MgO2 oxygen fertilizations had the potential to keep absorbing nutrients such as N and P by flooded snap bean plants, reduce flooding damage, and minimize the yield loss. These novel methods of oxygen fertilization may mitigate economic loss and benefit agriculture often facing different hypoxic stresses.
Materials and methods
Plant materials and soil
Snap bean (P. vulgaris. cv. ‘Bronco’) is a primary vegetable crop and widely cultivated in USA. The ‘Bronco’ seeds are commercially available for research and breeding and were purchased from a seed company, HOSS TOOLS located in Norman Park, GA (GPS coordinates: Latitude: 31.2462 and Longitude -83.6549) before the study was started in spring 2015. Miracle-Gro® garden soil was procured from Lowes in Gainesville, FL.
In Trial 1, snap bean seeds were soaked in 0.15% H2O2 for 24 h and rinsed with deionized (DI) water for 5 times, and then were placed into a 12-cm petri dish with moistened filter paper and sealed inside a zip-lock plastic bag and incubated at 33 °C overnight. After seedling emergence, each seedling was transplanted into a 5.1-cm diameter plastic mesh basket. The baskets (Net Pots Net Cups, Heavy Duty Plastic Net Pot with Wide Rim Design) were placed in an aeroponic system to allow for rapid root growth and development. The aeroponic system consisted of a tank half-filled with 10% Hoagland solution26, a misting pump, tubing, and three sprinklers that continuously misted the roots. After two weeks, the seedlings with three true leaves were transferred from the aeroponic system to a hydroponic system where the roots were submerged in a 1000 mL 0.2 mM CaSO4 solution for measurement use. The use of 0.2 mM CaSO4 solution was to protect the membranes, since calcium is essential for the intactness and selectivity of biological membranes27.
In trial 2, two sizes of pots were used for different purposes. The smaller pots with 7.6 cm in diameter × 8.9 cm in height was for seed germination. The larger pots with 15 cm in diameter × 16.5 cm in height for production. Miracle-Gro® garden soil was used, and the fertilizers were applied per pot as follows: NH4NO3, 2.92 g; triple superphosphate, 1.65 g; potassium chloride, 0.44 g per pot.
Experimental design
The trials were all conducted in the Horticultural Sciences Department, University of Florida/IFAS, Gainesville, Florida. The hydroponic trials had three sub-trials. Trial 1–1 and 1–2 had the same treatments with four replicates. The trial 1–1 was conducted in an environmental-controlled growth chamber with a temperature range of 23–27 °C, 16-h daylight and 8-h dark. The trial 1–2 and trial 1–3 were conducted in a standard greenhouse with a temperature range of 23–27 °C. The trial 2 was conducted in a high tunnel under a natural atmosphere. The treatments were imposed by (1) flooding control without bubbling or applying H2O2 after the pre-set time (approximately 100 μM DO level at the beginning, no oxygen supplement), (2) aeration (continuous oxygen supply with an air pump), and (3) H2O2 (application of 529 μM H2O2 at 0 h). The growth medium was 1 L DI water with 0.22 g L−1 KH2PO4, 0.373 g L−1 KNO3, 0.02 g L−1 CaSO4, 0.012 gL−1 MgSO4, 0.29 mg L−1 H3BO3, 0.18 mg L−1 MnCl2∙4H2O, 0.02 mg L−1 ZnSO4∙7H2O, 0.008 mg L−1 CuSO4∙5H2O, 0.002 mg L−1 H2MoO4∙H2O, 0.65 mg L−1 Ethylenediaminetetraacetic acid (EDTA), and 0.62 mg L−1 FeSO4∙7H2O. For trial 1–1, 2 mL samples were collected from the center of pots every 2 h for 10 h. The samples were analyzed for ortho-P (EPA Method 365.3), and NO3− (EPA Method 352.1) using a Seal AQ-2 discrete analyzer (2006 SEAL Analytical Ltd. Mequon, WI) (Fig. 1).
For trials 1–2, Neulog™ Dissolved oxygen sensors, Neulog NO3− sensors, and Neulog NH4+ sensors were set up for each container to monitor concentration changes (Fig. 7). Those three sensors were combined and installed in each container, and aluminum foil was used to hold the sensors and plants and block the sunlight. The data were monitored every 10 min for 20 h and recorded with NeuLog™ Dissolved Oxygen sensor, Nitrate logger sensors, and Ammonium logger sensors (Neulog brand dissolved oxygen sensor, EISCO Scientific, Rochester, NY, USA).
Trials 1–3 used the same sensors and settings as trials 1–2 to monitor the consistently dynamic nitrogen changes for 96 h (96 h). The growth medium was 1 L DI water with the same ingredients as trial 1–1 but used 0.57 g L−1 NH4H2PO4 and 1.8 g L−1 KNO3 instead of 0.22 g L−1 KH2PO4 and 0.373 g L−1 KNO3. Additionally, 0.57 g L−1 NH4H2PO4 and 1.8 g L−1 KNO3 were applied every 24 h to provide 5 mg L−1 of NO3− and 5 mg L−1 of NH4+. The data were recorded every 30 min for 4 days (96 h).
In trial 2, a completely randomized design was used with 4 replications. There were two SOFs at different rates, i.e., CaO2 at 0, 1, 2, and 4 g per pot and MgO2 at 0, 2, 4, and 8 g per pot. The seeds were planted in 7.6 cm pots for two weeks and were transferred to the 15.2-cm pots (internal volume: 2,622 cm3) after germination. The SOF, CaO2 or MgO2 was mixed with the soil (approximate 2,000 cm3 per pot), the mixed soil was added into the 15.2-cm pots, and then the seedlings were transplanted into the pots, respectively. One seedling was planted per pot. The fertilizer rates were based on the vegetable production handbook of Florida28. The N, P and K application rates were 112 kg ha−1, 134 kg ha−1 and 134 kg ha−1 for the whole season. After one week, both groups with CaO2 and MgO2 were each divided into two subgroups. One subgroup was individually flooded in 18.9 L buckets filled with water to simulate the flooding conditions for two days, while another subgroup was not flooded.
The data of leaf greenness representing leaf chlorophyll content determined by a SPAD meter (Knoica-Minolta, Osaka, Japan), plant height, ADH activities, and shoot biomass were measured when the flooded plants started wilting. To collect yield data, this trial was identically repeated and the yields were measured 55 DAP.
Root ADH activity
In trial 2, two days after flooding, approximately 1 g of roots (4–5 cm from the tips) was harvested per treatment and placed in a container filled with liquid nitrogen. Root ADH activity was determined according to a modification of the method30 as follows. The root tips (1 g) were homogenized in an extraction solution buffer (10 ml) composed of 7.88 g L−1 Tris–HCl (pH 8.0), 0.29 g L−1 EDTA, 0.5 μg L−1 DTT, and 0.08 mg L−1 β-mercaptoethanol. Then, the extraction solution of the ADH enzyme was centrifuged at 4 °C at 15,000 rpm for 10 min. 100 μL of enzyme solution was added to 900 μL of a reaction solution composed of 7.88 g L−1 Tris–HCl (pH 9.0), 0.29 g L−1 EDTA, and 0.66 g L−1 NAD. The mixture was incubated in 1.5–mL microcentrifuge tubes in a water bath at 30 °C for 3 min. To start the measurable reaction, 100 µL of 100% ethanol was added to the 900 µL reaction mixture. Measurements were taken after 15 s, and the absorbance at A340 was recorded every 15 s for two minutes. After a reaction time of 1 min in the cuvette, reading at 340 nm was made with a spectrophotometer (Eppendorf BioSpectrometer®, Hauppauge, NY.USA) to determine the concentration of NADH. The activity was calculated using a value of 6.22 mmol−1 cm−1 as the molar extinction coefficient of NADH at 340 nm31.
Statistical analysis
All statistical analyses were performed using R Studio statistical software (RSTUDIO, INC., Boston, MA). One-way ANOVA was applied to test the significance of SPAD readings, plant heights, shoot biomass, yield, and ADH activities. Comparisons of means for significant effects were determined using Tukey HSD tests. The data collected were all subjected to statistical analysis through ANOVA, and the value of (HSD at 5% was calculated to compare every two means. Differences among the means and correlation coefficients were deemed statistically significant at the p < 0.05 level.
Permission statement
All the experiments on plants, including the collection of snap bean materials, ‘Bronco’ used as the cultivar, were strictly performed in accordance with relevant guidelines, regulations, and legislation.
References
Wadman-van Schravendijk, H. & van Andel, O.M. Interdependence of growth, water relations, and abscisic acid level in Phaseolus vulgaris during waterlogging. Physiol. Plant. 63, 215–220 (1985).
Holbrook, N. M. & Zwieniecki, M. A. Plant biology: water gate. Nature 425, 361–361 (2003).
Ma, M., Cen, W., Li, R., Wang, S. & Luo, J. The molecular regulatory pathways and metabolic adaptation in the seed germination and early seedling growth of rice in response to low O2 stress. Plants 9, 1363 (2020).
Zhang, Y., Liu, G., Dong, H. & Li, C. Waterlogging stress in cotton: Damage, adaptability, alleviation strategies, and mechanisms. Crop J. 9, 257–270 (2021).
Li, Y. et al. Effect of soil aeration on root morphology and photosynthetic characteristics of potted tomato plants (Solanum lycopersicum) at different NaCl salinity levels. BMC Plant Biol. 19, 1–15 (2019).
Tian, L. X., Zhang, Y. C., Chen, P. L., Zhang, F. F., Li, J., Yan, F., & Feng, B. L. (2021). How does the waterlogging regime affect crop yield? A global meta-analysis. Front. Plant Sci., 12.
Zabalza, A. et al. Regulation of respiration and fermentation to control the plant internal oxygen concentration. Plant Physiol. 149, 1087–1098 (2009).
Ma, Z., Bykova, N. V. & Igamberdiev, A. U. Cell signaling mechanisms and metabolic regulation of germination and dormancy in barley seeds. The Crop J. 5, 459–477 (2017).
Drew, M.C. Oxygen deficiency and root metabolism: Injury and acclimation under hypoxia and anoxia. Ann. Rev. Plant Physiol. Plant Mole. Biol. 48, 223–250 (1997).
Kato-Noguchi, H. Evaluation of the importance of lactate for the activation of ethanolic fermentation in the lettuce in anoxia. Physiol. Plant. 109, 28–33 (2000).
Morimoto, K. & Yamasue, Y. Differential ability of alcohol fermentation between the seeds of flooding-tolerant and flooding-susceptible varieties of Echinochloa crus-galli. Weed Biol. Manag. 7, 62–69 (2007).
Johnson, J. R., Cobb, B. G. & Drew, M. C. Hypoxic induction of anoxia tolerance in roots of adh1 null Zea mays L. Plant Physiol. 105, 61–67 (1994).
Liu, G. D. & Porterfield, D. M. Oxygen enrichment with magnesium peroxide for minimizing hypoxic stress of flooded corn. J. Plant Nutr. Soil Sci. 177, 733–740 (2014).
Liu, G.D., Li, Y.C., Migliaccio, K., Olczyk, T. & Alva, A. Oxygen fertilization improves growth and nitrogen-use efficiency of flooded Italian basil (Ocimum Basilicum L.). Intl. J. Vegs. Sci. 19, 217–227 (2013).
Gil, P. M., Ferreyra, R. E., Barrera, C. M., Zuniga, C. E. & Gurovich, L. R. Effects of injecting hydrogen peroxide into heavy clay loam soil on plant water status, net CO2 assimilation, biomass, and vascular anatomy of avocado trees. Chil. J. Agric. Res. 69, 97–106 (2009).
Jampeetong, A. & Brix, H. Oxygen Stress in Salvinia natans: Interactive effects of oxygen availability and nitrogen source. Environ. Exp. Bot. 66, 153–159 (2009).
Lakitan, B., Wolfe, D. W. & Zobel, R. W. Flooding affects snap bean yield and genotypic variation in leaf gas exchange and root growth response. J. Am. Soc. Hort. Sci. 117, 711–716 (1992).
Masclaux-Daubresse, C. et al. Nitrogen uptake, assimilation and remobilization in plants: Challenges for sustainable and productive agriculture. Ann. Bot. 105, 1141–1157 (2010).
Stitt, M. Nitrate regulation of metabolism and growth. Curr. Opin. Plant Biol. 2, 178–186 (1999).
Wray, J. L. Molecular approaches to the analysis of nitrite assimilation. Plant Cell Environ. 11, 369–382 (1998).
Campbell, W.H. Nitrate reductase structure, function and regulation. Annu. Rev. Plant Physiol. Plant Mol. Biol. 50, 277–303 (1999).
Klok, E. J. et al. Expression profile analysis of the low-oxygen response in Arabidopsis root cultures. Plant Cell 14, 2481–2494 (2002).
Reggiani, R. & Bertani, A. Anaerobic amino acid metabolism. Russ. J. Plant Physiol. 50, 733–736 (2003).
Aslam, M. & Huffaker, R. C. Role of nitrate and nitrite in the induction of nitrite reductase in leaves of barley seedlings. Plant Physiol. 91, 1152–1156 (1989).
Schaffer, P., Andersen, B. C. & Ploetz, R. C. Responses of fruit crops to flooding. Hort. Rev. 13, 257–313 (1993).
Hoagland, R. R. & Arnon, D. I. The water culture method for growing plants without soil. Calif. Agric. Exp. Station Circ. 347, 1–32 (1950).
Lynch, J., Cramer, G. R. & Lauchli, A. Salinity reduces membrane-associated calcium in corn root protoplasts. Plant Physiol. 83, 390–394 (1987).
Mathews L. P., Peter J. D., Shinsuke A., Hugh A. S., et al. Vegetable production handbook of Florida, 2021–2022 edition. The Institute of Food and Agricultural Sciences, University of Florida. https://doi.org/10.32473/edis-cv292-2021.
Hoel, B. O. Use of a hand-held chlorophyll meter in winter wheat: Evaluation of different measuring positions on the leaves. Acta Agric. 48, 222–228 (1998).
Chung, H. J. & Ferl, R. J. Arabidopsis alcohol dehydrogenase expression in both shoots and roots is conditioned by root growth environment. Plant Physiol. 121, 429–436 (1999).
Schomburg, K. T. et al. Computational biotechnology: Prediction of competitive substrate inhibition of enzymes by buffer compounds with protein–ligand docking. J. Biotechn. 161, 391–401 (2012).
Acknowledgements
Ed Hanlon, Ashok Alva at the University of Florida, and Mary Dixon at Auburn University reviewed and helped improve the manuscript.
Author information
Authors and Affiliations
Contributions
G.D.L, D.L., A-L.P., and K.T.M. conceptualized the experiments. D.L. conducted the experiments, analyzed the data, and wrote the first draft of the manuscript. All authors contributed to editing and improving the manuscript. G.D.L finalized the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, D., Paul, AL., Morgan, K.T. et al. Effects of oxygen fertilization on damage reduction in flooded snap bean (Phaseolus vulgaris L.). Sci Rep 12, 4282 (2022). https://doi.org/10.1038/s41598-022-08165-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-08165-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.