Abstract
To compare the postoperative estimated-glomerular-filtration-rate (eGFR) and parenchymal changes between cold ischemia and zero/selective ischemia for a T1a mass. We analyzed 104 patients who underwent open partial nephrectomy with cold ischemia (53) or zero/selective ischemia (51) for T1a between 2008 and 2018 to determine postoperative renal function changes and associated factors. Postoperative renal function was expressed as (postoperative-eGFR − preoperative-eGFR)/preoperative-eGFR × 100%. Parenchymal enhancement and thicknesses of the ipsilateral kidney as tissue changes were measured on postoperative CT to identify the correlation with the renal function change. Patients with 10% or 25% decrease in eGFR were significantly more in the cold ischemia group (p = 0.032, p = 0.006). On multivariable analysis, preoperative eGFR, ischemic type, and percent change of parenchymal thickness were identified to be significantly associated with postoperative 12 months renal function (B = − 0.367, p = 0.020; B = 6.788, p = 0.042; B = 0.797, p = 0.029). Change in parenchymal thickness was negatively correlated with changes in postoperative renal function (r = − 0.277, p = 0.012). Changes in eGFR were associated with a decrease in parenchymal thickness and the type of ischemic technique. Zero/selective ischemia during partial nephrectomy may have an advantage in preserving postoperative renal function compared to cold ischemia.
Similar content being viewed by others
Introduction
For T1a renal masses, partial nephrectomy is strongly suggested. Numerous studies have stressed the quality of ischemia to maximize remaining renal function, and since then various techniques have been implemented1,2. The selective vascular clamping technique, in which the surgeon clamps the branches of renal arteries to the mass during resection, has been suggested3,4. The induction of ice or cold water was implemented to minimize renal damage during clamping of the renal hila5. In addition, partial nephrectomy without any clamping, so-called “zero ischemia” can prevent ischemic damage. The zero and selective ischemia technique would prevent changes in hyper-filtration, acceleration of nephropathy, and decrease in perfusion2,6.
Selective vascular clamping and off-clamping preserve renal perfusion of normal parenchyma but bleeding can be encountered during resection7,8. Cold ischemia technique reduces bleeding and lessens the time of renorrhaphy but can cause damage to the normal parenchyma. Dong et al. found that cold ischemia preserved ~ 7% more renal function than warm ischemia8. Bertolo et al. proved that postoperative renal function between off-clamp and on-clamp technique was significantly different at 12 and 24-months postoperatively9. And also, radiological factors relating to a renal mass, such as diameter, volume, and RENAL score, were identified to be closely related to postoperative renal function1,8,10. However, there exist only a few studies that provide a comparative analysis between the ischemic techniques.
We aimed to compare the ischemic techniques based on postoperative renal function. The two urologists provided data on radiological findings such as parenchymal thickness and parenchymal enhancement based on preoperative and postoperative computed tomography (CT) scan, and we also examined their correlation with postoperative renal function to uncover the effects of ischemia techniques on normal parenchyma11,12.
Patients and methods
Patient cohort
Our retrospective review was conducted of 189 patients who underwent open partial nephrectomy between 2008 and 2018. The institutional review board (IRB) of the Seoul National University-Seoul Metropolitan Government Boramae Medical Center approved this study (IRB No. 10-2019-47). Because this study retrospectively analyzed anonymized data, Informed consent was waived by the IRB. All study protocols were conducted in compliance with the principles of the Declaration of Helsinki guidelines.
Among the 189 patients, patients with clinical T1 renal mass, who underwent open partial nephrectomy, were considered suitable for the study. As a result, open partial nephrectomy was performed in 53 patients with cold ischemia and 51 patients with zero or selective ischemia. The patient cohort was divided into two groups: the cold ischemia group and zero/selective ischemia group. Serum creatinine was measured in the same center and eGFR was calculated by MDRD-2 (Modification of Diet in Renal Disease) equation. Postoperative eGFRs were compared to preoperative eGFR based on percent change of eGFR: (preoperative eGFR − postoperative eGFR)/preoperative eGFR × 100%. Serum creatinine was measured on the postoperative 3, 6, and 12 months after surgery. eGFR at 12-months postoperatively was considered as long-term follow-up eGFR. Since the decrease in renal function after partial nephrectomy is reported to be about 10% globally, we identified patients with a decrease of more than 10% in both groups13. And one definition of CKD progression in a KDIGO CKD guideline was described with a 25% or greater drop from baseline GFR14. Thus, patients with greater than 25% GFR decrement as a significant functional decline were also identified in both groups. The RENAL score was evaluated at preoperative CT within 3 months before surgery.
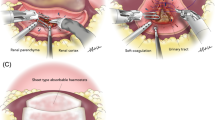
Before tumor resection, the kidneys were mobilized to expose the tumor to face the operator. Before each vessel clamping procedure, 100 mL of 20% d-mannitol was administered intravenously. The tumor was excised including the thin normal parenchyma after securing a safety margin of several millimeters around the tumor. The deep layer with the opened collecting system and blood vessels was closed, and the superficial layer with the resected renal stump was closed. For the cold ischemia technique, renal artery clamping was prepared while soaking cold saline with ice slush around the kidney. The main hilar vessels were entirely clamped and core cooling was maintained for 8 min. And the main hilar vessel was de-clamped after tumor resection and renorrhaphy. The zero ischemia technique was performed without hilar clamping or core cooling. For the selective ischemia, the only branch to the renal mass were exclusively clamped after all renal vessels were identified. We considered patients operated by selective ischemia technique and by zero ischemia techniques as the same group since the percent change of postoperative renal function at 12 months was not statistically distinct in both the groups.
Radiological findings
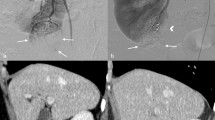
Parenchymal enhancement and parenchymal thickness were gauged on preoperative CT and postoperative CT images taken at 6 months after surgery by two different urologists15. The parenchymal thickness and parenchymal enhancement were measured at the hilar level of the ipsilateral kidney without overlapping the renal mass8. Parenchymal enhancement was calculated on the arterial phase of 3D-kidney CT. Parenchymal enhancement was normalized by defining it as the ratio of Hounsfield unit of parenchyma to that of the aorta11,12,16. The parenchymal thickness and parenchymal enhancement, measured by different urologists, were averaged.
Statistical analysis
Continuous data were expressed as means and standard deviations or median with interquartile range (IQR). Univariate and multivariable linear regression analysis were used to determine significant variables, which were correlated with postoperative eGFR. The variables with p < 0.05 on univariate analysis were dealt with on multivariate analysis and backward elimination was used. The Pearson correlation coefficients of postoperative eGFR, parenchymal enhancement, and parenchymal thickness were determined with a 95% confidence interval. A two-sided p value < 0.05 was defined as statistically significant and all data were analyzed with SPSS version 22.0.
Ethical approval
The institutional review board of the Seoul National University-Seoul Metropolitan Government Medical Center approved this study (IRB No. 10-2019-47).
Results
Of the 104 patients, 53 patients (51.0%) underwent partial nephrectomy by cold ischemia technique and 51 patients (49.0%) by zero/selective ischemia. Table 1 shows the clinicopathologic characteristics of the patients. Postoperative percent change of eGFR between the two groups demonstrated statistical difference only after 12 months (cold ischemia group vs. zero/selective ischemia group: 93.0 ± 20.8 vs. 98.4 ± 20.4%, p = 0.204; 95.1 ± 21.6 vs. 98.1 ± 14.3%, p = 0.449; and 93.5 ± 20.6 vs. 101.3 ± 15.2%, p = 0.038 at 3, 6, and 12 months after the surgery, respectively) (Fig. 1). The patients with more than 10% eGFR decrease at 12 months had a significant difference with 25 and 12 patients in the cold ischemia and the zero/selective ischemia group, respectively (p = 0.032). Eight patients with more than 25% eGFR decrease were identified only in the cold ischemia group (p = 0.006). The multivariate analyses identified that preoperative eGFR, ischemic type, and percent change of parenchymal thickness were significantly related as predictive factors of percent change of postoperative eGFR at 12 months (B = − 0.367 (− 0.598 ~ − 0.137), p = 0.020; B = 6.788 (0.982–13.789), p = 0.042; B = 0.797 (0.086–1.508), p = 0.029. respectively, Table 2). Moreover, zero/selective ischemia demonstrated a more significantly preventable effect on renal damage than cold ischemia.
Changes of renal function according to ischemia type during partial nephrectomy. (a) eGFR. Preoperative and postoperative eGFR were plotted for patients grouped according to the time. (b) Percent change of eGFR. Percent change of eGFRs were defined as (preoperative eGFR − postoperative eGFR)/preoperative eGFR × 100% and plotted for patients grouped according to the time. Number of patients with (c) > 25% eGFR decrease (p < 0.006) and (d) > 10% eGFR decrease (p = 0.032). eGFR, estimated glomerular filtration rate.
We compared the radiological variables of both groups (Table 3) to explore the relationship between renal function and radiological measurements. The difference in parenchymal thickness before and after surgery and postoperative parenchymal thickness were not significantly different between the two groups (p = 0.236, p = 0.199). However, the decrement in parenchymal thickness and changes in renal function were significantly negatively correlated with postoperative renal function (R = − 0.277, p = 0.012). There was no significant difference in parenchymal enhancement between the two groups at 12 months after surgery (p = 0.579).
Discussion
Renal ischemia types for preserving renal function in nephron sparing surgery has been a controversial topic for decades. Several factors including ischemia times of less than 25 min are known to be important for preserving renal function4. Many studies are being conducted on the effect of ischemic type on renal function. Several studies on robotic-assisted or laparoscopic partial nephrectomy have reported that techniques such as off-clamping, selective-clamping, or on-clamping do not affect functional outcomes17,18,19. On the contrary, some studies provide supporting evidence on the hypothesis that ischemia techniques such as cold ischemia, off-clamping, or selective vascular clamping are beneficial to preserve postoperative renal function1,6,20,21,22,23,24. Riccardo Bertolo et al. endeavored to compare the functional outcomes between off-clamped and on-clamped robot-assisted partial nephrectomy, and showed that the off-clamp is more favorable to preserve postoperative eGFR than clamping the renal hilum9. To preserve renal function, various ischemia techniques have been proposed, but the difference among them is not yet well understood2,18. Our results could support the hypothesis that zero/selective ischemia technique helps to preserve renal function even in T1a kidney cancer and could prevent a significant decrease (> 10% or > 25%) in renal function for some patients, which is comparable to the decrease in eGFR after radical nephrectomy25.
The main result of this study is that the zero/selective ischemia technique could be more advantageous for renal function preservation than the cold ischemia technique. The mean percent change of eGFR decrease in our study was a 6.5% decrease in cold ischemia, but a no significant decrease in zero/selective ischemia. Cold ischemia was believed to protect renal function during the longer ischemic time of up to 3 h, without causing any renal injury26. Funahashi et al. also showed that the cold ischemia technique allowed lengthening the ischemic time by up to 58 min without causing any renal injury27. However, Riccardo Bertolo et al. found that off-clamp showed a tendency towards better renal function recovery after partial nephrectomy than cold ischemia and warm ischemia technique9. Boga et al. also reviewed that impairment of renal function in selective renal artery clamping technique and off-clamping technique was less significant than on-clamping22. Our study rather argues that zero/selective ischemia technique can be regarded as superior to cold ischemia technique.
The decrement in eGFR after partial nephrectomy appeared to be affected by excising the normal functional portion of the ipsilateral kidney, ischemia-related damage, and mechanical trauma10. Volume loss is caused by surgical excision near the tumor margins. ischemia–reperfusion injury is caused by clamping of renal hila and traumatic damage can be caused by partial edema of renal parenchyma and alternation in vascularity near the mass. Since RENAL scores of both the patient cohorts have no statistical significance, the ischemia-related renal injury led to the difference in both the ischemia techniques, rather than the excision of normal parenchyma. The ischemia-related renal injury might lead to the difference in percent change of postoperative eGFR between both the ischemia techniques; cold ischemia reduces ischemia-related functional decline but zero/selective ischemia has almost no effect on adjacent normal tissues.
Many studies support the hypothesis that a postoperative decrease in renal function results from ischemic damage of normal parenchyma or volume reduction of normal tissue during partial nephrectomy7,10,13,28. Also, renal parenchymal preservation was strongly correlated with renal functional preservation1,3,8,10,29. However, Zhang et al. reported that the truncated parenchymal volume was more important than the atrophic change due to ischemia because the opposite pole volume did not decrease at 12 months after the on-clamping surgery30. Our study showed that decrement in the parenchymal thickness of normal parenchyma was negatively correlated with postoperative renal function (R = − 0.277, p = 0.012), Also, decrease in parenchymal thickness in zero/selective ischemia type was lower than in cold ischemia, although statistical significance was marginal. Since the parenchymal thickness was measured far from the tumor, we may cautiously claim that ischemic damage causes the atrophy of normal parenchyma, thereby resulting in a decline in parenchymal thickness. Zero/selective vascular clamping may help to preserve normal parenchyma and therefore provides potential benefits for functional outcomes in comparison to cold ischemia13. Matthias et al. showed that the off-clamping technique statistically marginally tended to maintain better microcirculation during partial nephrectomy than on-clamping31.
Similarly, to the outcomes of earlier studies, preoperative eGFR, RENAL score, and BMI were identified to be associated with postoperative renal function. The size was not associated with postoperative renal function in our study. The length of renal masses was so small that changes in the postoperative residual volume of the kidney probably did not significantly vary with the size32. Moreover, previous studies included patients with various sizes of renal tumors such as clinical T1b and T2 renal masses but our study concentrated on only clinical T1a renal tumors. Instead, the RENAL score was significantly associated with postoperative renal function33. Likewise, the location of renal mass had more influence on the postoperative residual volume of the kidney due to the tiny size rather than the effect of size; apparently, the postoperative renal function varied with the RENAL score. The effects of BMI on postoperative renal function were controversial, but this study supports the contention that BMI affects postoperative renal function25.
This study also has several limitations. First, it is a retrospective study and a small number of patients were included in this study. Therefore, preoperative factors such as RENAL score did not differ between the two groups, but potential confounding factors may not have been completely controlled. Second, we did not analyze the preserved parenchymal volume, which is often used as a quantitative predictor of renal function after partial nephrectomy using CT scan. However, it was reported that the factor of parenchymal thickness and the factor of preserved parenchymal volume were significantly related, and that the thickness was also associated with an ischemic time8. Third, we observed bilateral renal function by eGFR and not individual renal function by renal scintigraphy. Measurements of split renal function can compare the effects of ischemia type on the ipsilateral kidney and the contralateral kidney in a more systematic manner. This study emphasized that zero/selective ischemia type could maintains renal function more effectively than cold ischemia and drastic changes in the renal function (> 10% or > 25% decrease of eGFR) in zero/selective ischemia were significantly reduced. In the future, more number of patients can make the study extra influential and clarified. Marginal difference in parenchymal thickness, for example, might be explained more clearly.
Conclusion
Open partial nephrectomy with selective or zero ischemia may have some benefit in preserving renal function at 12 months after surgery compared to open partial nephrectomy with cold ischemia. Atrophic changes in normal parenchyma at 12 months after surgery were significantly associated with decreased renal function, but there was no significant difference in the level of atrophic changes in normal parenchyma between the two groups. Therefore, it is necessary to analyze a larger volume of patients or to study other factors such as preserved parenchymal volume for the possibility that the postoperative difference in renal function is due to the difference in the atrophic change of the parenchyma according to the ischemic type. The choice of the zero/selective ischemia technique may have potential benefits in preserving renal function at 12 months postoperatively in patients undergoing open partial nephrectomy.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Dong, W. et al. Ischemia and functional recovery from partial nephrectomy: Refined perspectives. Eur. Urol. Focus 4(4), 572–578 (2018).
Greco, F. et al. Ischemia techniques in nephron-sparing surgery: A systematic review and meta-analysis of surgical, oncological, and functional outcomes. Eur. Urol. 75(3), 477–491 (2019).
Volpe, A. et al. Renal ischemia and function after partial nephrectomy: A collaborative review of the literature. Eur. Urol. 68(1), 61–74 (2015).
Simone, G. et al. Indications, techniques, outcomes, and limitations for minimally ischemic and off-clamp partial nephrectomy: A systematic review of the literature. Eur. Urol. 68(4), 632–640 (2015).
Klatte, T. et al. A literature review of renal surgical anatomy and surgical strategies for partial nephrectomy. Eur. Urol. 68(6), 980–992 (2015).
Smith, G. L. et al. Non-clamped partial nephrectomy: Techniques and surgical outcomes. BJU Int. 107(7), 1054–1058 (2011).
Sharfuddin, A. A. & Molitoris, B. A. Pathophysiology of ischemic acute kidney injury. Nat. Rev. Nephrol. 7(4), 189–200 (2011).
Simmons, M. N. et al. Association between warm ischemia time and renal parenchymal atrophy after partial nephrectomy. J. Urol. 189(5), 1638–1642 (2013).
Bertolo, R. et al. Development and internal validation of a nomogram for predicting renal function after partial nephrectomy. Eur. Urol. Oncol. 2(1), 106–109 (2019).
Simmons, M. N. et al. Functional recovery after partial nephrectomy: Effects of volume loss and ischemic injury. J. Urol. 187(5), 1667–1673 (2012).
Kumar, P. et al. Qualitative and quantitative CECT features for differentiating renal primitive neuroectodermal tumor from the renal cell carcinoma and its subtypes. Br. J. Radiol. 92(1094), 20180738 (2019).
Blomley, M. & Dawson, P. The quantification of renal function with enhanced computed tomography. Br. J. Radiol. 69(827), 989–995 (1996).
Mir, M. C. et al. Decline in renal function after partial nephrectomy: Etiology and prevention. J. Urol. 193(6), 1889–1898 (2015).
Levin, A. et al. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. Suppl. 3, 1–150 (2013).
Quinlan, M. et al. Renal cell carcinoma follow-up-is it time to abandon ultrasound?. Curr. Urol. 13(1), 19–24 (2019).
Waseda, Y. et al. Predictive ability of renal cortex enhancement in dynamic computed tomography for residual renal function after nephroureterectomy: Comparison with 99mTc-diethylenetriaminopentacetic acid renography and validation study. J. Urol. 26(2), 266–272 (2019).
Antonelli, A. et al. Is off-clamp robot-assisted partial nephrectomy beneficial for renal function? Data from the CLOCK trial. BJU Int. 129(2), 217–224 (2022).
Anderson, B. G. et al. Comparing off-clamp and on-clamp robot-assisted partial nephrectomy: A prospective randomized trial. Urology 126, 102–109 (2019).
Martin, G. L. et al. Comparison of total, selective, and nonarterial clamping techniques during laparoscopic and robot-assisted partial nephrectomy. J. Endourol. 26(2), 152–156 (2012).
Xu, J. et al. Segmental artery clamping versus main renal artery clamping in nephron-sparing surgery: Updated meta-analysis. World J. Surg. Oncol. 18(1), 210 (2020).
Cacciamani, G. E. et al. Impact of renal hilar control on outcomes of robotic partial nephrectomy: Systematic review and cumulative meta-analysis. Eur. Urol. 5(4), 619–635 (2019).
Boga, M. S. & Sonmez, M. G. Long-term renal function following zero ischemia partial nephrectomy. Res. Rep. Urol. 11, 43–52 (2019).
Deng, W. et al. Off-clamp partial nephrectomy has a positive impact on short- and long-term renal function: A systematic review and meta-analysis. BMC Nephrol. 19(1), 188 (2018).
Mina-Riascos, S. H., Vitagliano, G. & Garcia-Perdomo, H. A. Effectiveness and safety of partial nephrectomy-no ischemia vs warm ischemia: Systematic review and meta-analysis. Investig. Clin. Urol. 61(5), 464–474 (2020).
Isharwal, S. et al. Impact of comorbidities on functional recovery from partial nephrectomy. J. Urol. 199(6), 1433–1439 (2018).
Novick, A. C. Renal hypothermia: In vivo and ex vivo. Urol. Clin. N. Am. 10(4), 637–644 (1983).
Funahashi, Y. et al. Comparison of warm and cold ischemia on renal function after partial nephrectomy. Urology 84(6), 1408–1413 (2014).
Venkatachalam, M. A. et al. Acute kidney injury: A springboard for progression in chronic kidney disease. Am. J. Physiol. Renal. Physiol. 298(5), F1078–F1094 (2010).
Mir, M. C., Pavan, N. & Parekh, D. J. Current paradigm for ischemia in kidney surgery. J. Urol. 195(6), 1655–1663 (2016).
Zhang, Z. et al. Analysis of atrophy after clamped partial nephrectomy and potential impact of ischemia. Urology 85(6), 1417–1422 (2015).
Maruschke, M. et al. Prognostic value of intraoperative measurements of renal tissue oxygenation and microcirculation on renal function in partial nephrectomy. Clin. Exp. Nephrol. 22(3), 735–742 (2018).
Bertolo, R. et al. cT1a renal masses less than 2 versus 2 cm or greater managed by robotic partial nephrectomy: A propensity score matched comparison of perioperative outcomes. J. Urol. 201(1), 56–61 (2019).
Watts, K. L. et al. Value of nephrometry score constituents on perioperative outcomes and split renal function in patients undergoing minimally invasive partial nephrectomy. Urology 99, 112–117 (2017).
Funding
The authors received no financial support for the research, authorship and publication of this article.
Author information
Authors and Affiliations
Contributions
J.L., Y.C.H. and H.J. conceptualized this study. J.L. and Y.C.H. performed the analyses and drafted the manuscript and revisions. S.Y., M.S.C., M.C.C., H.S. and H.J. were responsible for the treatment of patients. J.L. and Y.C.H. collected the patients’ data and analyzed statistically. J.L., Y.C.H., S.Y., M.S.C., M.C.C., H.S. and H.J. validated the analysis and reviewed the manuscript. H.J. supervised this study, and confirmed final manuscript. All authors approved the results of the data analysis and the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lee, J., Hwang, Y.C., Yoo, S. et al. Changes in kidney function according to ischemia type during partial nephrectomy for T1a kidney cancer. Sci Rep 12, 4223 (2022). https://doi.org/10.1038/s41598-022-07919-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-07919-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.